Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Analysis of Ni–ZnO Monolayer
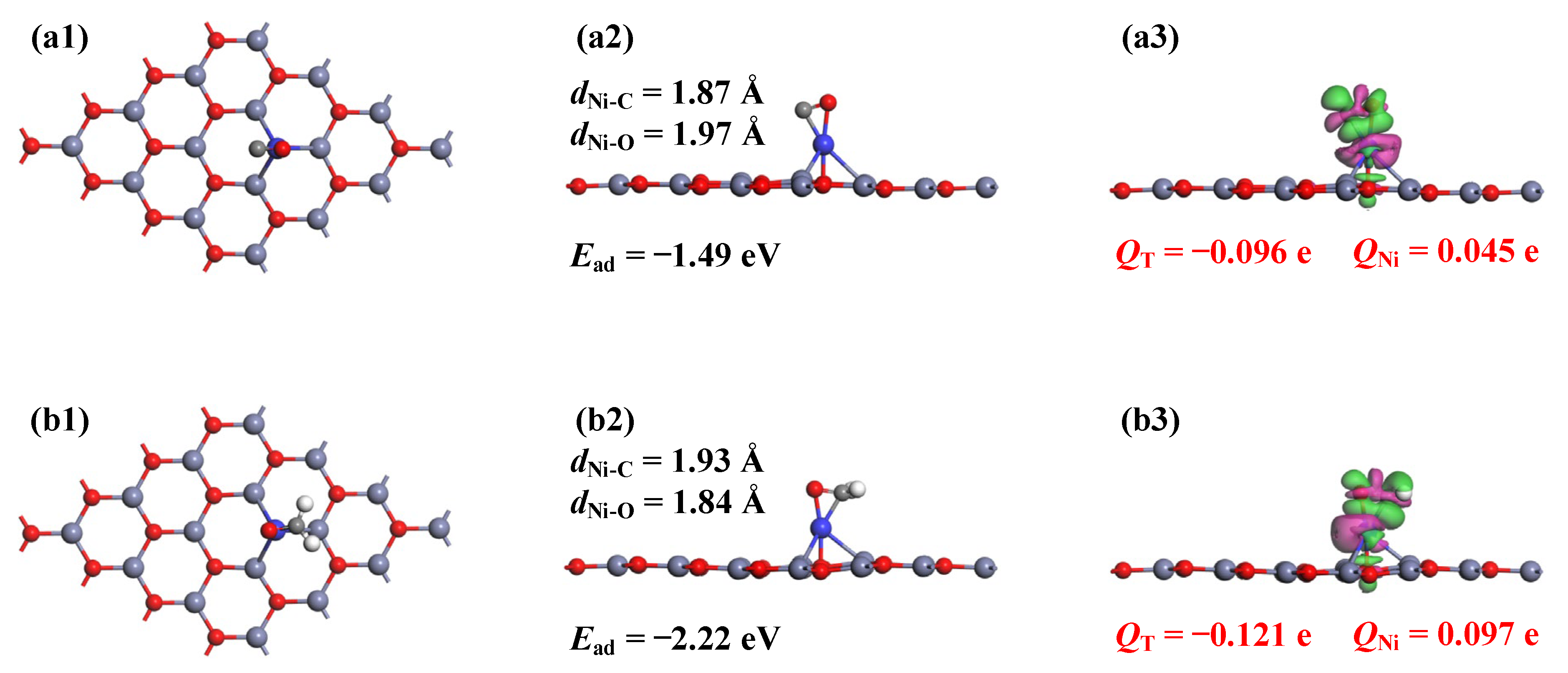
3.2. Analysis of Gas Adsorptions
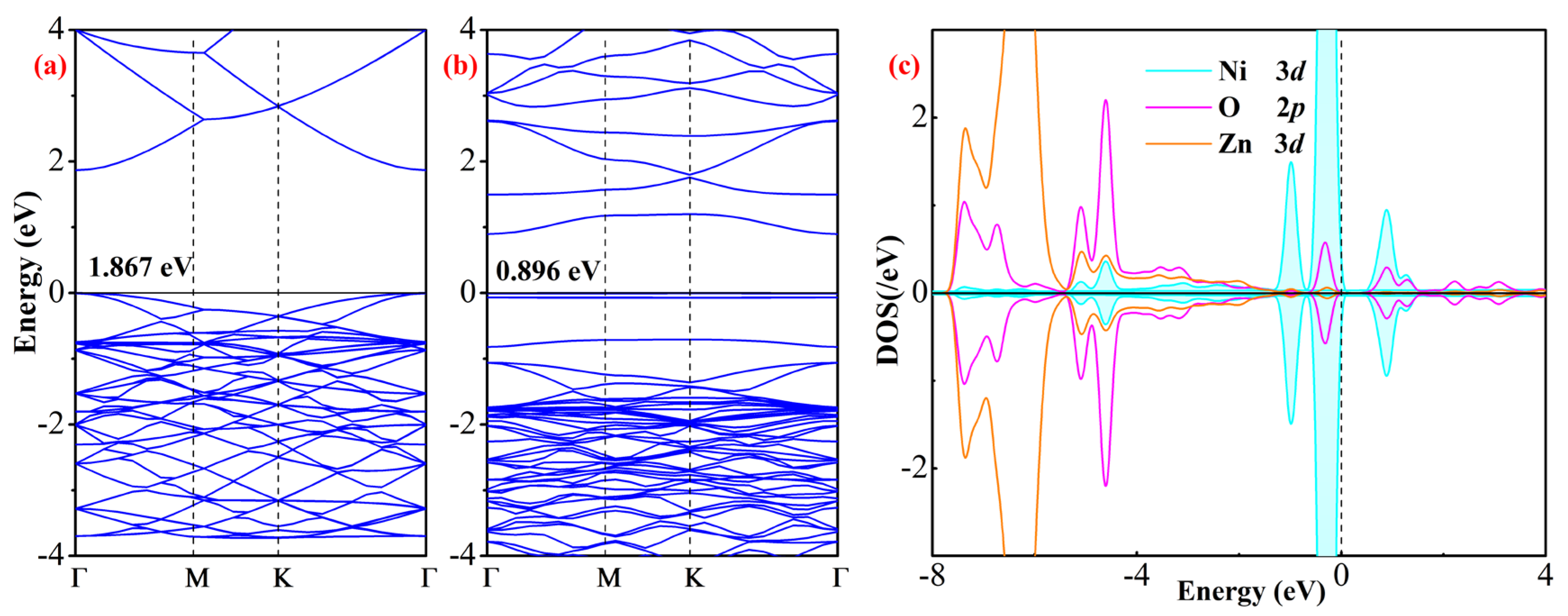
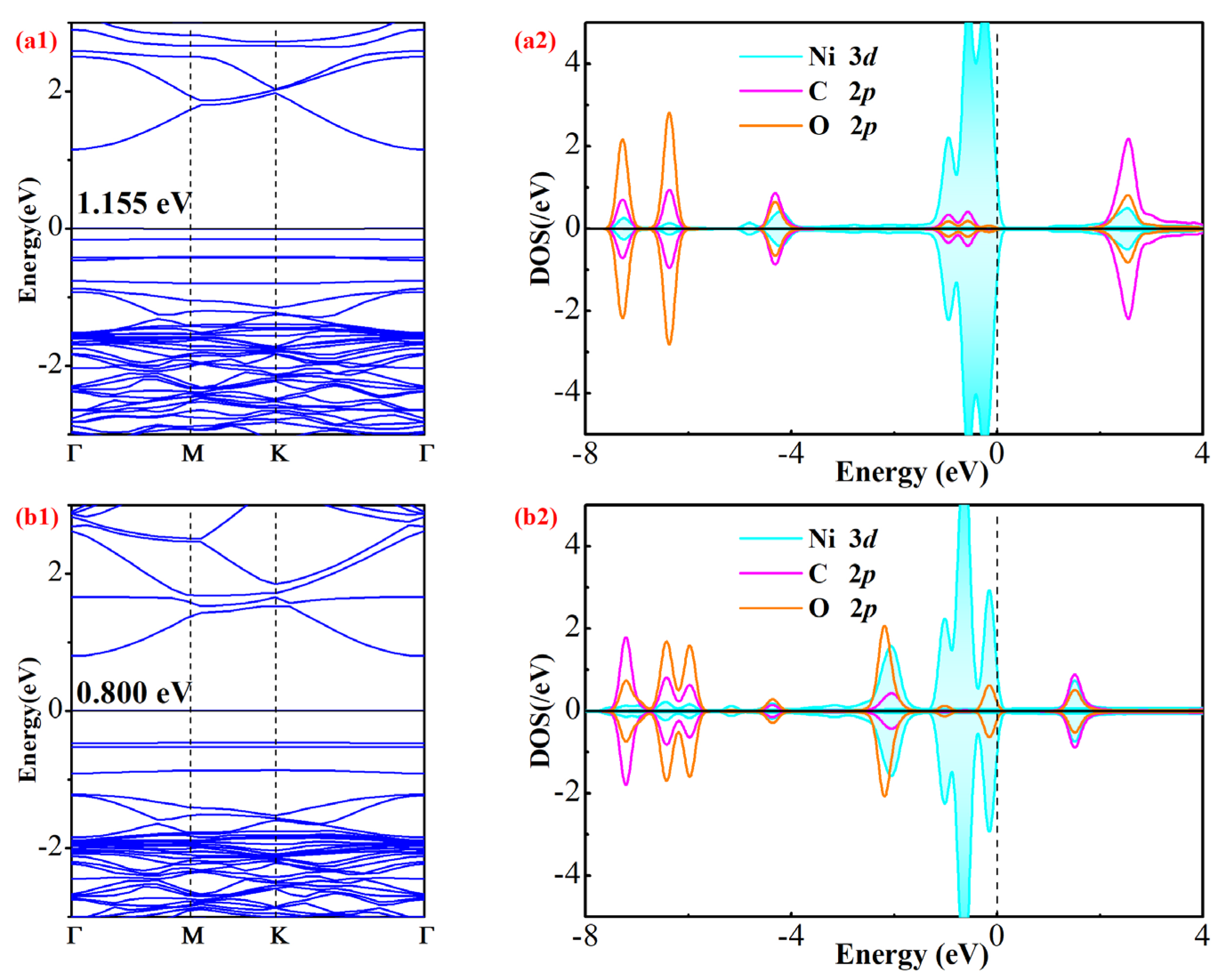
3.3. Electronic Property Analysis
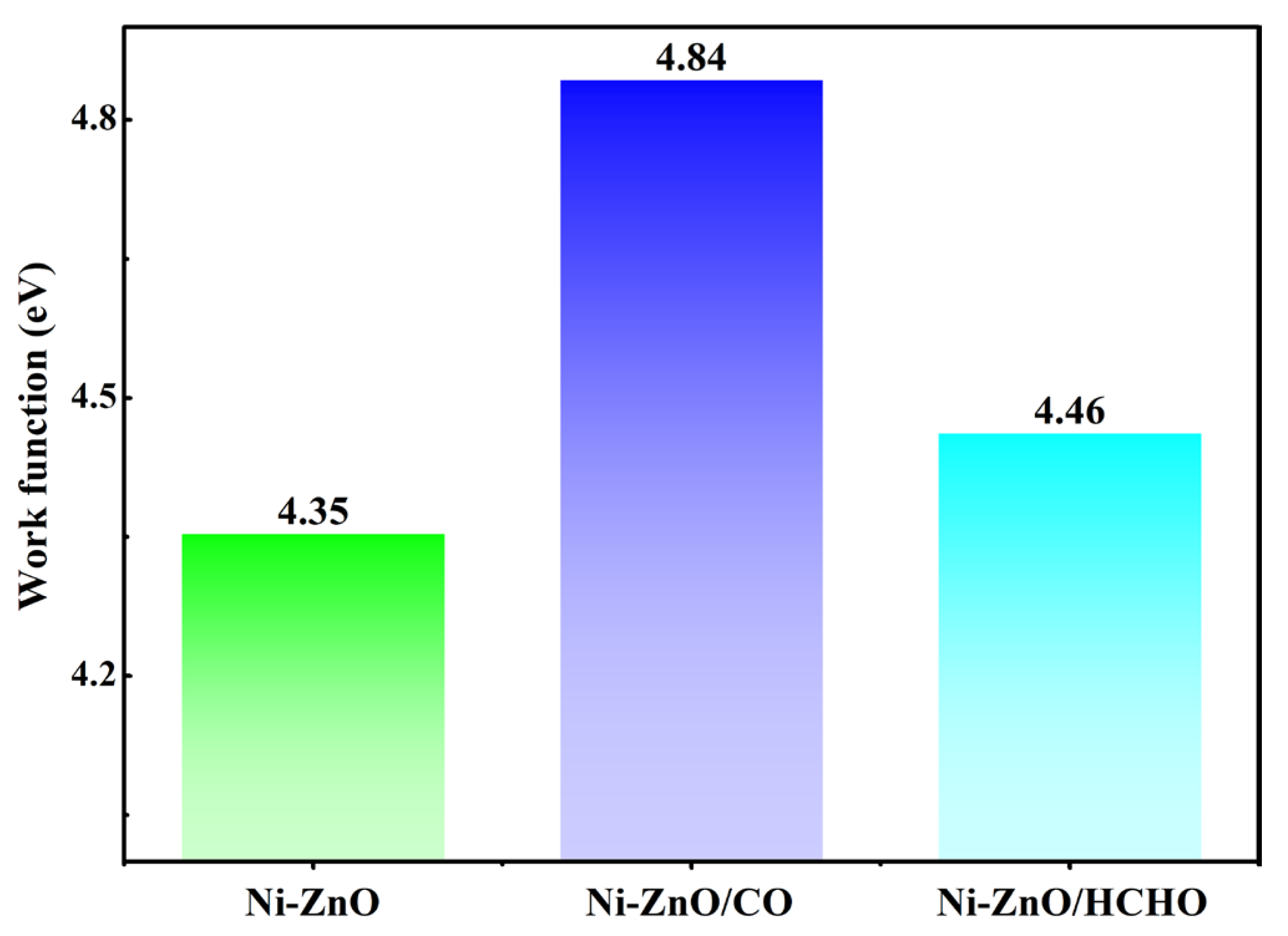
3.4. Gas Sensor Explorations
4. Conclusions
- (i)
- The TO site is identified as the most preferred site for Ni-decorating on the pristine ZnO surface, with the Eb of −1.75 eV;
- (ii)
- Chemisorption is determined for CO and HCHO systems given the admirable Ead of −1.49 and −2.22 eV for their adsorption on the Ni–ZnO surface;
- (iii)
- BS and WF analysis reveal the potential of Ni–ZnO monolayer as a resistance-type or Kelvin Probe gas sensor for CO and HCHO detection with high sensitivity and selectivity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, T.; Fu, C.; Gong, Y.; Tong, Y.; Zhang, J.; Wang, Y. First-principles Insights into Cu-Decorated GaN Monolayers for Sensing CO and HCHO in Dry-Type Transformers. ACS Omega 2021, 6, 19127–19133. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wang, J.; Si, Q.; Shi, T.; Ma, S. Cu-decorated ZnO monolayer as a promising gas sensor in dry-type transformers: A first-principles study. Comput. Theor. Chem. 2021, 1204, 113429. [Google Scholar] [CrossRef]
- Li, Y.; Guan, Y.J.; Li, T.Y. Calculation of Thermal Performance in Amorphous Core Dry-Type Transformers. Adv. Mater. Res. 2014, 986–987, 1771–1774. [Google Scholar] [CrossRef]
- Xiong, L.; Zhao, Y.; Yang, Z.; Song, D.; He, W. Temperature rise analysis and calculation of cast resin dry-type transformers. Gaodianya Jishu/High Volt. Eng. 2013, 39, 265–271. [Google Scholar]
- Wang, Y.; Feng, C.; Fei, R.; Luo, Y. Thermal-ageing characteristics of dry-type transformer epoxy composite insulation. High Perform. Polym. 2020, 32, 095400832090643. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, L.; Xiong, L.; Zhao, Y.L.; Yang, Z.K.; Wei, H.E. On-line Temperature Monitoring and State Evaluation System for 10 kV Dry-type Transformers. Electr. Meas. Instrum. 2012, 49, 33. [Google Scholar]
- Park, C.Y.; Park, D.W.; Choi, J.S.; Kil, G.S. A Study on PD Detection Methods for Cast-resin Dry-type Transformers. J. Korean Inst. Electr. Electron. Mater. Eng. 2009, 22, 786–791. [Google Scholar]
- Patel, K.; Roondhe, B.; Dabhi, S.D.; Jha, P.K. A new flatland buddy as toxic gas scavenger: A first principles study. J. Hazard. Mater. 2018, 351, 337–345. [Google Scholar] [CrossRef]
- Cui, H.; Kai, Z.; Zhang, Y.; Ye, H.; Chen, X. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron. Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 2019, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Bradley, D. Graphene gas sensor. Mater. Today 2012, 15, 233. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Xiaoxing, Z.; Lei, Y.; Xiaoqing, W.; Weihua, H. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar]
- Cui, H.; Jia, P.; Peng, X.; Hu, X. Geometric, Electronic and Optical Properties of Pt-Doped C3N Monolayer Upon NOx Adsorption: A DFT Study. IEEE Sens. J. 2021, 21, 3602–3608. [Google Scholar] [CrossRef]
- Luo, L.; Sosnowchik, B.D.; Lin, L. Local vapor transport synthesis of zinc oxide nanowires for ultraviolet-enhanced gas sensing. Nanotechnology 2010, 21, 495502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xiong, Z.; Cui, Y.; Luo, H.; Gao, Y. Adsorption of C6H6 and C7H8 onto pristine and metal (Pd, Pt)-mediated ZnO monolayers: Electronic and gas sensing properties. Appl. Surf. Sci. 2021, 542, 148767. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Yue, L.-J.; Gong, F.-L.; Li, F.; Zhang, H.-L.; Chen, J.-L.J.V. Highly enhanced H2S gas sensing and magnetic performances of metal doped hexagonal ZnO monolayer. Vacuum 2017, 141, 109–115. [Google Scholar] [CrossRef]
- Qu, Y.; Ding, J.; Fu, H.; Peng, J.; Chen, H.J.A.S.S. Adsorption of CO, NO, and NH3 on ZnO monolayer decorated with noble metal (Ag, Au). Appl. Surf. Sci. 2020, 508, 145202. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, Q.; Yang, L.; Cui, H. Enhanced NOx adsorption and sensing properties of MoTe2 monolayer by Ni-doping: A first-principles study. Surf. Interfaces 2021, 26, 101372. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, F.; Deng, Y.; Volinsky, A.A.J.O. Structural, electrochemical and optical properties of Ni doped ZnO: Experimental and theoretical investigation. Optik 2020, 219, 165204. [Google Scholar] [CrossRef]
- Wu, L.; Hou, T.; Wang, Y.; Zhao, Y.; Guo, Z.; Li, Y.; Lee, S.-T. First-principles study of doping effect on the phase transition of zinc oxide with transition metal doped. J. Alloy. Compd. 2012, 541, 250–255. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Liu, F.; Jia, J.; Li, X.; Cao, L. C2H2 gas sensor based on Ni-doped ZnO electrospun nanofibers. Ceram. Int. 2013, 39, 2883–2887. [Google Scholar] [CrossRef]
- Li, P.; Hong, Q.; Wu, T.; Cui, H. SOF2 sensing by Rh-doped PtS2 monolayer for early diagnosis of partial discharge in the SF6 insulation device. Mol. Phys. 2021, 119, e1919774. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X. Ru-InN Monolayer as a Gas Scavenger to Guard the Operation Status of SF6 Insulation Devices: A First-Principles Theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Jr, D.S.R.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Møller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 171. [Google Scholar]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A Mater. Sci. Process. 2018, 124, 194. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Aretouli, K.E.; Tsipas, P.; Tsoutsou, D.; Marquez-Velasco, J.; Xenogiannopoulou, E.; Giamini, S.A.; Vassalou, E.; Kelaidis, N.; Dimoulas, A. Two-dimensional semiconductor HfSe2 and MoSe2/HfSe2 van der Waals heterostructures by molecular beam epitaxy. Appl. Phys. Lett. 2015, 106, 699. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Wang, Q.; Li, T.; Tang, Z.; Yang, G.; He, C.; Lu, Z. CO catalytic oxidation on Al-doped graphene-like ZnO monolayer sheets: A first-principles study. J. Mater. Chem. C 2015, 3, 9964–9972. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Murphy, L.R.; Meek, T.L.; Allred, A.L.; Allen, L.C. Evaluation and test of pauling’s electronegativity scale. J. Phys. Chem. A 2000, 104, 5867–5871. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A first principles investigation of the mechanical properties of g-ZnO: The graphene-like hexagonal zinc oxide monolayer. Comput. Mater. Sci. 2013, 68, 320–324. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I–V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Han, S.W.; Cha, G.B.; Park, Y.; Hong, S.C. Hydrogen physisorption based on the dissociative hydrogen chemisorption at the sulphur vacancy of MoS2 surface. Sci. Rep. 2017, 7, 7152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, Z.M.; Yang, J.; Li, S.; Jiang, Q. Enhancement of CO detection in Al doped graphene. Chem. Phys. Lett. 2008, 461, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Wang, Q.; Li, T.; He, C.; Ma, B.; Tang, Y.; Lu, Z.; Yang, Z. Repairing sulfur vacancies in the MoS2 monolayer by using CO, NO and NO2 molecules. J. Mater. Chem. C 2016, 4, 7093–7101. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, M.; Ma, D.; Lv, P.; Li, S.; Yang, Z.; Lu, Z.; Yang, M.; Ma, D.; Lv, P. CO oxidation on Mn-N4 porphyrin-like carbon nanotube: A DFT-D study. Appl. Surf. Sci. 2017, 426, 1232–1240. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Xiong, H.; Li, Y.; Tang, J.; Xiao, S.; Zhang, D. A First-Principles Study of the SF6 Decomposed Products Adsorbed Over Defective WS2 Monolayer as Promising Gas Sensing Device. IEEE Trans. Device Mater. Reliab. 2019, 19, 473–483. [Google Scholar] [CrossRef]
- A, X.H.; A, Y.G.; B, K.L.; A, L.X. Comparison of sensing and electronic properties of C2H2 on different transition metal oxide nanoparticles (Fe2O3, NiO, TiO2) modified BNNT (10, 0). Appl. Surf. Sci. 2020, 521, 146463. [Google Scholar]
- Cui, Z.; Wang, X.; Li, E.; Ding, Y.; Sun, C.; Sun, M. Alkali-metal-adsorbed g-GaN monolayer: Ultralow work functions and optical properties. Nanoscale Res. Lett. 2018, 13, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.J.S.; Chemical, A.B. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Verlag, S. Semiconductor Physical Electronics. Semicond. Phys. Electron. 2006, 28, 363–364. [Google Scholar]
- Zhang, D.; Wu, Z.; Zong, X.; Zhang, Y. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens. Actuators B Chem. 2018, 274, 575–586. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.; Zhang, Y.; Zhang, X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Feng, Z.; Xie, Y.; Chen, J.; Yu, Y.; Zheng, S.; Zhang, R.; Li, Q.; Chen, X.; Sun, C.; Zhang, H. Highly sensitive MoTe2 chemical sensor with fast recovery rate through gate biasing. 2D Mater. 2017, 4, 025018. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, J.; Zhang, Y. Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Volt. 2019, 4, 242–258. [Google Scholar] [CrossRef]
- Qazi, M.; Koley, G.; Park, S.; Vogt, T. NO2 detection by adsorption induced work function changes in In2O3 thin films. Appl. Phys. Lett. 2007, 91, 043113. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, Y.; Wei, Z.; Wang, Q.; Liang, Z.; Yuan, T. Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory. Chemosensors 2022, 10, 307. https://doi.org/10.3390/chemosensors10080307
Zhang J, Wang Y, Wei Z, Wang Q, Liang Z, Yuan T. Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory. Chemosensors. 2022; 10(8):307. https://doi.org/10.3390/chemosensors10080307
Chicago/Turabian StyleZhang, Jin, Yuqing Wang, Zhuo Wei, Qi Wang, Zhengbo Liang, and Tian Yuan. 2022. "Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory" Chemosensors 10, no. 8: 307. https://doi.org/10.3390/chemosensors10080307
APA StyleZhang, J., Wang, Y., Wei, Z., Wang, Q., Liang, Z., & Yuan, T. (2022). Ni-Decorated ZnO Monolayer for Sensing CO and HCHO in Dry-Type Transformers: A First-Principles Theory. Chemosensors, 10(8), 307. https://doi.org/10.3390/chemosensors10080307




