Nanowire Gas Sensor to Support Optical and Volatile Changes in the Production Chain of Fruit Jams
Abstract
:1. Introduction
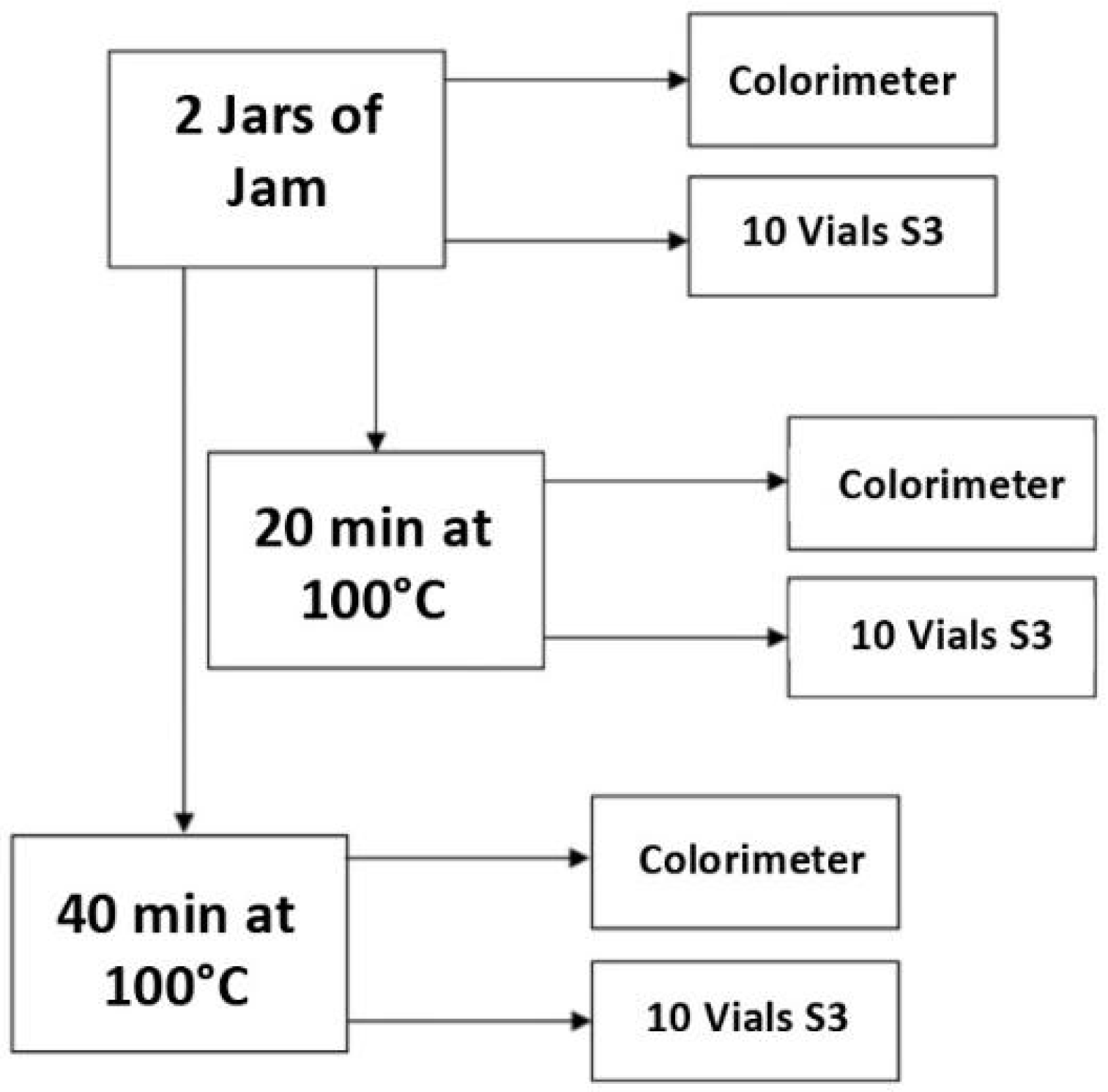
2. Materials and Methods
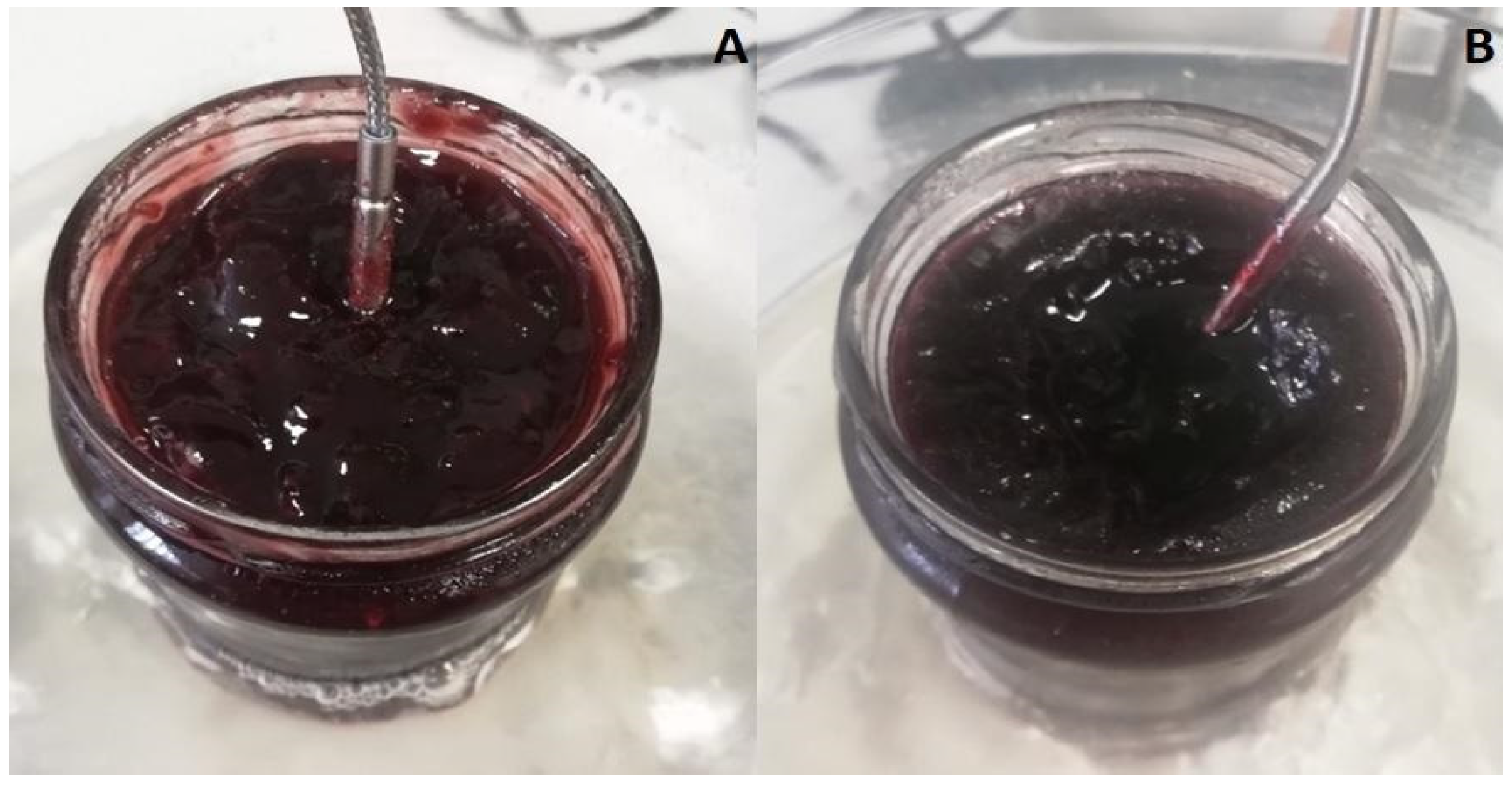
2.1. Sample Preparation
2.2. CIELab Optical Measurements
- L*: defines the brightness, this parameter could change from 0 to 100; therefore the color can change from white (100) to black (0).
- a*: first color coordinate, which defines the color from green (negative values) to red (positive values).
- b*: second color coordinate, which defines it from blue (negative values) to yellow (positive values).
2.3. Small Sensor Systems (S3)
- Pneumatic components, such as actuators, pumps, and pipes that make the sample inside the vial pass through the instrument;
- Electronic parts that manage the pneumatic components, flow systems, and collect the data produced by the sensors by sending it to the dedicated online web app;
- Sensor chamber, the main part, which can accommodate up to 10 sensors and is thermally insulated in order to have a controlled environment within which only VOC samples can enter.
2.4. Data Analysis
3. Results and Discussion
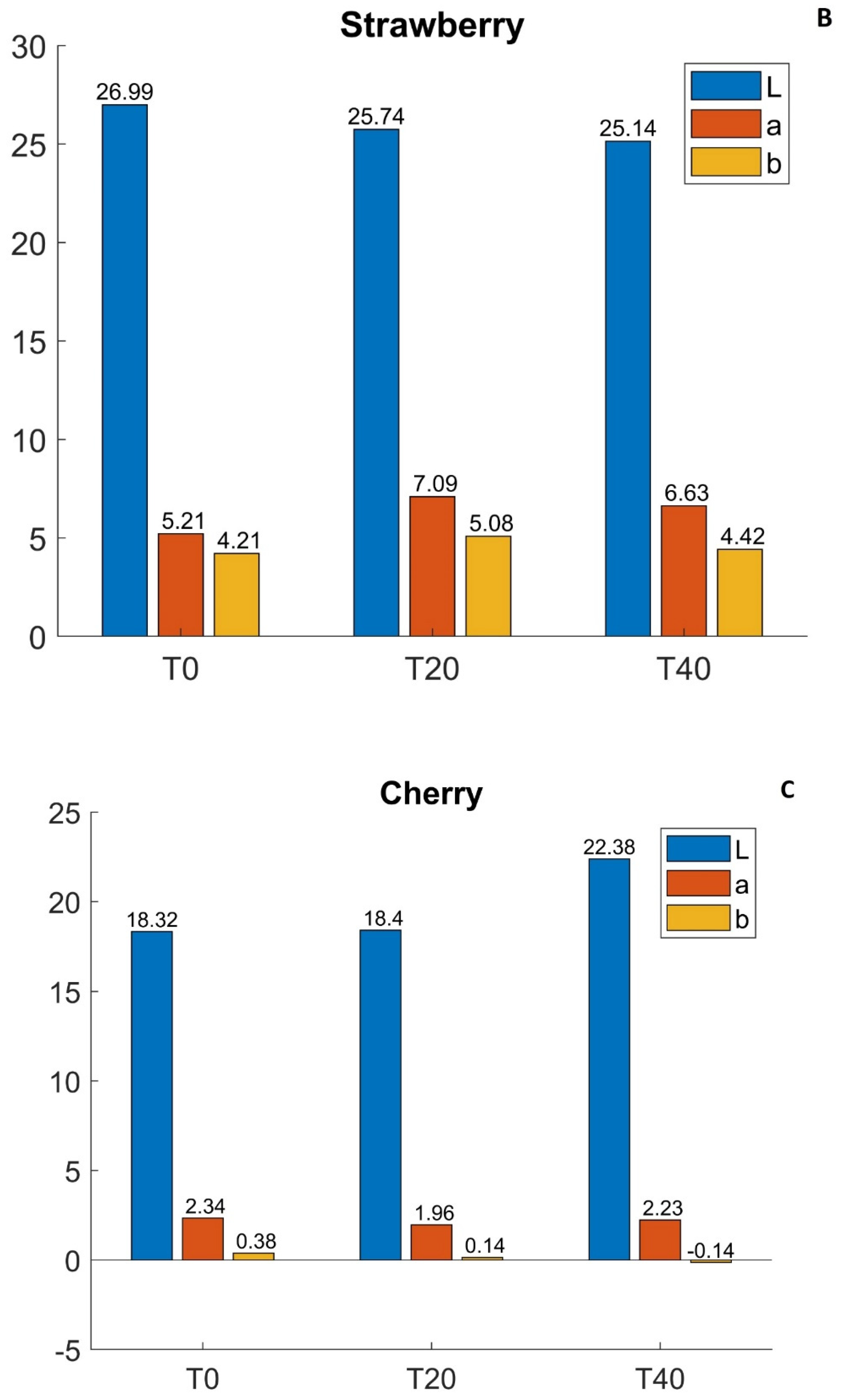
3.1. CIELab Optical Measurements
- before cooking (T0);
- after 20 min of cooking (T20);
- after 40 min of cooking (T40).
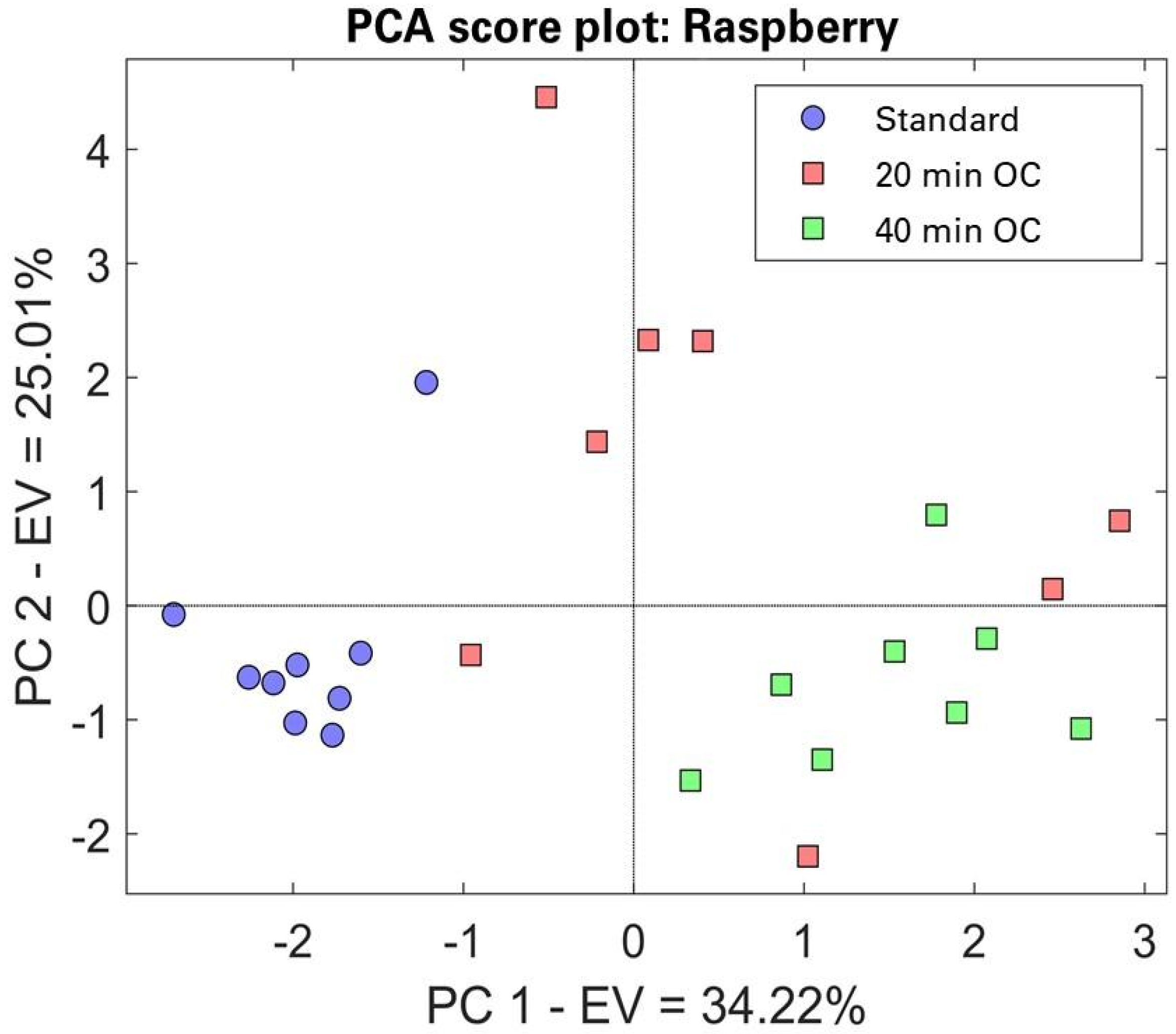
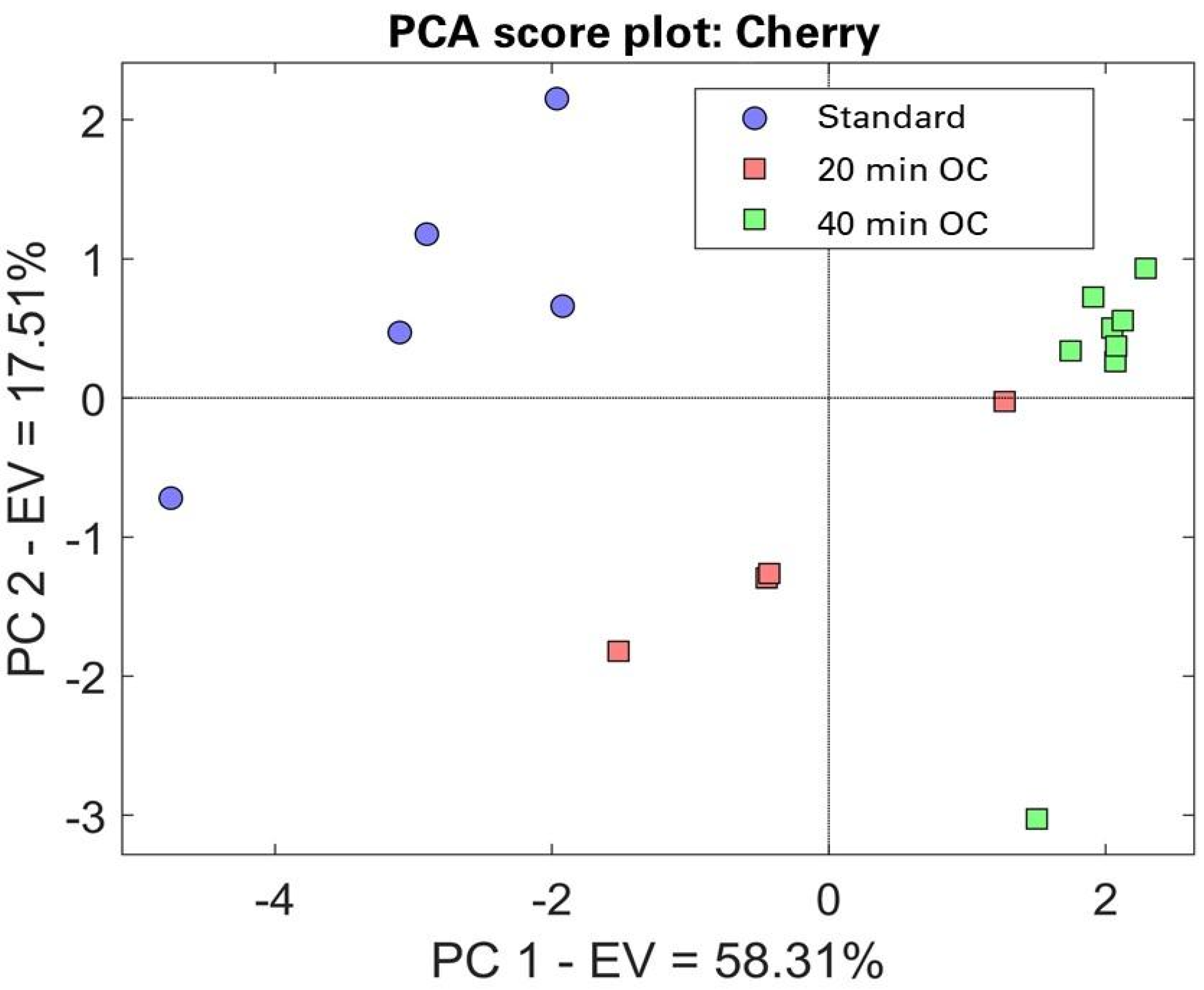
3.2. Small Sensor Systems (S3)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, D.A. Jams and preserves, Methods of Manufacture. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 3409–3415. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Zottele, I.; Piccoli, P.; Tamanini, A.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanomaterial Gas Sensors for Online Monitoring System of Fruit Jams. Foods 2019, 8, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Producers and the Fair-Trade Distribution Systems: What Are the Benefits and Problems? Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/sd.420 (accessed on 13 July 2020).
- Ellis, G.P. The Maillard Reaction. In Advances in Carbohydrate Chemistry, 2nd ed.; Wolfrom, M.L., Ed.; Elservier: Amsterdam, The Netherlands, 1959; Volume 14, pp. 63–134. [Google Scholar]
- Reineccius, G. Kinetics of flavor formation during Maillard browning. In Flavor Chemistry: Thirty Years of Progress, 2nd ed.; Teranishi, R., Wick, E.L., Hornstein, I., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 346–352. [Google Scholar]
- Barta, J.; Balla, C.; Vatai, G. Dehydration Preservation of Fruits. In Handbook of Fruits and Fruit Processing, 2nd ed.; Sinha, N.K., Sidhu, J.S., Barta, J., Wu, J.S.B., Cano, M.P., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 133–151. [Google Scholar]
- Friedman, M. Food Broening and Its Prevention: An Overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Delatour, T.; Huertas-Pérez, J.F.; Dubois, M.; Theurillat, X.; Desmarchelier, A.; Ernest, M.; Stadler, R.H. Thermal degradation of 2-furoic acid and furfuryl alcohol as pathways in the formation of furan and 2-methylfuran in food. Food Chem. 2020, 303, 125406. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, V.; Núñez-Carmona, E.; Comini, E.; Ponzoni, A.; Zappa, D.; Pirrotta, O.; Pulvirenti, A. A Novel Electronic Nose as Adaptable Device to Judge Microbiological Quality and Safety in Foodstuff. BioMed Res. Int. 2014, 2014, 529519. [Google Scholar] [CrossRef] [PubMed]
- Aimonino, D.R.; Comba, L.; Gay, P. Image processing for the determination of jam browning kinetics. In Proceedings of the Image Analysis for Agricultural Products and Processes, Workshop on Image analysis for agricultural processes and products, Potsdam, Germany, 27–28 August 2009; Bornim, E.V., Ed.; Institut fuer Agrartechnik, Bornimer Agrartechnische Berichte: Potsdam, Germany, 2009; pp. 132–137. [Google Scholar]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Comini, E.; Sberveglieri, G. k-NN and k-NN-ANN Combined Classifier to Assess MOX Gas Sensors Performances Affected by Drift Caused by Early Life Aging. Chemosensors 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. An Array of MOX Sensors and ANNs to Assess Grated Parmigiano Reggiano Cheese Packs’ Compliance with CFPR Guidelines. Biosensors 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Abbatangelo, M.; Zappa, D.; Comini, E.; Sberveglieri, G.; Sberveglieri, V. Nanostructured MOS Sensor for the Detection, Follow up, and Threshold Pursuing of Campylobacter Jejuni Development in Milk Samples. Sensors 2020, 20, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappa, D.; Bertuna, A.; Comini, E.; Kaur, N.; Poli, N.; Sberveglieri, V.; Sberveglieri, G. Metal oxide nanostructures: Preparation, characterization and functional applications as chemical sensors. Beilstein J. Nanotechnol. 2017, 8, 1205–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzoni, A.; Zappa, D.; Comini, E.; Sberveglieri, V.; Faglia, G.; Sberveglieri, G. Metal oxide nanowire gas sensors: Application of conductometric and surface ionization architectures. Chem. Eng. Trans. 2012, 30, 31–36. [Google Scholar] [CrossRef]
- Cano, M.P.; Marín, M.A. Pigment composition and color of frozen and canned kiwi fruit slices. J. Agric. Food Chem. 1992, 40, 2121–2146. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Eskin, N.A.M. Biochemistry of food processing: Browning reactions in foods. In Biochemistry of Foods, 2nd ed.; Eskin, N.A.M., Ed.; Academic Press: London, UK, 1990; pp. 240–295. [Google Scholar] [CrossRef]
- Lespinard, A.R.; Bambicha, R.R.; Mascheroni, R.H. Quality parameters assessment in kiwi jam during pasteurization. Modelling and optimization of the thermal process. Food Bioprod. Process. 2012, 90, 799–808. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.N.; Liu, L.-X.; Tang, X.-J.; Lei, S.-M.; Meng, X.-S.; Liu, Y.-G. Dynamics of microbial communities, physicochemical factors and flavor in rose jam during fermentation. LWT 2022, 155, 112920. [Google Scholar] [CrossRef]
- Umar, R.; Naz, A.; Razzaq, K.; Raza, N.; Farooq, U.; Sharif, M.; Naz, N.; Ahmad, S.; Waheed, U. Physiochemical comparison of black and green grapes varieties and sensory evaluation of jam in Punjab, Pakistan. Int. J. Agric. Ext. 2022, 10, 219–231. [Google Scholar]
- Estoquia, L.Q.; Teleron, J.I. Operations Research on Product Development, Sensory Evaluation, Consumer’s Acceptability of Coco Sweet Jam. Int. J. Adv. Eng. Res. Sci. 2022, 7, 289–292. [Google Scholar]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Support Tea Supply Chain. Sensors 2021, 21, 4266. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Carmona, E.N.; Sberveglieri, G.; Genzardi, D.; Sberveglieri, V. A New Kind of Chemical Nanosensors for Discrimination of Espresso Coffee. Chemosensors 2022, 10, 186. [Google Scholar] [CrossRef]



| Fruit Jam Kind | Ingredients | Sample Description | |
|---|---|---|---|
| Raspberry | Raspberry; grape sugar; gelling agent: pectin; concentrated elderberry juice. Fruit used 100 g per 100 g. | Standard | 10 |
| 20 min overcooked | 10 | ||
| 40 min overcooked | 10 | ||
| Strawberry | Strawberry; grape sugar; gelling agent: pectin; acidity corrector: citric acid; concentrated elderberry juice. Fruit used 70 g per 100 g. | Standard | 10 |
| 20 min overcooked | 10 | ||
| 40 min overcooked | 10 | ||
| Cherry | Cherries; sugar; concentrated lemon juice; gelling agent: pectin; acidity corrector: calcium citrate. Fruit used 50 g per 100 g of product. | Standard | 10 |
| 20 min overcooked | 10 | ||
| 40 min overcooked | 10 | ||
| Materials | Type of Sensor | Working Temperature (°C) |
|---|---|---|
| SnO2 + Au | RGTO | 400 |
| SnO2 + Au | RGTO | 300 |
| CuO | Nanowire | 350 |
| SnO2 + Au | Nanowire | 350 |
| SnO2 | Nanowire | 350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Carmona, E.; Greco, G.; Genzardi, D.; Piccoli, P.; Zottele, I.; Tamanini, A.; Sberveglieri, G.; Sberveglieri, V. Nanowire Gas Sensor to Support Optical and Volatile Changes in the Production Chain of Fruit Jams. Chemosensors 2022, 10, 345. https://doi.org/10.3390/chemosensors10090345
Núñez-Carmona E, Greco G, Genzardi D, Piccoli P, Zottele I, Tamanini A, Sberveglieri G, Sberveglieri V. Nanowire Gas Sensor to Support Optical and Volatile Changes in the Production Chain of Fruit Jams. Chemosensors. 2022; 10(9):345. https://doi.org/10.3390/chemosensors10090345
Chicago/Turabian StyleNúñez-Carmona, Estefanía, Giuseppe Greco, Dario Genzardi, Pierpaolo Piccoli, Ivano Zottele, Armando Tamanini, Giorgio Sberveglieri, and Veronica Sberveglieri. 2022. "Nanowire Gas Sensor to Support Optical and Volatile Changes in the Production Chain of Fruit Jams" Chemosensors 10, no. 9: 345. https://doi.org/10.3390/chemosensors10090345
APA StyleNúñez-Carmona, E., Greco, G., Genzardi, D., Piccoli, P., Zottele, I., Tamanini, A., Sberveglieri, G., & Sberveglieri, V. (2022). Nanowire Gas Sensor to Support Optical and Volatile Changes in the Production Chain of Fruit Jams. Chemosensors, 10(9), 345. https://doi.org/10.3390/chemosensors10090345









