A Novel Voltammetric Electronic Tongue Based on Nanocomposites Modified Electrodes for the Discrimination of Red Wines from Different Geographical Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Wine Samples
2.3. Preparation of Nanocomposites Modified Electrodes
2.3.1. Preparation of Poly-Aspartic Acid/Nickel Oxide/GCE
2.3.2. Preparation of Copper/Poly-Tryptophan/Nafion/GCE
2.3.3. Preparation of Copper/Poly-Neutral Red/GCE
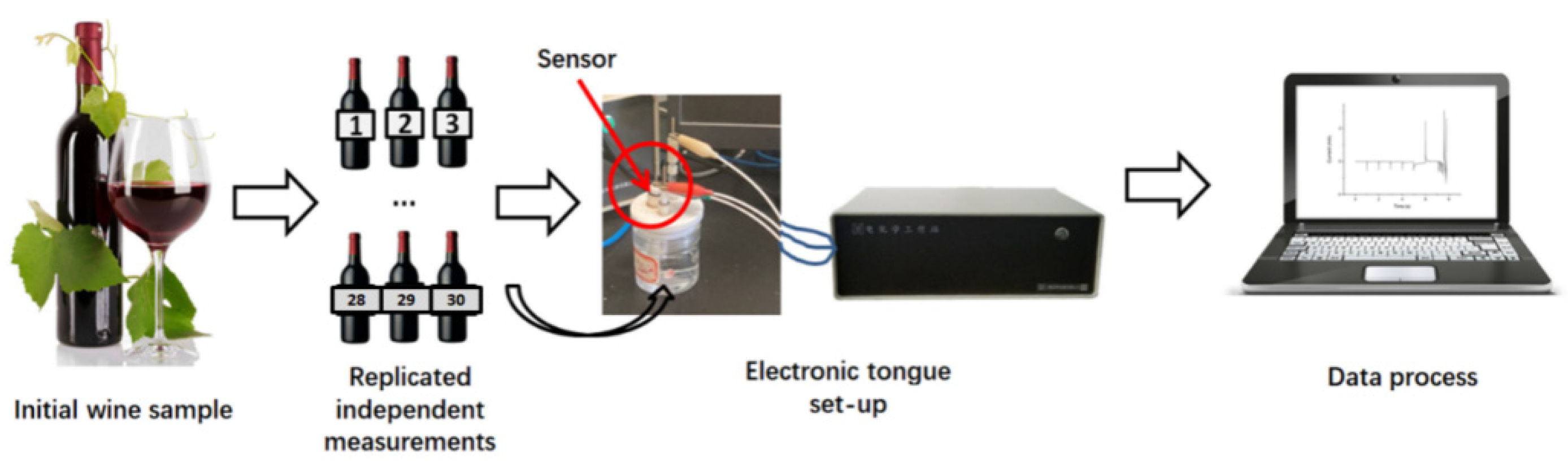
2.4. Experiment Procedures
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Working Electrodes
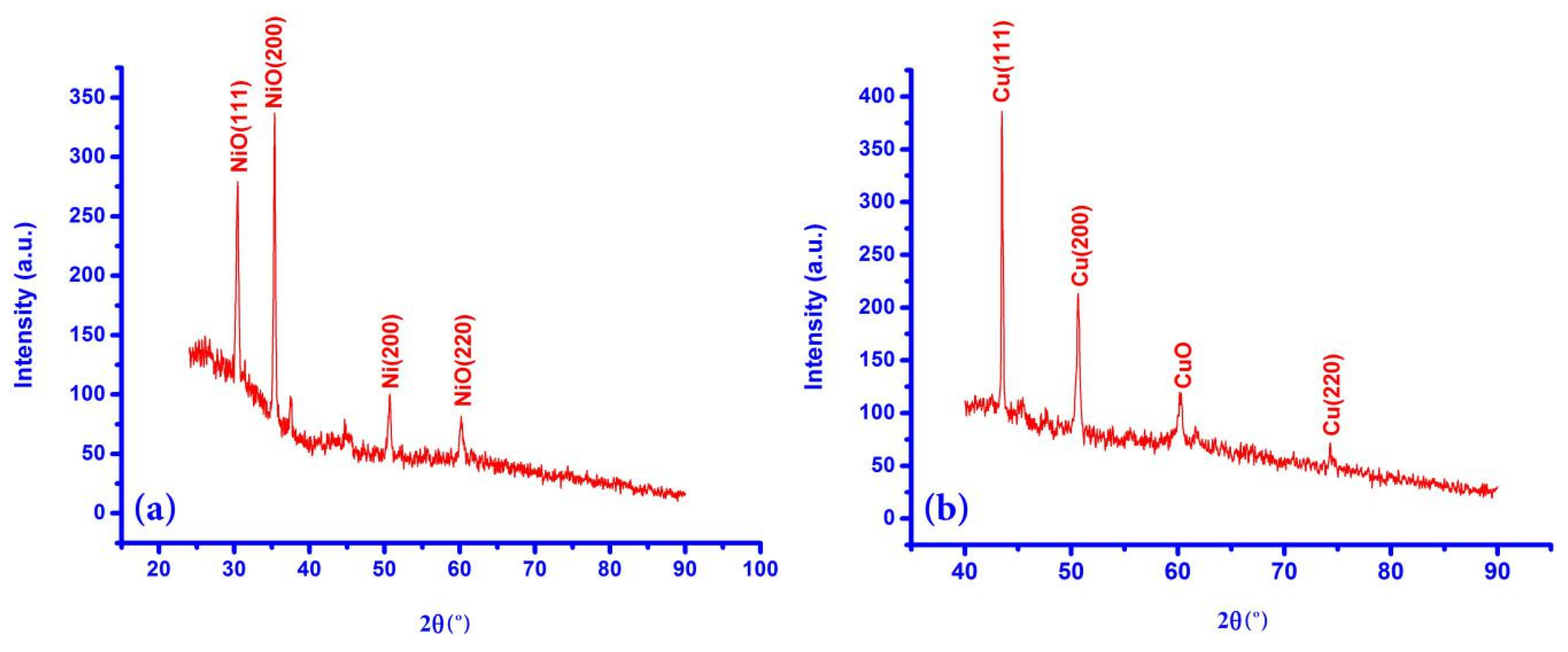
3.1.1. XRD Patterns of Working Electrodes
3.1.2. Morphologies of Working Electrodes
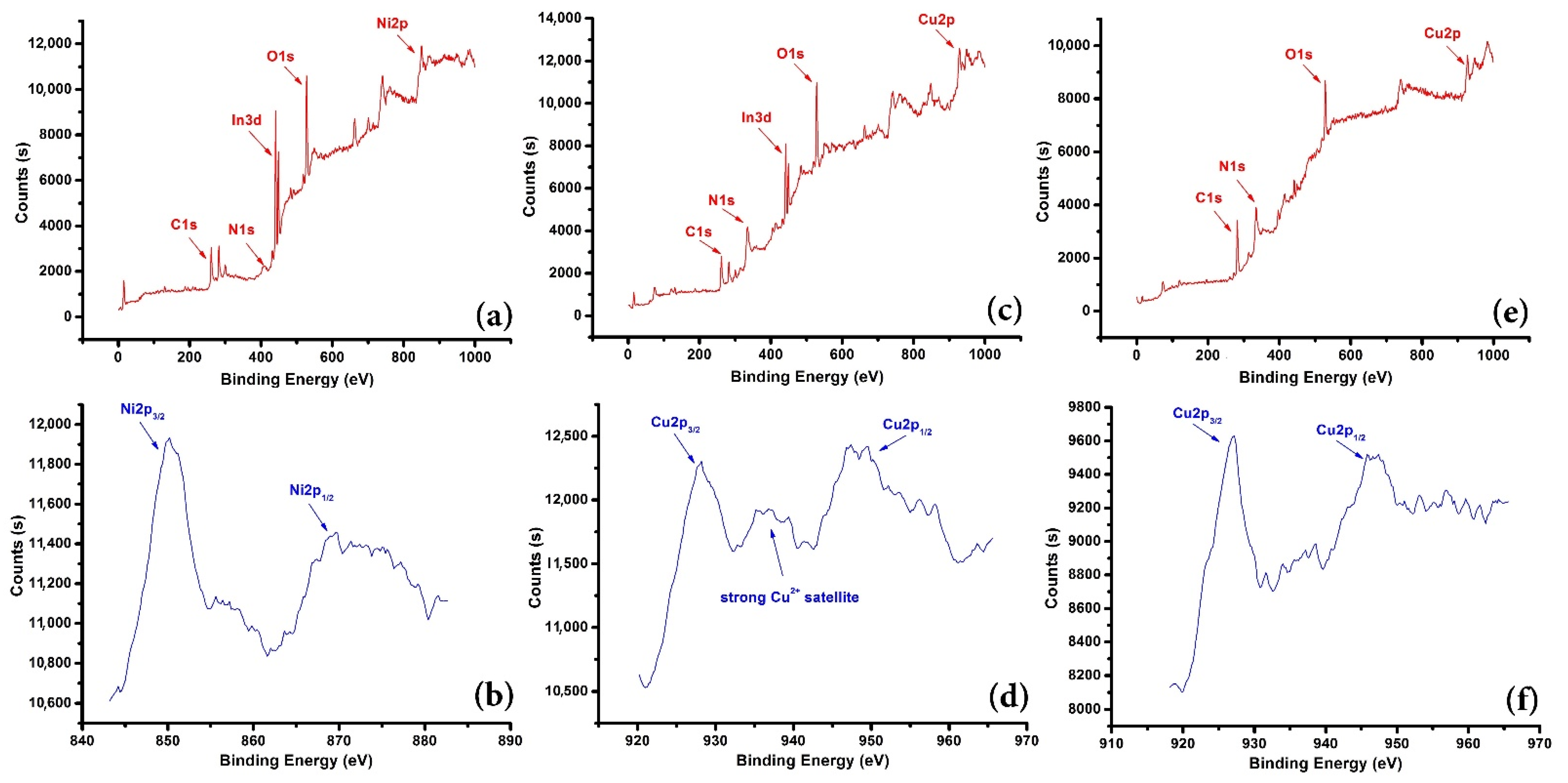
3.1.3. XPS Pattern of Working Electrodes
3.2. Electrochemical Behaviors of Target Substances at Working Electrodes
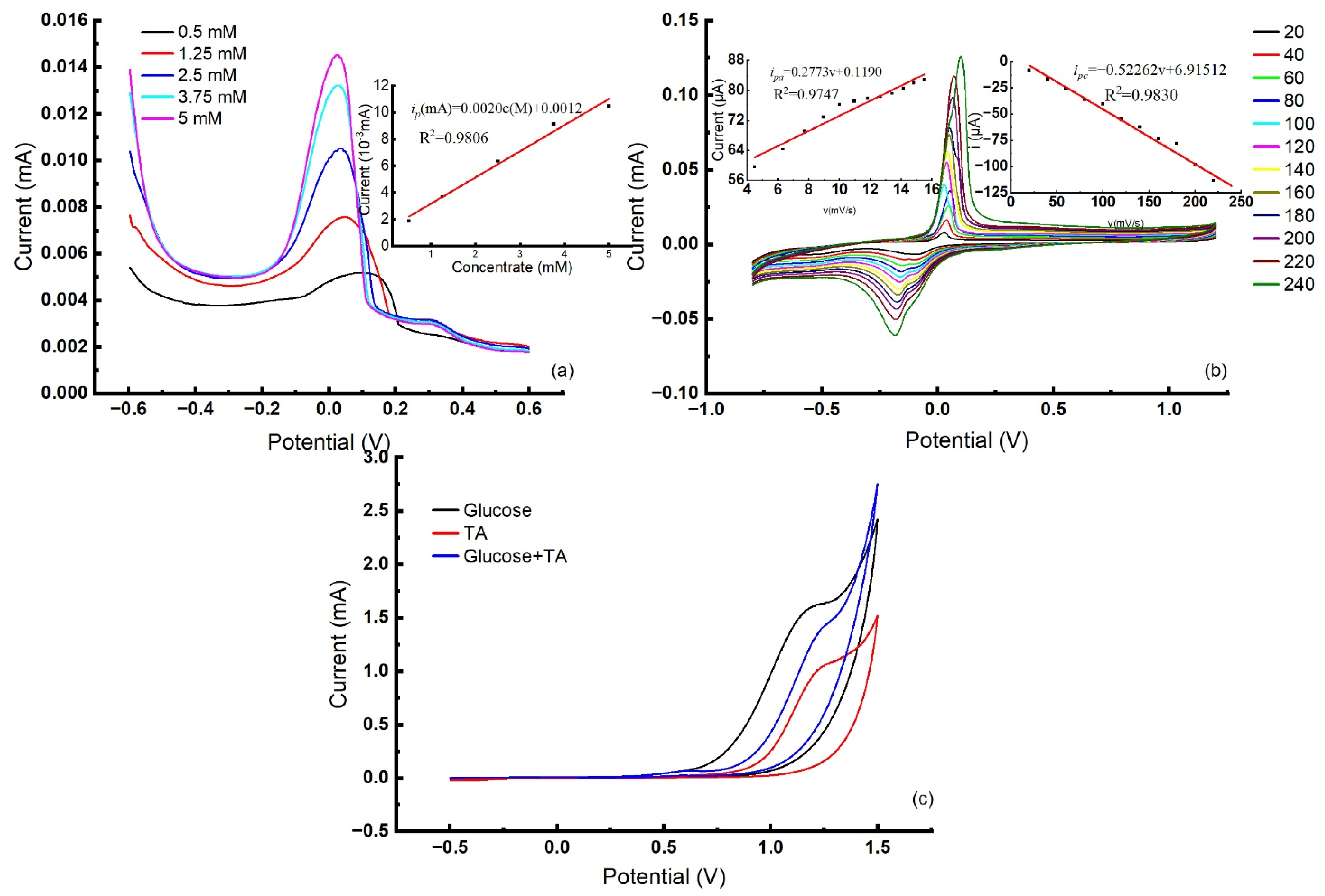
3.2.1. Electrochemical Behaviors of Glu at PASP/NiO/GCE
3.2.2. Electrochemical Behaviors of TA at Cu/PTRP/Nafion/GCE
3.2.3. Electrochemical Behaviors of Glu and TA at Cu/PNR/GCE
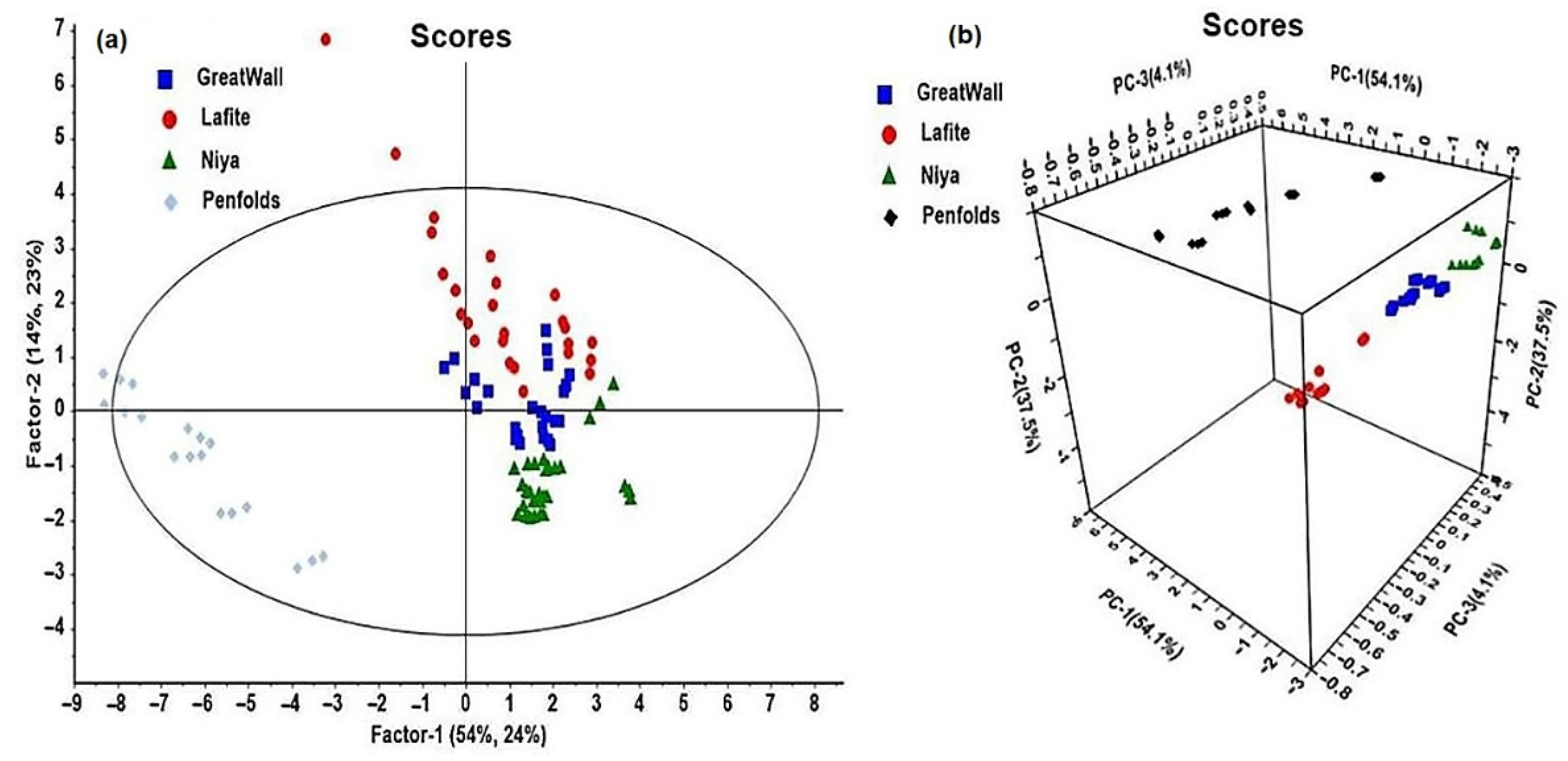
3.3. Tests on Wine Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bronzi, B.; Brilli, C.; Beone, G.M.; Fontanella, M.C.; Ballabio, D.; Todeschini, R.; Consonni, V.; Grisoni, F.; Parri, F.; Buscema, M. Geographical identification of Chianti red wine based on ICP-MS element composition. Food Chem. 2020, 315, 126248. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Pérez-Jiménez, M.; Pozo-Bayón, M.A. Oral persistence of esters is affected by wine matrix composition. Food Res. Int. 2020, 135, 109286. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shuxia, H.; Junhong, Y.; Shumin, H.; Zhaoxia, Y.; Shuli, H.; Yuxin, Z. Beer taste evaluation using electronic tongue and the relationship between sensor information and flavor compounds. Food Ferment. Ind. 2019, 45, 196–201. [Google Scholar]
- Kutyła-Olesiuk, A.; Wesoły, M.; Wróblewski, W. Hybrid electronic tongue as a tool for the monitoring of wine fermentation and storage process. Electroanalysis 2018, 30, 1983–1989. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.G.; Veloso, A.C.A.; Peres, A.M.; Pereira, J.A. Impact of thermal sterilization on the physicochemical-sensory characteristics of Californian-style black olives and its assessment using an electronic tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Walsh, E.A.; Diako, C.; Smith, D.M.; Ross, C.F. Influence of storage time and elevated ripening temperature on the chemical and sensory properties of white Cheddar cheese. J. Food Sci. 2020, 85, 268–278. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Han, F.; Zhang, D.; Aheto, J.H.; Feng, F.; Duan, T. Integration of a low-cost electronic nose and a voltammetric electronic tongue for red wines identification. Food Sci. Nutr. 2020, 8, 4330–4339. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Schmidtke, L.M.; Reis, A.; Domingues, M.R.M.; Delgadillo, I.; Debus, B.; Kirsanov, D.; Legin, A. Measurements of the effects of wine maceration with oak chips using an electronic tongue. Food Chem. 2017, 229, 20–27. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Moreno-Barón, L.; Pividori, M.I.; Alegret, S.; del Valle, M. A voltammetric electronic tongue made of modified epoxy-graphite electrodes for the qualitative analysis of wine. Microchim. Acta 2010, 169, 261–268. [Google Scholar] [CrossRef]
- Milovanovic, M.; Žeravík, J.; Obořil, M.; Pelcová, M.; Lacina, K.; Cakar, U.; Petrovic, A.; Glatz, Z.; Skládal, P. A novel method for classification of wine based on organic acids. Food Chem. 2019, 284, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, C.; de Oliveira, P.R.; Bonet San Emeterio, M.; González-Calabuig, A.; del Valle, M.; Salvio Mangrich, A.; Humberto Marcolino Junior, L.; Bergamini, M.F. Voltammetric electronic tongue based on carbon paste electrodes modified with biochar for phenolic compounds stripping detection. Electroanalysis 2019, 31, 2238–2245. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T. NiCo2O4 Nanorods decorated MoS2 nanosheets synthesized from deep eutectic solvents and their application for electrochemical sensing of glucose in red wine and honey. J. Electrochem. Soc. 2019, 166, H404–H411. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Z.; Xu, C.; Zhang, K.; Wang, W.; Zhang, Z. Comparative analysis of organic acids in wine grape fruits from Xinjiang. Food Sci. Technol. Res. 2020, 36, 249–254. [Google Scholar]
- Yalcin, D.; Özçalık, O.; Altıok, E.; Bayraktar, O. Characterization and recovery of tartaric acid from wastes of wine and grape juice industries. J. Therm. Anal. Calorim. 2008, 94, 767–771. [Google Scholar] [CrossRef]
- Wang, H.; Ni, Z.J.; Ma, W.P.; Song, C.B.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Effect of sodium sulfite, tartaric acid, tannin, and glucose on rheological properties, release of aroma compounds, and color characteristics of red wine. Food Sci. Biotechnol. 2019, 28, 395–403. [Google Scholar] [CrossRef]
- Donmez, S. A novel electrochemical glucose biosensor based on a poly (L-aspartic acid)-modified carbon-paste electrode. Prep. Biochem. Biotechnol. 2020, 50, 961–967. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, F.F.; Wei, T.; Pei, L.Z. Facile synthesis of Cu bismuthate nanosheets and sensitive electrochemical detection of tartaric acid. J. Alloys Compd. 2017, 723, 1062–1069. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Zhang, W.; Wei, Z. Application of the voltammetric electronic tongue based on nanocomposite modified electrodes for identifying rice wines of different geographical origins. Anal. Chim. Acta 2019, 1050, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, J.; Shao, W.; Wang, J. Fabrication and application of three-dimensional nanocomposites modified electrodes for evaluating the aging process of Huangjiu (Chinese rice wine). Food Chem. 2022, 372, 131158. [Google Scholar] [CrossRef] [PubMed]



| Sample | Brand | Denominations of Origins | Type of Grape |
|---|---|---|---|
| S1 | Greatwall | Shacheng, Hebei, China | Cabernet Sauvignon |
| S2 | Lafite | France | Cabernet Sauvignon |
| S3 | Penfolds | Australia | Cabernet Sauvignon, Shiraz |
| S4 | Niya | Changji, Xinjiang, China | Cabernet Sauvignon |
| Method | Parameter 1 | Training Accuracy | Prediction Accuracy |
|---|---|---|---|
| BPNN | 18-64-64-4 | 100% | 95.8% |
| DNN | 18-64-8-64-4 | 100% | 94.5% |
| RF | 25 | 100% | 91.7% |
| SVM | radial basis function | 98.9% | 79.2% |
| KNN | 5 | 90.6% | 75% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Qiu, S.; Wei, Z. A Novel Voltammetric Electronic Tongue Based on Nanocomposites Modified Electrodes for the Discrimination of Red Wines from Different Geographical Origins. Chemosensors 2022, 10, 332. https://doi.org/10.3390/chemosensors10080332
Zheng Z, Qiu S, Wei Z. A Novel Voltammetric Electronic Tongue Based on Nanocomposites Modified Electrodes for the Discrimination of Red Wines from Different Geographical Origins. Chemosensors. 2022; 10(8):332. https://doi.org/10.3390/chemosensors10080332
Chicago/Turabian StyleZheng, Ziwei, Shanshan Qiu, and Zhenbo Wei. 2022. "A Novel Voltammetric Electronic Tongue Based on Nanocomposites Modified Electrodes for the Discrimination of Red Wines from Different Geographical Origins" Chemosensors 10, no. 8: 332. https://doi.org/10.3390/chemosensors10080332
APA StyleZheng, Z., Qiu, S., & Wei, Z. (2022). A Novel Voltammetric Electronic Tongue Based on Nanocomposites Modified Electrodes for the Discrimination of Red Wines from Different Geographical Origins. Chemosensors, 10(8), 332. https://doi.org/10.3390/chemosensors10080332








