Ba-Modified ZnO Nanorods Loaded with Palladium for Highly Sensitive and Rapid Detection of Methane at Low Temperatures
Abstract
:1. Introduction
2. Materials and Methods
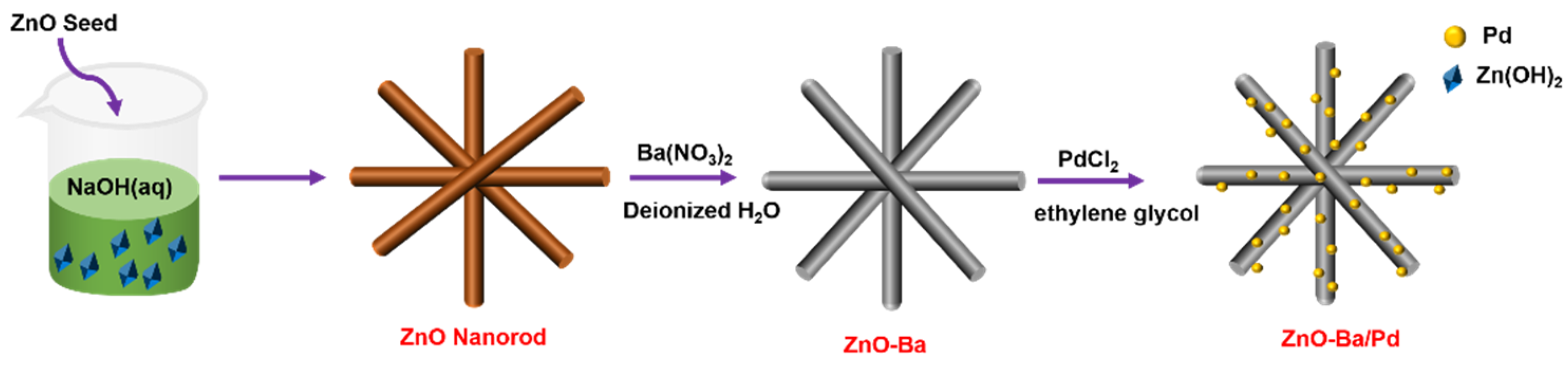
2.1. Synthesis
2.2. Characterization
2.3. Sensor Fabrication and Response Measurement
2.4. Catalytic Measurements
3. Results
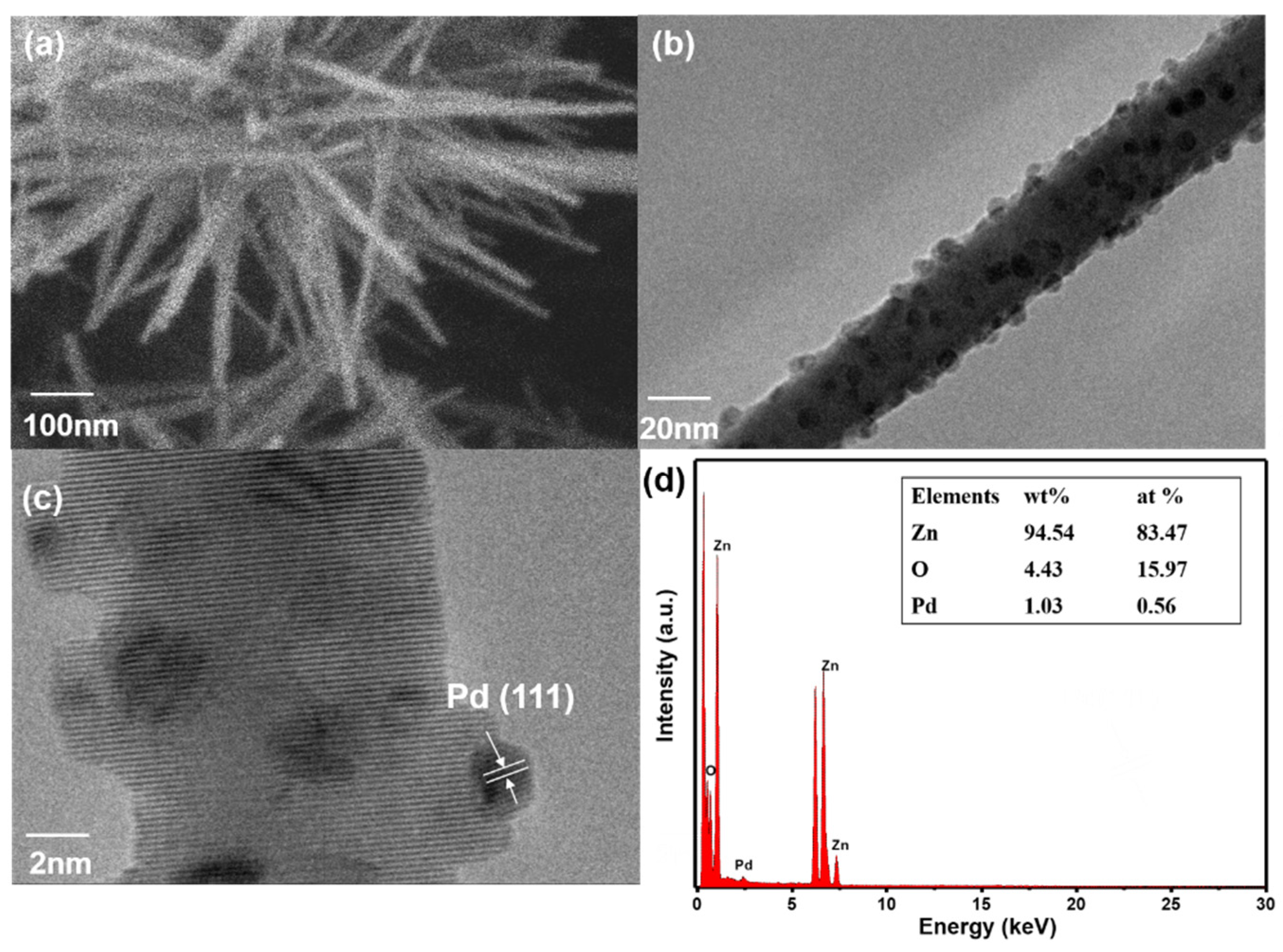
3.1. Structural and Morphological Characteristics
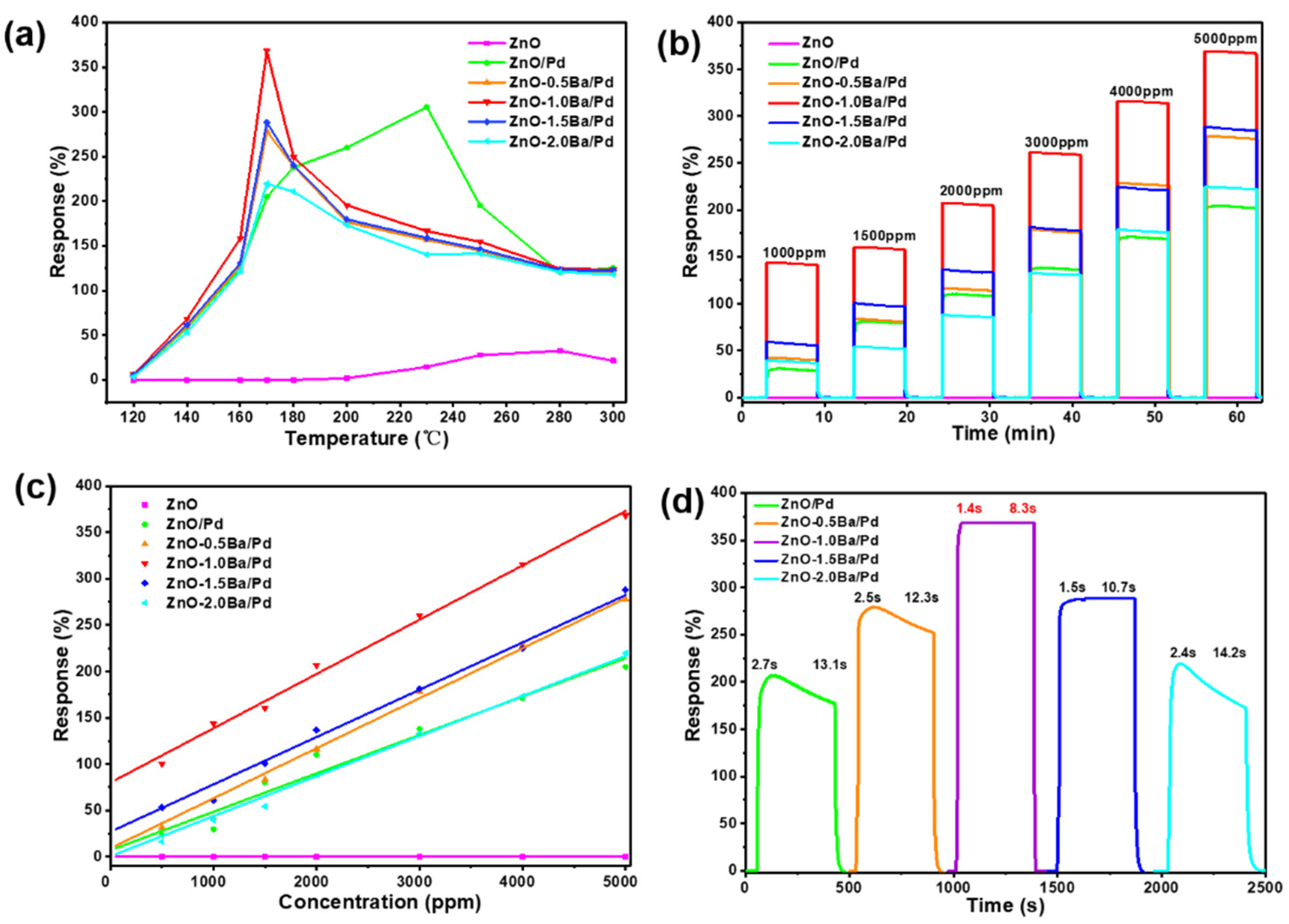
3.2. Gas Sensing Properties
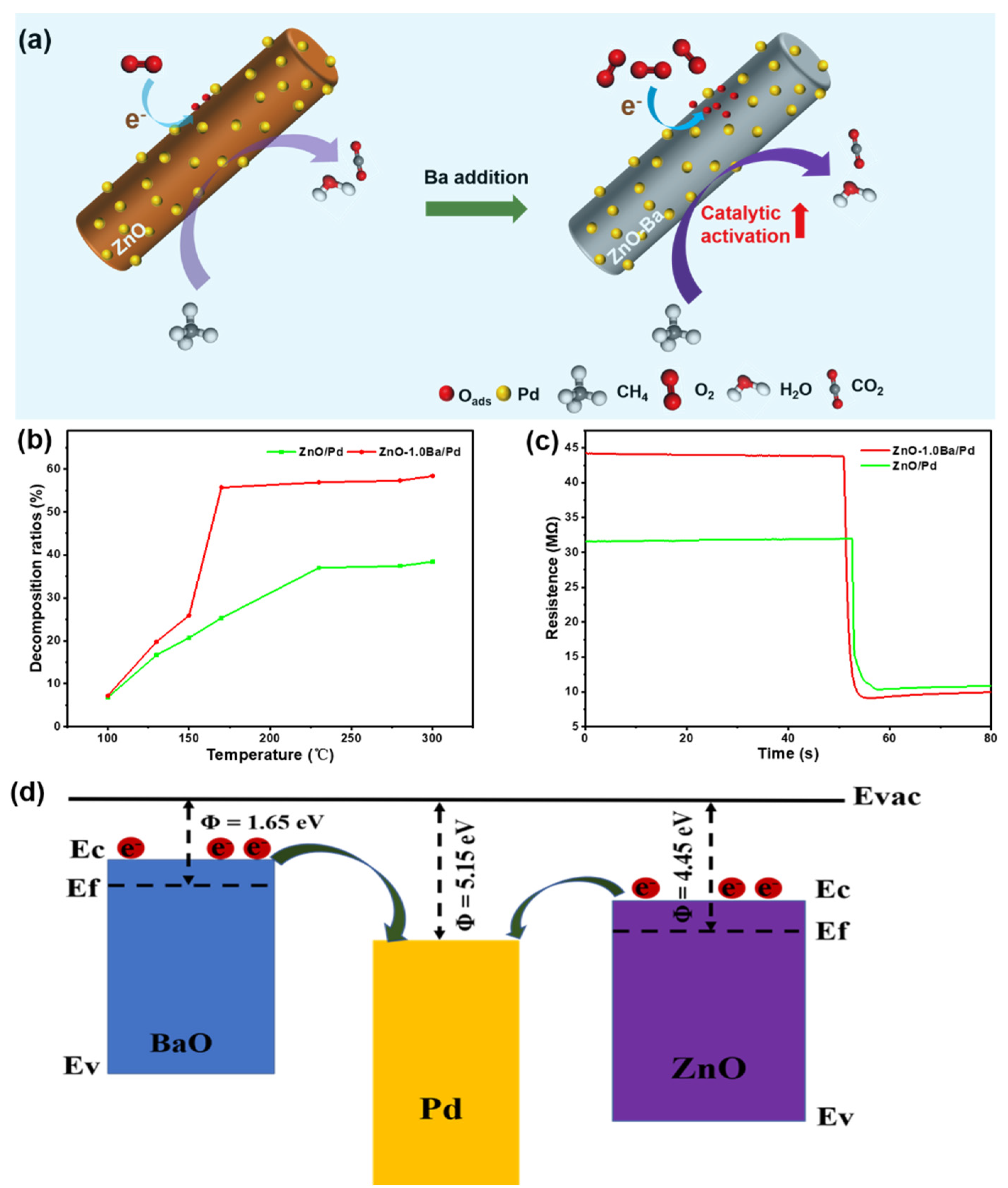
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Liu, C.; Wang, D.; He, R. Advances in the development of methane sensors with gas-sensing materials. Chin. Sci. Bull. 2019, 64, 1456–1470. [Google Scholar] [CrossRef]
- Hong, T.; Culp, J.T.; Kim, K.-J.; Devkota, J.; Sun, C.; Ohodnicki, P.R. State-of-the-art of methane sensing materials: A review and perspectives. TrAC-Trend Anal. Chem. 2020, 125, 115820. [Google Scholar] [CrossRef]
- Qin, T.; Niu, Y.; Qiao, X.; Guo, W.; Zhang, C.; Yang, Z.; Zhang, Y. Integrated coaxial graphene-based yarn with multidimensional architecture for self-powered photoelectrochemical methane sensor. Sens. Actuators B 2022, 365, 131965. [Google Scholar] [CrossRef]
- Dosi, M.; Lau, I.; Zhuang, Y.; Simakov, D.S.A.; Fowler, M.W.; Pope, M.A. Ultrasensitive Electrochemical Methane Sensors Based on Solid Polymer Electrolyte-Infused Laser-Induced Graphene. ACS Appl. Mater. Interfaces 2019, 11, 6166–6173. [Google Scholar] [CrossRef]
- Jiao, M.-Z.; Chen, X.-Y.; Hu, K.-X.; Qian, D.-Y.; Zhao, X.-H.; Ding, E.-J. Recent developments of nanomaterials-based conductive type methane sensors. Rare Met. 2021, 40, 1515–1527. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, W.; Qin, T.; Qiao, X.; Xiao, Z.; Yang, Z. Ultrasensitive self-powered photoelectrochemical detection of methane based on a coaxial integrated carbonene fiber. Environ. Sci. Nano 2022, 9, 2086–2093. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuators B 2020, 304, 127334. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Debeda, H.; Massok, P.; Lucat, C.; Ménil, F.; Aucouturier, J.L. Methane sensing: From sensitive thick films to a reliable selective device. Meas. Sci. Technol. 1997, 8, 99. [Google Scholar] [CrossRef]
- Lawrence, N.S. Analytical detection methodologies for methane and related hydrocarbons. Talanta 2006, 69, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Intc. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Yuan, H.; Aljneibi, S.; Yuan, J.; Wang, Y.; Liu, H.; Fang, J.; Tang, C.; Yan, X.; Cai, H.; Gu, Y.; et al. ZnO Nanosheets Abundant in Oxygen Vacancies Derived from Metal-Organic Frameworks for ppb-Level Gas Sensing. Adv. Mater. 2019, 31, e1807161. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Pan, A.; Gardner, D.W.; Zhao, S.; Davey, A.K.; Li, Z.; Zhao, L.; Carraro, C.; Maboudian, R. Well-connected ZnO nanoparticle network fabricated by in-situ annealing of ZIF-8 for enhanced sensitivity in gas sensing application. Sens. Actuators B 2021, 344, 130180. [Google Scholar] [CrossRef]
- Luo, S.; Chen, R.; Wang, J.; Xie, D.; Xiang, L. Designed synthesis of ZnO/Pd@ZIF-8 hybrid structure for highly sensitive and selective detection of methane in the presence of NO2. Sens. Actuators B 2021, 344, 130220. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Luo, S.; Xiang, L.; Li, W.; Xie, D. Unraveling photoexcited electron transfer pathway of oxygen vacancy-enriched ZnO/Pd hybrid toward visible light-enhanced methane detection at a relatively low temperature. Appl. Catal. B Environ. 2020, 264, 118554. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches for the improvement of conductometric gas sensor parameters. Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Li, C.; Song, B.-Y.; Lv, M.-S.; Chen, G.-L.; Zhang, X.-F.; Deng, Z.-P.; Xu, Y.-M.; Huo, L.-H.; Gao, S. Highly sensitive and selective nitric oxide sensor based on biomorphic ZnO microtubes with dual-defects assistance at low temperature. Chem. Eng. J. 2022, 446, 136846. [Google Scholar] [CrossRef]
- Qin, C.; Wang, B.; Li, P.; Sun, L.; Han, C.; Wu, N.; Wang, Y. Metal-organic framework-derived highly dispersed Pt nanoparticles-functionalized ZnO polyhedrons for ppb-level CO detection. Sens. Actuators B 2021, 331, 129433. [Google Scholar] [CrossRef]
- Hieu, N.M.; Lam, D.V.; Hien, T.T.; Chinh, N.D.; Quang, N.D.; Hung, N.M.; Van Phuoc, C.; Lee, S.-M.; Jeong, J.-R.; Kim, C.; et al. ZnTe-coated ZnO nanorods: Hydrogen sulfide nano-sensor purely controlled by pn junction. Mater. Des. 2020, 191, 108628. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Santos-Carballal, D.; Terasa, M.-I.; Magariu, N.; Zappa, D.; Comini, E.; Pauporté, T.; Siebert, L.; Faupel, F.; et al. Tailoring the selectivity of ultralow-power heterojunction gas sensors by noble metal nanoparticle functionalization. Nano Energy 2021, 88, 106241. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surf. Interfaces 2021, 24, 101110. [Google Scholar] [CrossRef]
- Koo, W.T.; Choi, S.J.; Kim, S.J.; Jang, J.S.; Tuller, H.L.; Kim, I.D. Heterogeneous Sensitization of Metal-Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Shang, Y.; Yang, X.; Zhang, S.; Wang, G.; Wang, Y.; Zhang, B.; Zhang, Z. Preparation of Pd/PdO@ZnO-ZnO nanorods by using metal organic framework templated catalysts for selective detection of triethylamine. Sens. Actuators B 2022, 350, 130840. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhang, N.; Zhao, X.; Zhang, Z.; Wang, Y. Self-sacrificial templated formation of ZnO with decoration of catalysts for regulating CO and CH4 sensitive detection. Sens. Actuators B 2021, 330, 129286. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Luo, N.; Wang, W.; Xu, J. Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis. Coatings 2022, 12, 677. [Google Scholar] [CrossRef]
- Du, J.; Guo, M.; Zhang, A.; Zhao, H.; Zhao, D.; Wang, C.; Zheng, T.; Zhao, Y.; Luo, Y. Performance, structure and kinetics of Pd catalyst supported in Ba modified γ-Al2O3 for low temperature wet methane oxidation. Chem. Eng. J. 2022, 430, 133113. [Google Scholar] [CrossRef]
- Klingstedt, F.; Karhu, H.; Neyestanaki, A.K.; Lindfors, L.E.; Salmi, T.; Väyrynen, J. Barium Promoted Palladium Catalysts for the Emission Control of Natural Gas Driven Vehicles and Biofuel Combustion Systems. J. Catal. 2002, 206, 248–262. [Google Scholar] [CrossRef]
- Auvray, X.; Lindholm, A.; Milh, M.; Olsson, L. The addition of alkali and alkaline earth metals to Pd/Al2O3 to promote methane combustion. Effect of Pd and Ca loading. Catal. Today 2018, 299, 212–218. [Google Scholar] [CrossRef]
- Friberg, I.; Sadokhina, N.; Olsson, L. Complete methane oxidation over Ba modified Pd/Al2O3: The effect of water vapor. Appl. Catal. B 2018, 231, 242–250. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Wang, H.; Li, H.; Wang, J.; Shen, M. Promoted Hydrothermal Stability of Pd/CeO2 Catalyst by Ba Doping. Catal. Lett. 2018, 148, 2596–2607. [Google Scholar] [CrossRef]
- Modwi, A.; Taha, K.K.; Khezami, L.; Al-Ayed, A.S.; Al-Duaij, O.K.; Khairy, M.; Bououdina, M. Structural and Electrical Characterization of Ba/ZnO Nanoparticles Fabricated by Co-precipitation. J. Inorg. Organomet. Polym. Mater. 2019, 30, 2633–2644. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Zheng, Y.; Zhan, Y.; Zhang, W.; Xiao, Y.; Zheng, Y.; Jiang, L. Catalytic methane oxidation performance over Pd/γ-Al2O3 catalyst optimized by the synergy of phosphorus and MOx (M = La, Ba and Zr). Fuel 2021, 299, 120933. [Google Scholar] [CrossRef]
- Lu, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Meng, M.; Zha, Y.; Ding, T. High-temperature close coupled catalysts Pd/Ce–Zr–M/Al2O3 (M = Y, Ca or Ba) used for the total oxidation of propane. Fuel 2010, 89, 2244–2251. [Google Scholar] [CrossRef]
- Liotta, L. Thermal stability, structural properties and catalytic activity of Pd catalysts supported on Al2O3–CeO2–BaO mixed oxides prepared by sol–gel method. J. Mol. Catal. A Chem. 2003, 204–205, 763–770. [Google Scholar] [CrossRef]
- Aghagoli, Z.; Ardyanian, M. Synthesis and study of the structure, magnetic, optical and methane gas sensing properties of cobalt doped zinc oxide microstructures. J. Mater. Sci. Mater. Electron. 2018, 29, 7130–7141. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Meng, X.; Zhang, Z.; Mu, S. High response methane sensor based on Au-modified hierarchical porous nanosheets-assembled ZnO microspheres. Mater. Chem. Phys. 2020, 250, 123027. [Google Scholar] [CrossRef]
- Basu, P.K.; Bhattacharyya, P.; Saha, N.; Saha, H.; Basu, S. The superior performance of the electrochemically grown ZnO thin films as methane sensor. Sens. Actuators B Chem. 2008, 133, 357–363. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhou, X.; Wu, X.; Han, N.; Chen, Y. Synthesis of Pd-loaded mesoporous SnO2 hollow spheres for highly sensitive and stable methane gas sensors. RSC Adv. 2018, 8, 24268–24275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Chang, H.; Li, P.; Liu, R. Characterization of nickel oxide decorated-reduced graphene oxide nanocomposite and its sensing properties toward methane gas detection. J. Mater. Sci. Mater. Electron. 2015, 27, 3723–3730. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Sun, G.; Zhang, B.; Wang, Y.; Cao, J.; Zhang, Z. Enhanced methane sensing properties of porous NiO nanaosheets by decorating with SnO2. Sens. Actuators B 2019, 288, 373–382. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Sun, G.; Zhang, B.; Wang, Y.; Zhang, Z. Enhanced CH4 sensitivity of porous nanosheets-assembled ZnO microflower by decoration with Zn2SnO4. Sens. Actuators B 2020, 304, 127374. [Google Scholar] [CrossRef]
- Lu, W.; Ding, D.; Xue, Q.; Du, Y.; Xiong, Y.; Zhang, J.; Pan, X.; Xing, W. Great enhancement of CH4 sensitivity of SnO2 based nanofibers by heterogeneous sensitization and catalytic effect. Sens. Actuators B 2018, 254, 393–401. [Google Scholar] [CrossRef]
- Min, B.-K.; Choi, S.-D. Undoped and 0.1 wt.% Ca-doped Pt-catalyzed SnO2 sensors for CH4 detection. Sens. Actuators B Chem. 2005, 108, 119–124. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.-S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Ghosh, M.; Veerender, P.; Datta, N.; Kaur, M.; Aswal, D.K.; Gupta, S.K. Growth and gas sensing characteristics of p- and n-type ZnO nanostructures. Sens. Actuators B 2011, 156, 875–880. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Z.; Tian, C.; Yuan, W.; Hua, Z.; Fan, S.; Wu, Y.; Tian, X. Selective detection of methane by HZSM-5 zeolite/Pd-SnO2 gas sensors. Sens. Actuators B 2020, 321, 128567. [Google Scholar] [CrossRef]


| Materials | Tem. (°C) | Con. (ppm) | Res. | Ref. |
|---|---|---|---|---|
| Pd–Ag/ZnO/Zn | 220 | 10,000 | 3.81 a | [38] |
| Au/ZnO | 250 | 100 | 4.16 a | [39] |
| ZnO/rGO | 190 | 1000 | 12.10% b | [40] |
| Pd–SnO2 | 300 | 250 | 4.88 a | [41] |
| Pd/ZnO | 200 | 100 | 8.56 a | [12] |
| NiO/rGO | 260 | 1000 | 15% b | [42] |
| SnO2/NiO | 330 | 500 | 15.2% b | [43] |
| Pd/s-ZnO | 190 | 500 | 6.44 a | [26] |
| Zn2SnO4/ZnO | 250 | 1000 | 27.2 a | [44] |
| Pt–SnO2 | 350 | 500 | 21% b | [45] |
| Pt–Ca/SnO2 | 400 | 5000 | 2.3 a | [46] |
| Ni2O3–SnO2 | 400 | 200 | 127% a | [47] |
| ZnO-1.0Ba/Pd | 170 | 5000 | 368.2% b | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Luo, S.; Chen, R.; Yu, J.; Xiang, L. Ba-Modified ZnO Nanorods Loaded with Palladium for Highly Sensitive and Rapid Detection of Methane at Low Temperatures. Chemosensors 2022, 10, 346. https://doi.org/10.3390/chemosensors10090346
Cai Y, Luo S, Chen R, Yu J, Xiang L. Ba-Modified ZnO Nanorods Loaded with Palladium for Highly Sensitive and Rapid Detection of Methane at Low Temperatures. Chemosensors. 2022; 10(9):346. https://doi.org/10.3390/chemosensors10090346
Chicago/Turabian StyleCai, Yijing, Shirui Luo, Renjie Chen, Junxia Yu, and Lan Xiang. 2022. "Ba-Modified ZnO Nanorods Loaded with Palladium for Highly Sensitive and Rapid Detection of Methane at Low Temperatures" Chemosensors 10, no. 9: 346. https://doi.org/10.3390/chemosensors10090346
APA StyleCai, Y., Luo, S., Chen, R., Yu, J., & Xiang, L. (2022). Ba-Modified ZnO Nanorods Loaded with Palladium for Highly Sensitive and Rapid Detection of Methane at Low Temperatures. Chemosensors, 10(9), 346. https://doi.org/10.3390/chemosensors10090346






