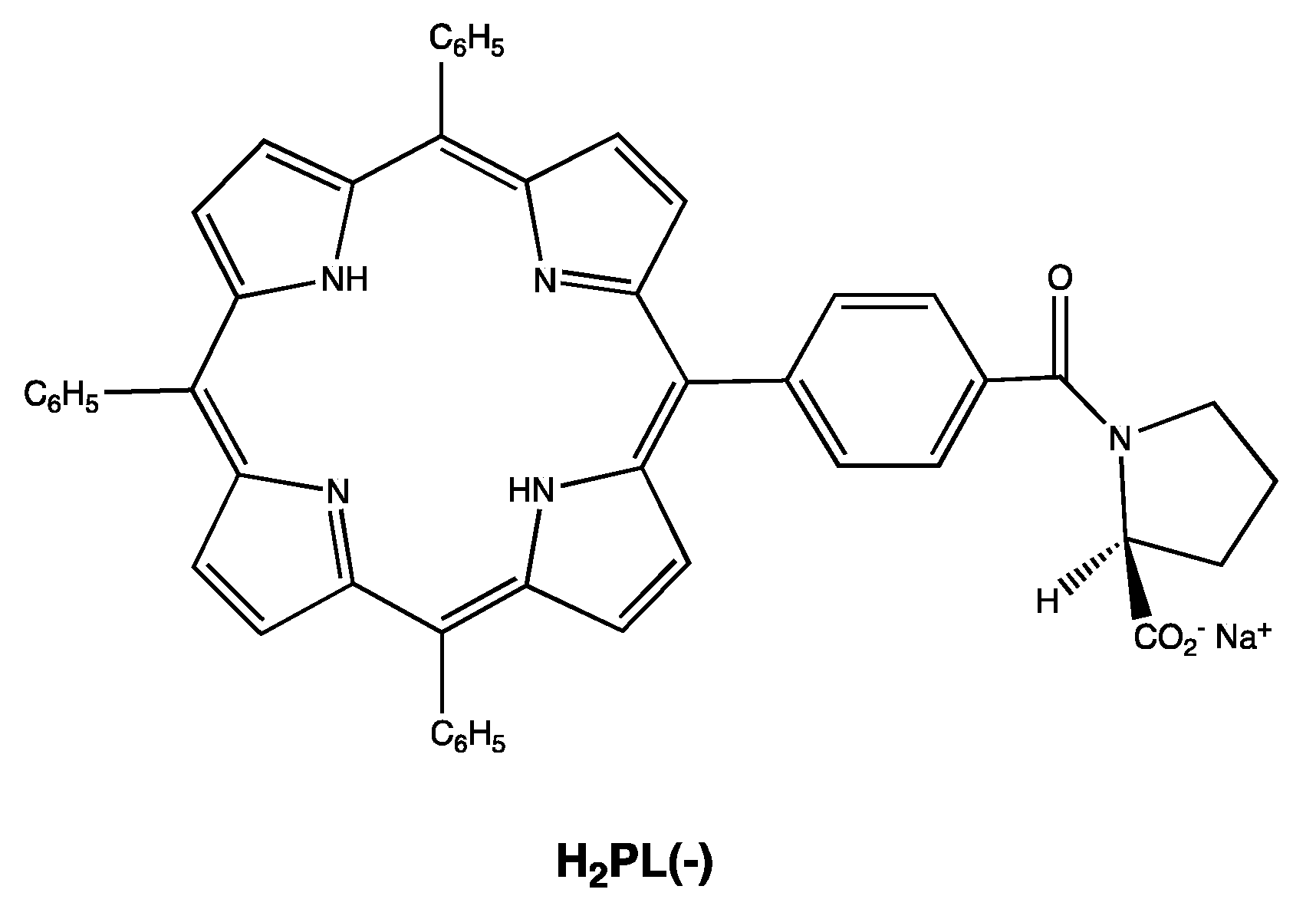
Proline Enantiomers Discrimination by (L)-Prolinated Porphyrin Derivative Langmuir–Schaefer Films: Proof of Concept for Chiral Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
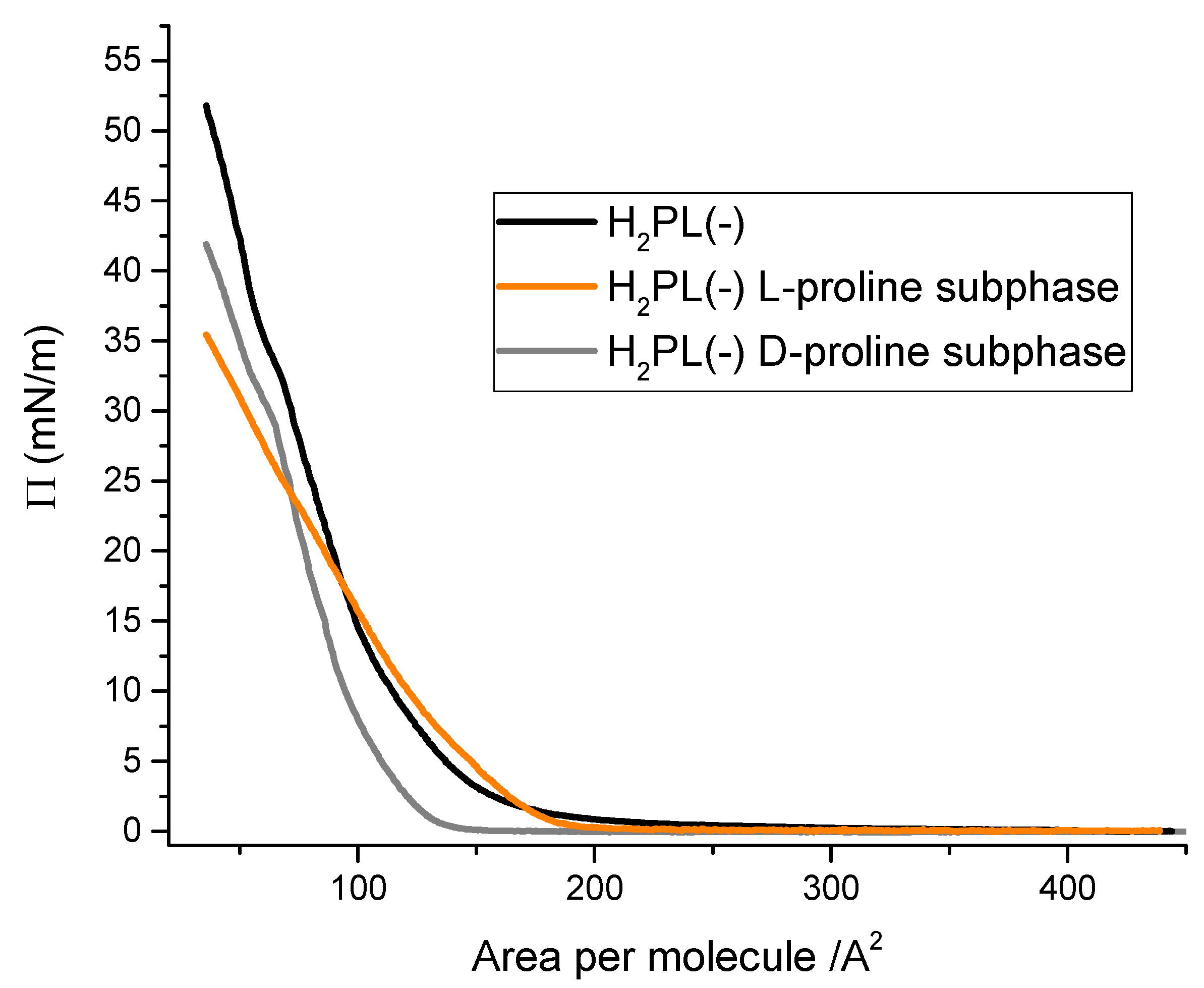
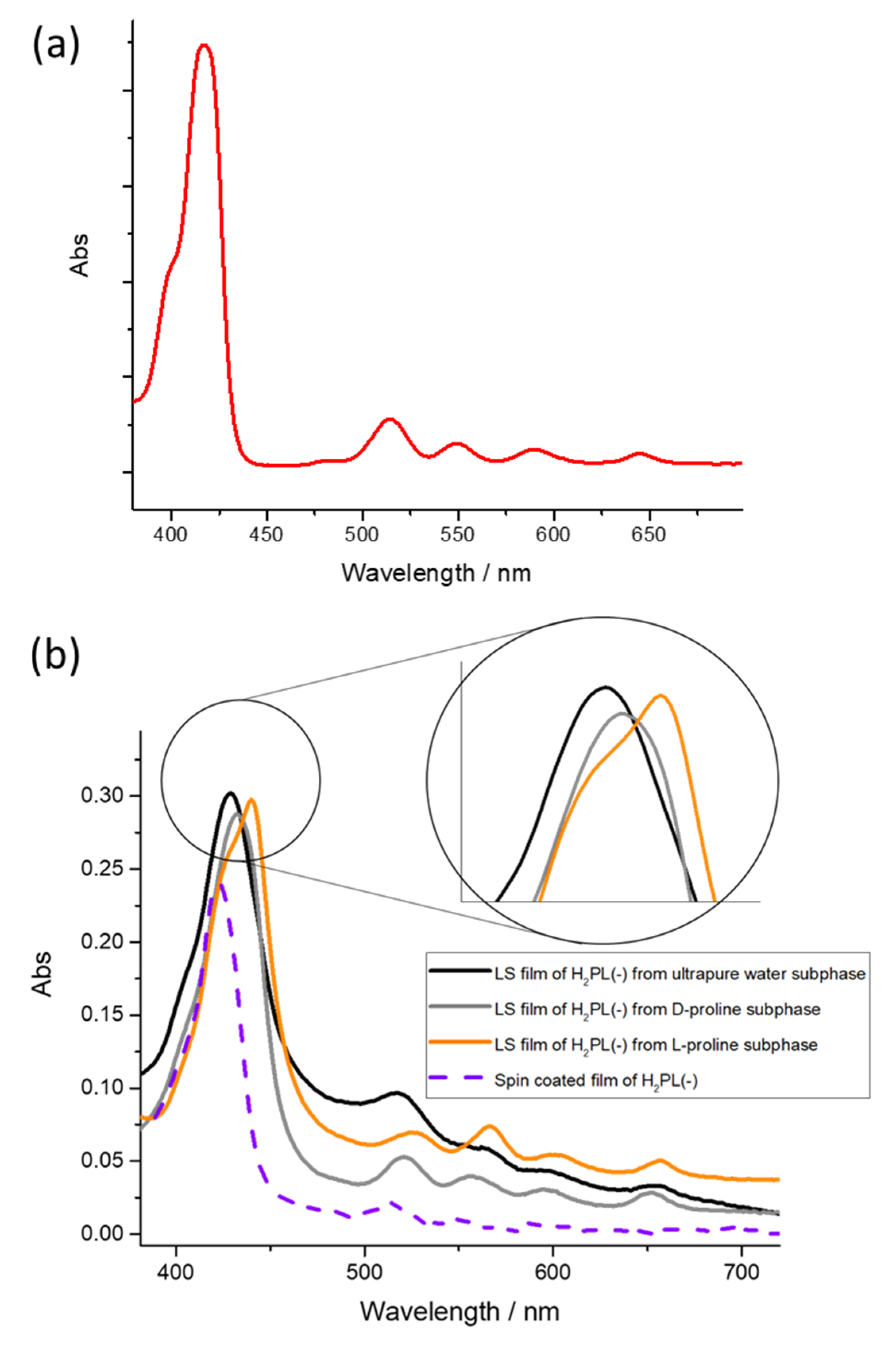
3.1. Langmuir Films Characterization and H2PL(-) Film Deposition
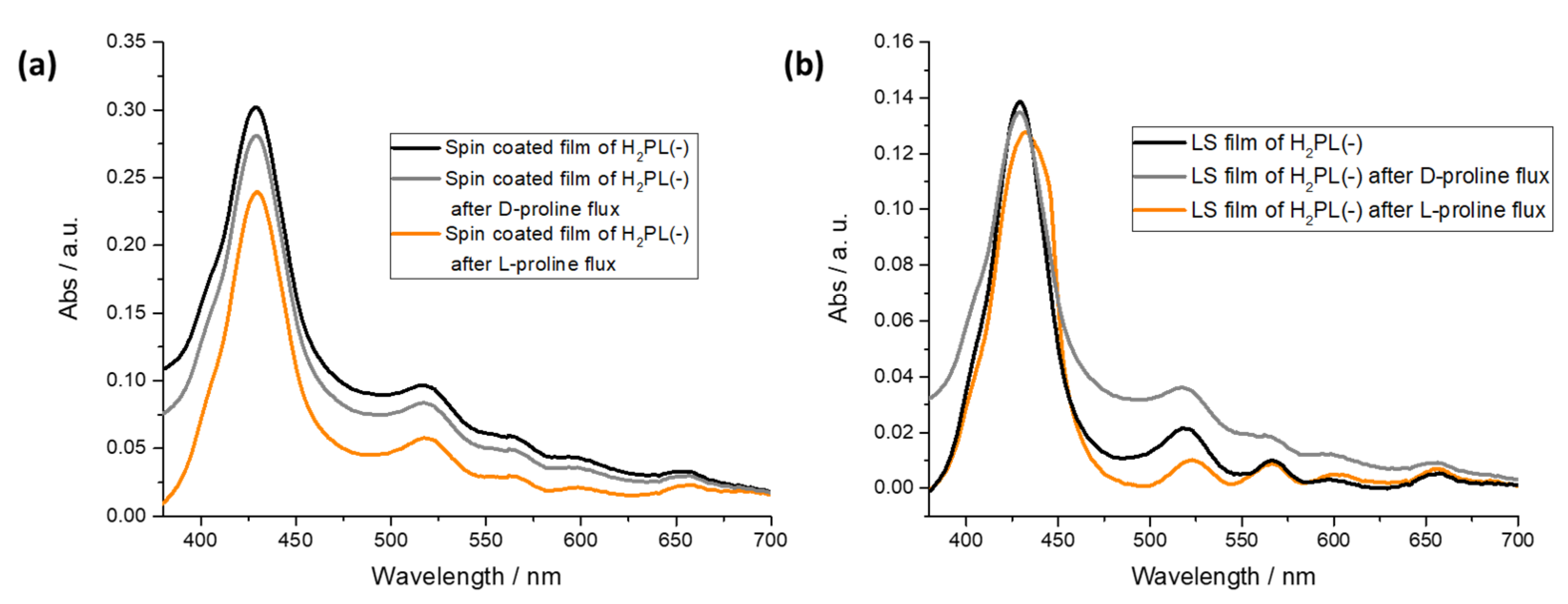
3.2. Physical Chemical Study of L- and D-Proline Interaction with H2PL(-) Langmuir and Langmuir Schaefer Films
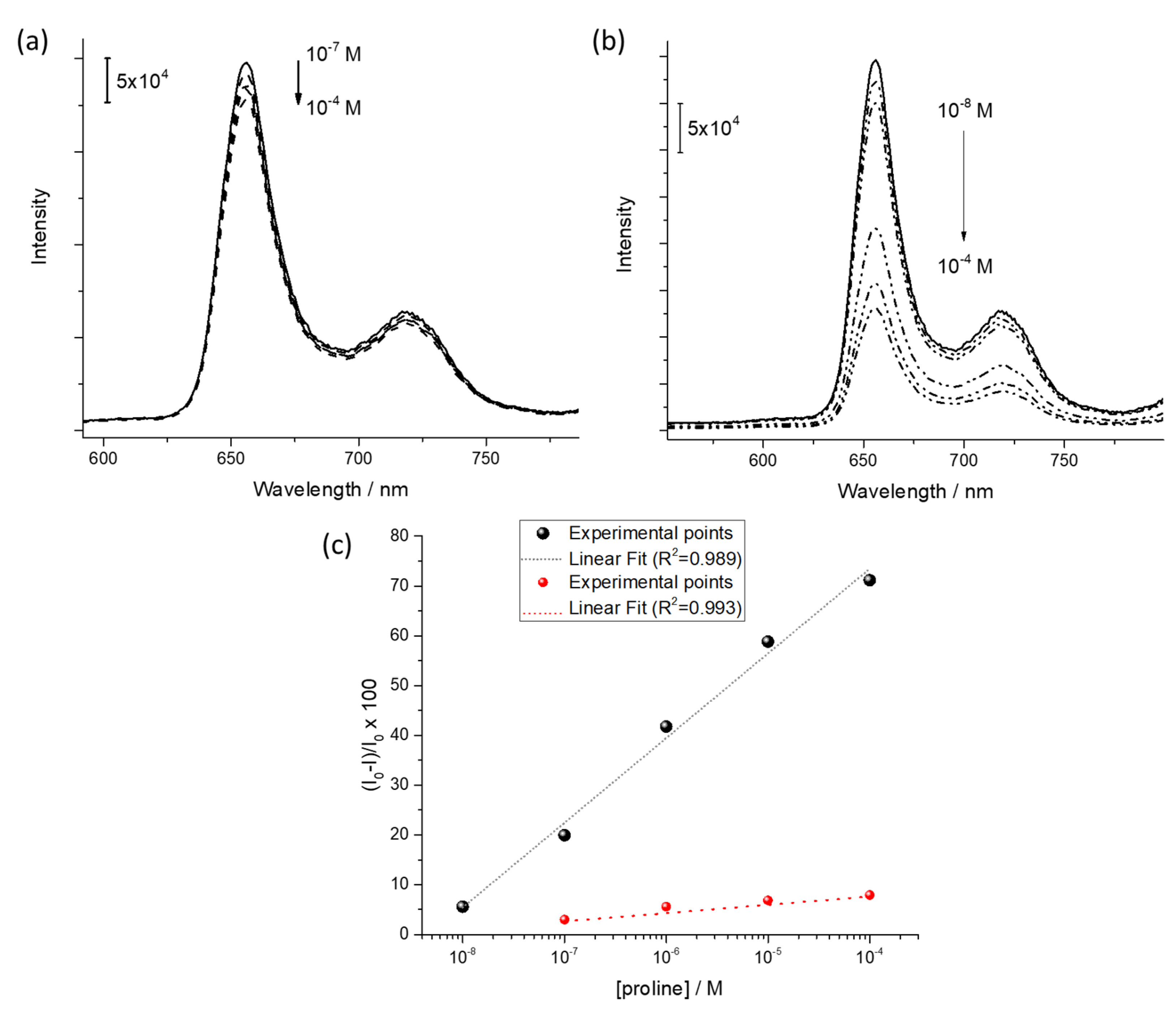
3.3. Preliminary Sensing Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vittorino, E.; Giancane, G.; Bettini, S.; Valli, L.; Sortino, S. Bichromophoric Multilayer Films for the Light-Controlled Generation of Nitric Oxide and Singlet Oxygen. J. Mater. Chem. 2009, 19, 8253–8258. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Senge, M.O. The Shape of Porphyrins. Coord. Chem. Rev. 2021, 431, 213760. [Google Scholar] [CrossRef]
- Singh, G.; Chandra, S. Unravelling the Structural-property Relations of Porphyrinoids with Respect to Photo- and Electro-chemical Activities. Electrochem. Sci. Adv. 2021, e2100149. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Lvova, L.; Di Natale, C.; Paolesse, R. Porphyrin-Based Chemical Sensors and Multisensor Arrays Operating in the Liquid Phase. Sens. Actuators B Chem. 2013, 179, 21–31. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-Based Sensor Nanoarchitectonics in Diverse Physical Detection Modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-L.; Cheng, Y.-H.; Xu, Z.; Chen, M.-L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Jiang, J.; Liu, M. Chiral Porphyrin Assemblies. Aggregate 2022, e198. [Google Scholar] [CrossRef]
- Van der Weegen, R.; Teunissen, A.J.P.; Meijer, E.W. Directing the Self-Assembly Behaviour of Porphyrin-Based Supramolecular Systems. Chem. Eur. J. 2017, 23, 3773–3783. [Google Scholar] [CrossRef]
- Negut, C.; Van Staden, R.-I.S.; Van Staden, J.F.V. Porphyrins-as Active Materials in the Design of Sensors. An Overview. ECS J. Solid State Sci. Technol. 2020, 9, 51005. [Google Scholar] [CrossRef]
- Porphyrin-Based Nanostructures for Sensing Applications. Available online: https://www.hindawi.com/journals/js/2009/856053/ (accessed on 2 May 2022).
- Buccolieri, A.; Hasan, M.; Bettini, S.; Bonfrate, V.; Salvatore, L.; Santino, A.; Borovkov, V.; Giancane, G. Ethane-Bridged Bisporphyrin Conformational Changes As an Effective Analytical Tool for Nonenzymatic Detection of Urea in the Physiological Range. Anal. Chem. 2018, 90, 6952–6958. [Google Scholar] [CrossRef]
- Nishiyama, F.; Yokoyama, T.; Kamikado, T.; Yokoyama, S.; Mashiko, S. Layer-by-Layer Growth of Porphyrin Supramolecular Thin Films. Appl. Phys. Lett. 2006, 88, 253113. [Google Scholar] [CrossRef]
- Al-Alwani, A.J.; Mironyuk, V.N.; Pozharov, M.V.; Gavrikov, M.V.; Glukhovskoy, E.G. Formation and Phase Behavior of Porphyrin/Arachidic Acid Mixed Systems and Morphology Study of Langmuir-Schaefer Thin Films. Soft Mater. 2022, 20, 310–321. [Google Scholar] [CrossRef]
- Arnold, D.P.; Manno, D.; Micocci, G.; Serra, A.; Tepore, A.; Valli, L. Gas-Sensing Properties of Porphyrin Dimer Langmuir–Blodgett Films. Thin Solid Film. 1998, 327–329, 341–344. [Google Scholar] [CrossRef]
- Giancane, G.; Bettini, S.; Valli, L.; Bracamonte, V.; Carraro, M.; Bonchio, M.; Prato, M. Supramolecular Organic–Inorganic Domains Integrating Fullerene-Based Acceptors with Polyoxometalate-Bis-Pyrene Tweezers for Organic Photovoltaic Applications. J. Mater. Chem. C 2021, 9, 16290–16297. [Google Scholar] [CrossRef]
- Manera, M.G.; Ferreiro-Vila, E.; García-Martín, J.M.; Cebollada, A.; García-Martín, A.; Giancane, G.; Valli, L.; Rella, R. Enhanced Magneto-Optical SPR Platform for Amine Sensing Based on Zn Porphyrin Dimers. Sens. Actuators B Chem. 2013, 182, 232–238. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M. Langmuir–Schaefer Films of a Set of Achiral Amphiphilic Porphyrins: Aggregation and Supramolecular Chirality. Soft Matter 2008, 4, 775–783. [Google Scholar] [CrossRef]
- Lin, L.; Wang, T.; Lu, Z.; Liu, M.; Guo, Y. In Situ Measurement of the Supramolecular Chirality in the Langmuir Monolayers of Achiral Porphyrins at the Air/Aqueous Interface by Second Harmonic Generation Linear Dichroism. J. Phys. Chem. C 2014, 118, 6726–6733. [Google Scholar] [CrossRef]
- Colozza, N.; Stefanelli, M.; Venanzi, M.; Paolesse, R.; Monti, D. Fabrication of Langmuir–Blodgett Chiral Films from Cationic (L)-Proline-Porphyrin Derivatives. In Porphyrin Science by Women; World Scientific: Singapore, 2021; pp. 878–884. ISBN 9789811223549. [Google Scholar]
- Bettini, S.; Grover, N.; Ottolini, M.; Mattern, C.; Valli, L.; Senge, M.O.; Giancane, G. Enantioselective Discrimination of Histidine by Means of an Achiral Cubane-Bridged Bis-Porphyrin. Langmuir 2021, 37, 13882–13889. [Google Scholar] [CrossRef]
- Randazzo, R.; Gaeta, M.; Gangemi, C.; Fragalà, M.; Purrello, R.; D’Urso, A. Chiral Recognition of L- and D- Amino Acid by Porphyrin Supramolecular Aggregates. Molecules 2018, 24, 84. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Jiang, H.; Wang, X. Recent Advances of BINOL-Based Sensors for Enantioselective Fluorescence Recognition. Analyst 2020, 145, 6769–6812. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, M.; Niu, L.; Liu, W. Enantioselective Environmental Toxicology of Chiral Pesticides. Chem. Res. Toxicol. 2015, 28, 325–338. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Li, Y.; Zhu, C.; Chen, Z.; Zheng, S.; Sun, H.; Liang, Y.; Jiang, G. Occurrence of Chiral Organochlorine Compounds in the Environmental Matrices from King George Island and Ardley Island, West Antarctica. Sci. Rep. 2015, 5, 13913. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of Drugs in German Sewage Treatment Plants and Rivers1Dedicated to Professor Dr. Klaus Haberer on the Occasion of His 70th Birthday.1. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- González-González, R.B.; Sharma, P.; Singh, S.P.; Américo-Pinheiro, J.H.P.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Persistence, Environmental Hazards, and Mitigation of Pharmaceutically Active Residual Contaminants from Water Matrices. Sci. Total Environ. 2022, 821, 153329. [Google Scholar] [CrossRef]
- Monti, D.; De Rossi, M.; Sorrenti, A.; Laguzzi, G.; Gatto, E.; Stefanelli, M.; Venanzi, M.; Luvidi, L.; Mancini, G.; Paolesse, R. Supramolecular Chirality in Solvent-Promoted Aggregation of Amphiphilic Porphyrin Derivatives: Kinetic Studies and Comparison between Solution Behavior and Solid-State Morphology by AFM Topography. Chem. Eur. J. 2010, 16, 860–870. [Google Scholar] [CrossRef]
- Stefanelli, M.; Savioli, M.; Zurlo, F.; Magna, G.; Belviso, S.; Marsico, G.; Superchi, S.; Venanzi, M.; Di Natale, C.; Paolesse, R.; et al. Porphyrins Through the Looking Glass: Spectroscopic and Mechanistic Insights in Supramolecular Chirogenesis of New Self-Assembled Porphyrin Derivatives. Front. Chem. 2020, 8, 587842. [Google Scholar] [CrossRef]
- Stefanelli, M.; Magna, G.; Zurlo, F.; Caso, F.M.; Di Bartolomeo, E.; Antonaroli, S.; Venanzi, M.; Paolesse, R.; Di Natale, C.; Monti, D. Chiral Selectivity of Porphyrin–ZnO Nanoparticle Conjugates. ACS Appl. Mater. Interfaces 2019, 11, 12077–12087. [Google Scholar] [CrossRef]
- Patriarca, E.J.; Cermola, F.; D’Aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The Multifaceted Roles of Proline in Cell Behavior. Front. Cell. Dev. Biol. 2021, 9, 2236. [Google Scholar] [CrossRef]
- Zeußel, L.; Aziz, C.; Schober, A.; Singh, S. PH-Dependent Selective Colorimetric Detection of Proline and Hydroxyproline with Meldrum’s Acid-Furfural Conjugate. Chemosensors 2021, 9, 343. [Google Scholar] [CrossRef]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front. Oncol. 2020, 10, 776. [Google Scholar] [CrossRef]
- Forlani, G.; Funck, D. A Specific and Sensitive Enzymatic Assay for the Quantitation of L-Proline. Front. Plant. Sci. 2020, 11, 582026. [Google Scholar] [CrossRef]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-511-62251-9. [Google Scholar]
- Rella, R.; Siciliano, P.; Quaranta, F.; Primo, T.; Valli, L.; Schenetti, L.; Mucci, A.; Iarossi, D. Gas Sensing Measurements and Analysis of the Optical Properties of Poly [3-(Butylthio)Thiophene] Langmuir–Blodgett Films. Sens. Actuators B Chem. 2000, 68, 203–209. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, L.; Liu, M. A Supramolecular Chiroptical Switch Exclusively from an Achiral Amphiphile. Adv. Mater. 2006, 18, 177–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, M. A General Method for Constructing Optically Active Supramolecular Assemblies from Intrinsically Achiral Water-Insoluble Free-Base Porphyrins. Chem. Eur. J. 2008, 14, 1793–1803. [Google Scholar] [CrossRef]
- Urtizberea, A.; Natividad, E.; Alonso, P.J.; Andrés, M.A.; Gascón, I.; Goldmann, M.; Roubeau, O. A Porphyrin Spin Qubit and Its 2D Framework Nanosheets. Adv. Funct. Mater. 2018, 28, 1801695. [Google Scholar] [CrossRef]
- Ulman, A. Part Two-Langmuir–Blodgett Films. In An Introduction to Ultrathin Organic Films; Ulman, A., Ed.; Academic Press: Cambridge, MA, USA, 1991; pp. 101–236. ISBN 978-0-08-092631-5. [Google Scholar]
- Ingrosso, C.; Curri, M.L.; Fini, P.; Giancane, G.; Agostiano, A.; Valli, L. Functionalized Copper(II)−Phthalocyanine in Solution and As Thin Film: Photochemical and Morphological Characterization toward Applications. Langmuir 2009, 25, 10305–10313. [Google Scholar] [CrossRef]
- Bliznyuk, V.N.; Assender, H.E.; Briggs, G.A.D. Surface Glass Transition Temperature of Amorphous Polymers. A New Insight with SFM. Macromolecules 2002, 35, 6613–6622. [Google Scholar] [CrossRef]
- Peltonen, J.P.K.; He, P.; Rosenholm, J.B. Influence of UV Irradiation on Unsaturated Fatty Acid Monolayers and Multilayer Films: X-Ray Diffraction and Atomic Force Microscopy Study. Langmuir 1993, 9, 2363–2369. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin−Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J-aggregate Formation of a Water-soluble Porphyrin in Acidic Aqueous Media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Lang, K.; Mosinger, J.; Wagnerová, D.M. Photophysical Properties of Porphyrinoid Sensitizers Non-Covalently Bound to Host Molecules; Models for Photodynamic Therapy. Coord. Chem. Rev. 2004, 248, 321–350. [Google Scholar] [CrossRef]
- Concepcion, J.J.; House, R.L.; Papanikolas, J.M.; Meyer, T.J. Chemical Approaches to Artificial Photosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 15560–15564. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Ghosh, M.; Nath, S.; Hajra, A.; Sinha, S. Fluorescence Self-Quenching of Tetraphenylporphyrin in Liquid Medium. J. Lumin. 2013, 141, 87–92. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giancane, G.; Pagano, R.; Naitana, M.L.; Magna, G.; Stefanelli, M.; Monti, D.; Paolesse, R.; Bettini, S.; Valli, L. Proline Enantiomers Discrimination by (L)-Prolinated Porphyrin Derivative Langmuir–Schaefer Films: Proof of Concept for Chiral Sensing Applications. Chemosensors 2022, 10, 331. https://doi.org/10.3390/chemosensors10080331
Giancane G, Pagano R, Naitana ML, Magna G, Stefanelli M, Monti D, Paolesse R, Bettini S, Valli L. Proline Enantiomers Discrimination by (L)-Prolinated Porphyrin Derivative Langmuir–Schaefer Films: Proof of Concept for Chiral Sensing Applications. Chemosensors. 2022; 10(8):331. https://doi.org/10.3390/chemosensors10080331
Chicago/Turabian StyleGiancane, Gabriele, Rosanna Pagano, Mario Luigi Naitana, Gabriele Magna, Manuela Stefanelli, Donato Monti, Roberto Paolesse, Simona Bettini, and Ludovico Valli. 2022. "Proline Enantiomers Discrimination by (L)-Prolinated Porphyrin Derivative Langmuir–Schaefer Films: Proof of Concept for Chiral Sensing Applications" Chemosensors 10, no. 8: 331. https://doi.org/10.3390/chemosensors10080331
APA StyleGiancane, G., Pagano, R., Naitana, M. L., Magna, G., Stefanelli, M., Monti, D., Paolesse, R., Bettini, S., & Valli, L. (2022). Proline Enantiomers Discrimination by (L)-Prolinated Porphyrin Derivative Langmuir–Schaefer Films: Proof of Concept for Chiral Sensing Applications. Chemosensors, 10(8), 331. https://doi.org/10.3390/chemosensors10080331











