Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy
Abstract
:1. Introduction
2. Design of Fluorescent Probes for Diabetes
3. Different Probes for Various Potential Biomarkers of Diabetic
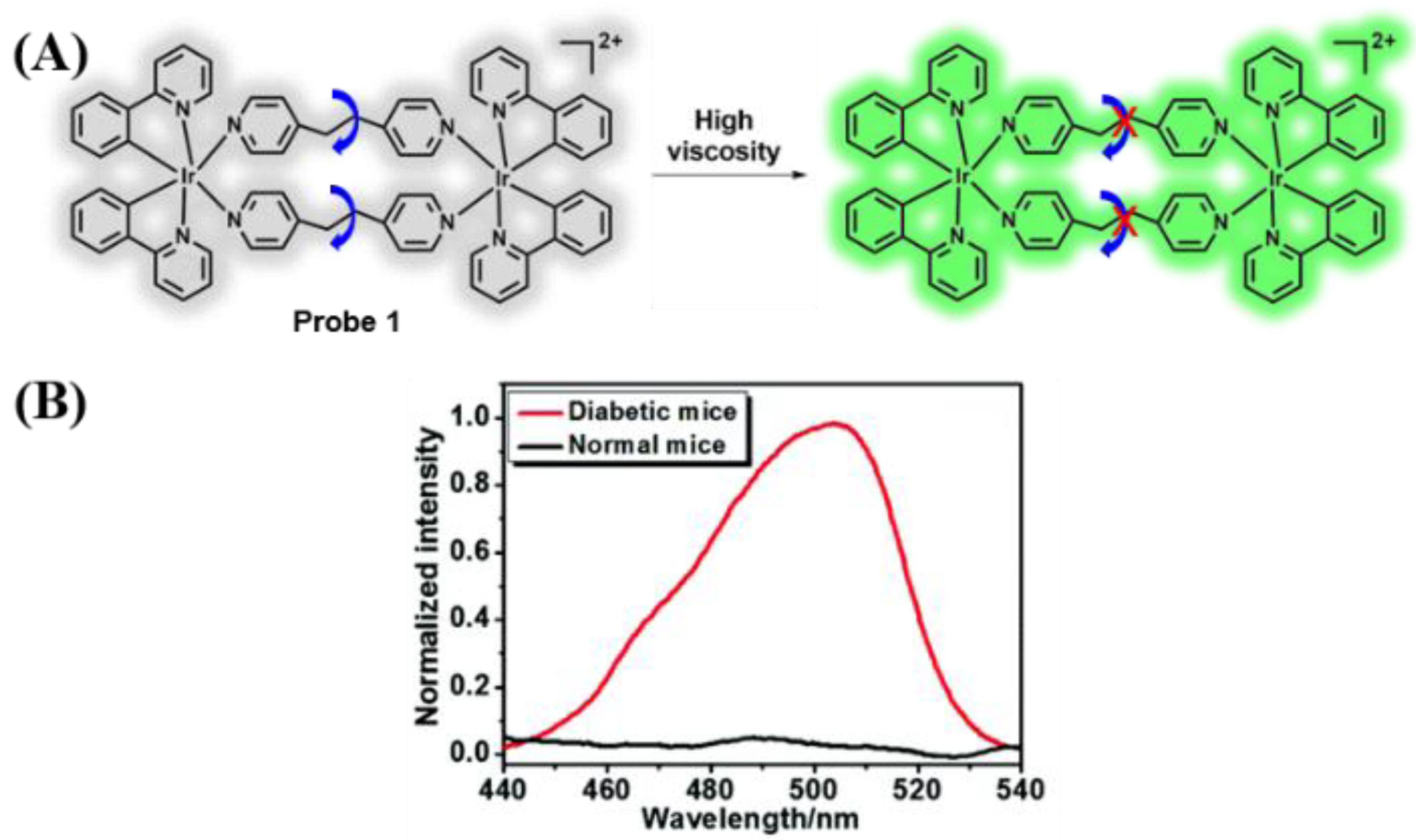
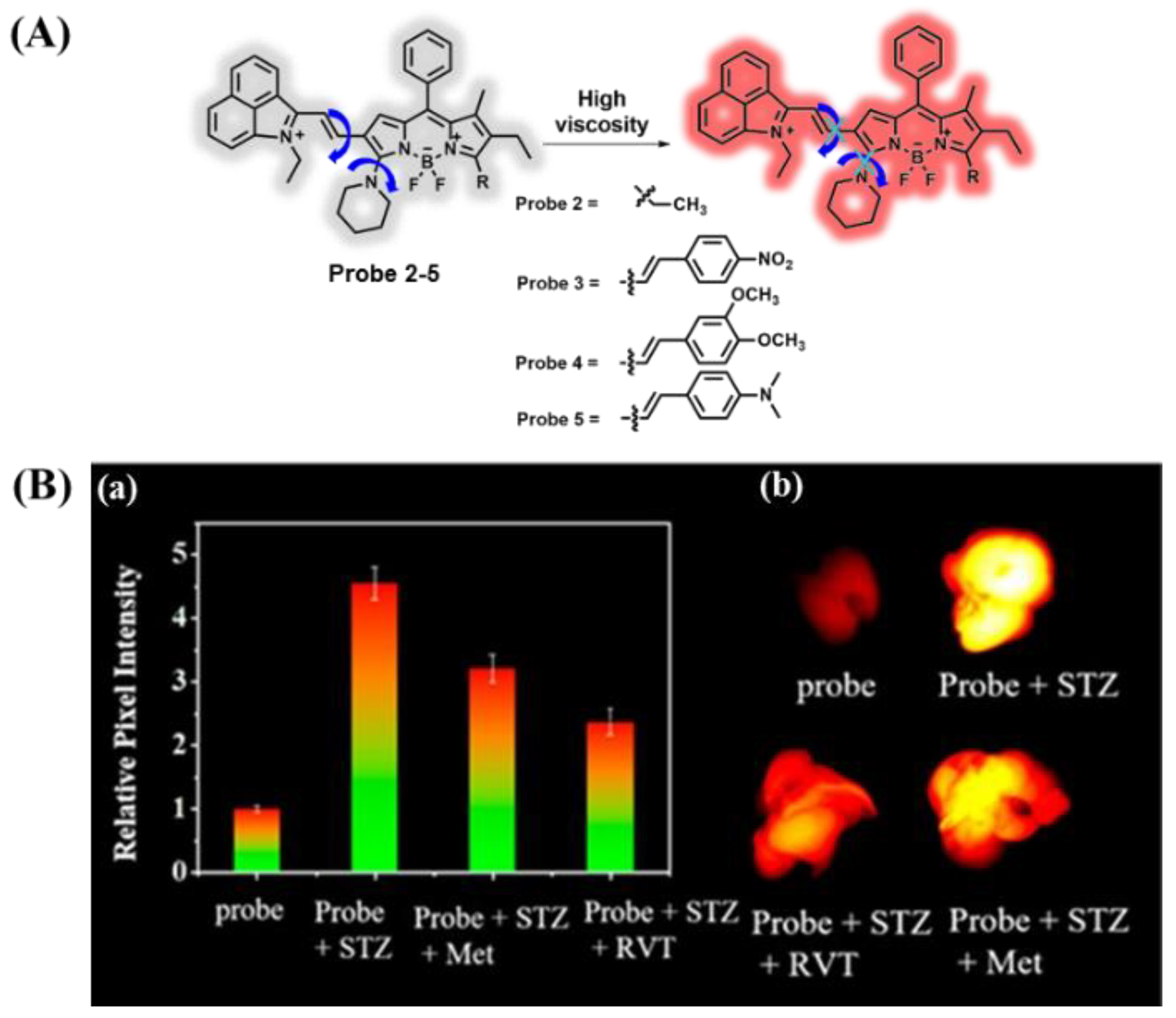
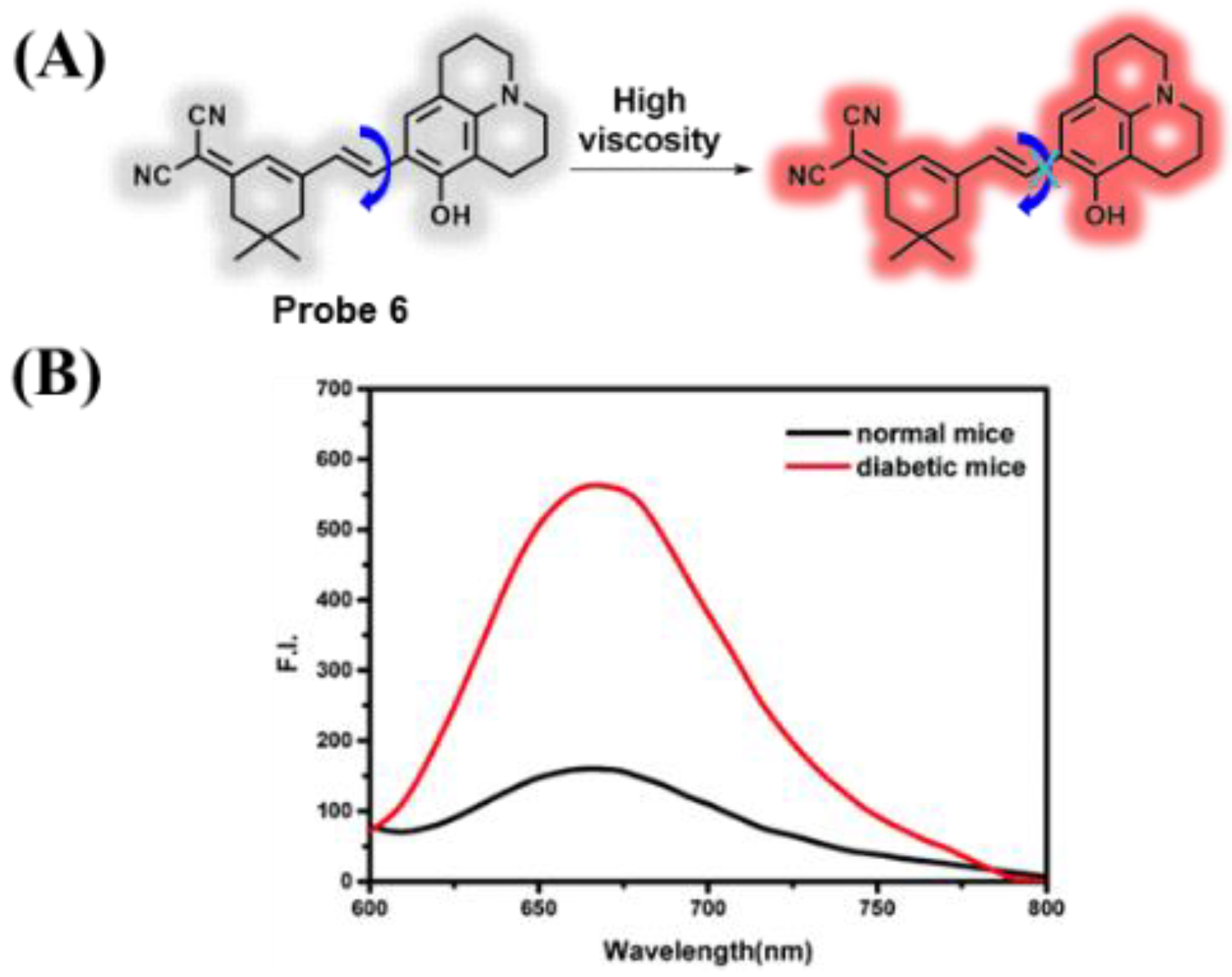
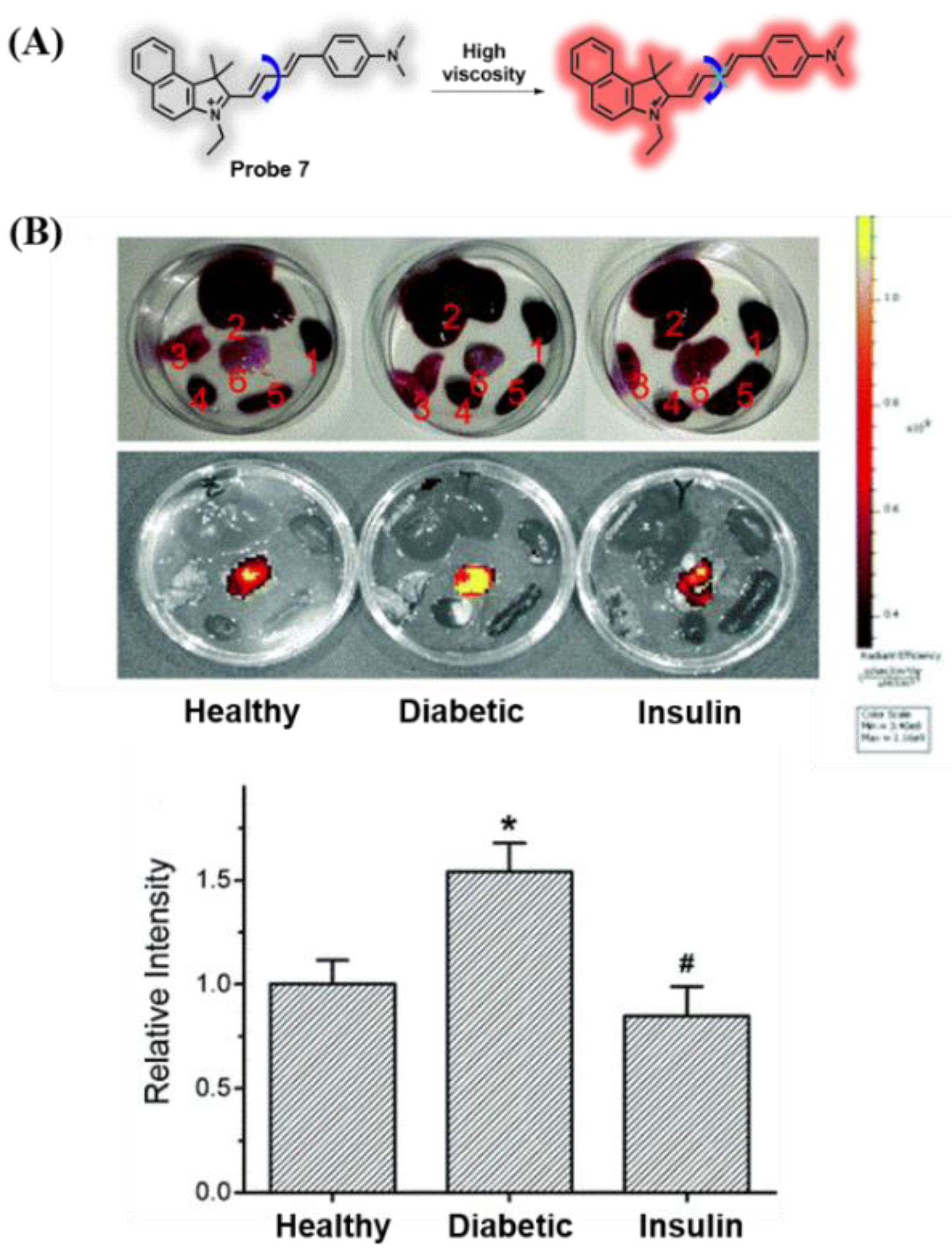
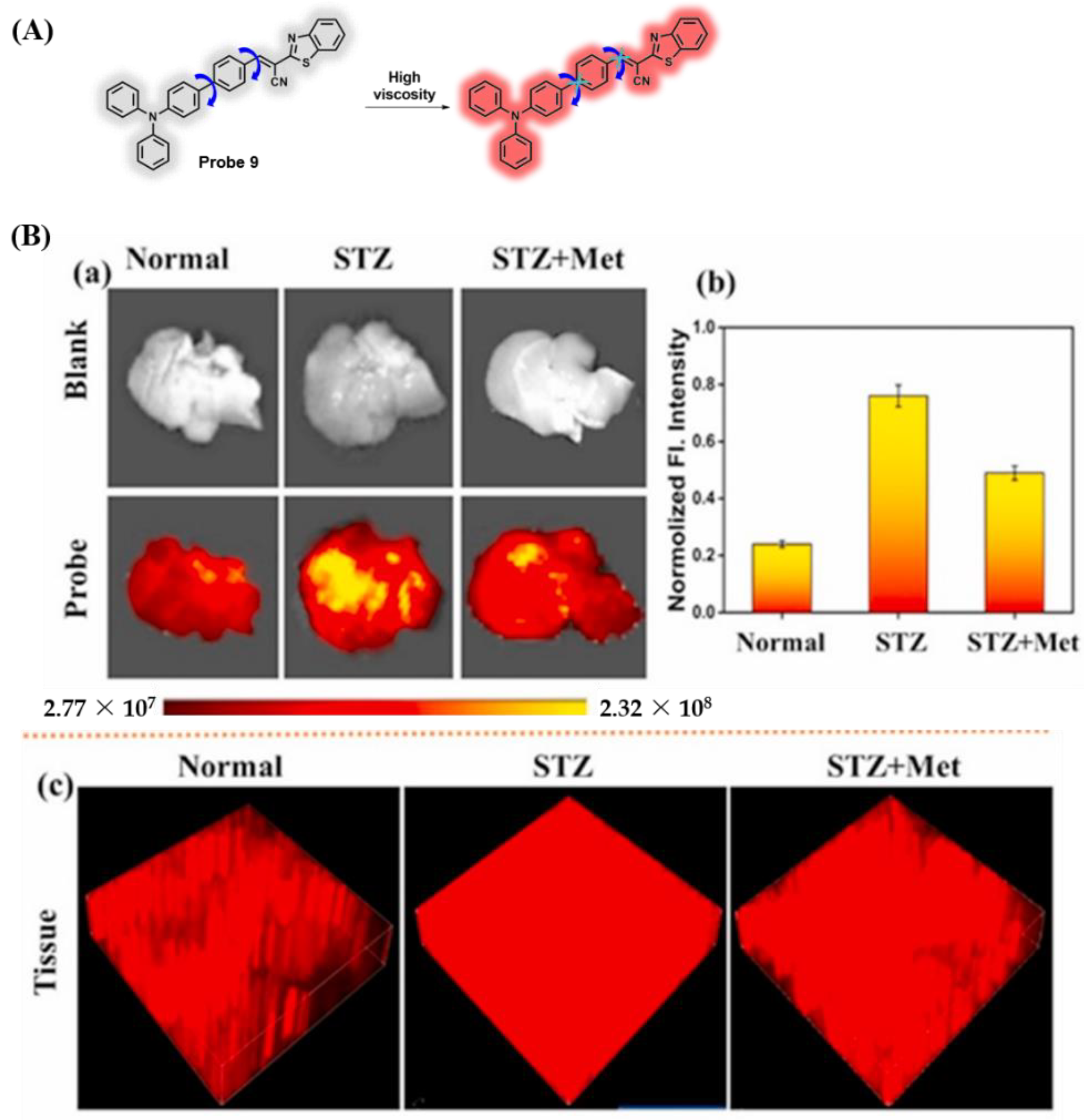
3.1. Probes Targeting Viscosity
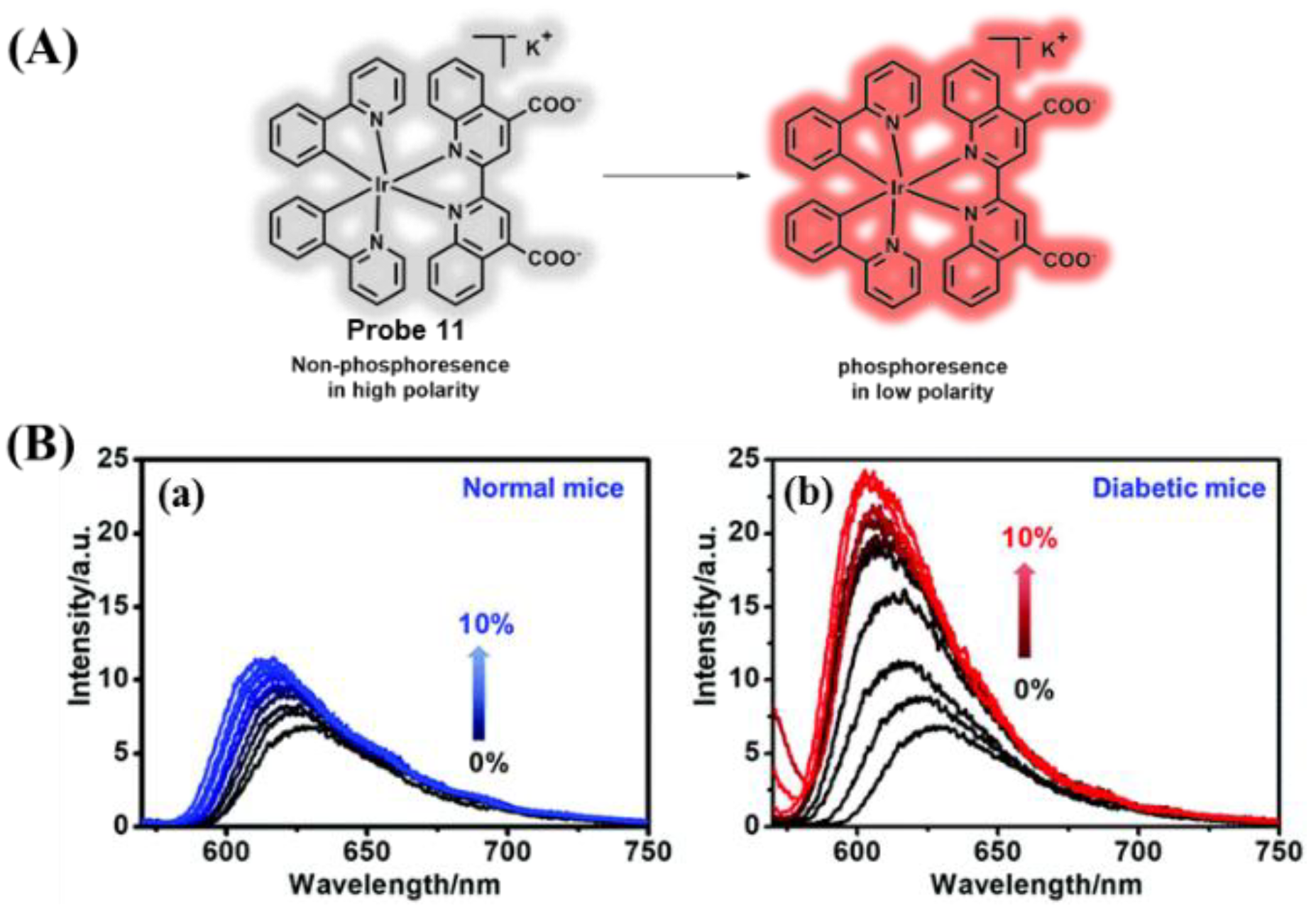
3.2. Probes Targeting Polarity
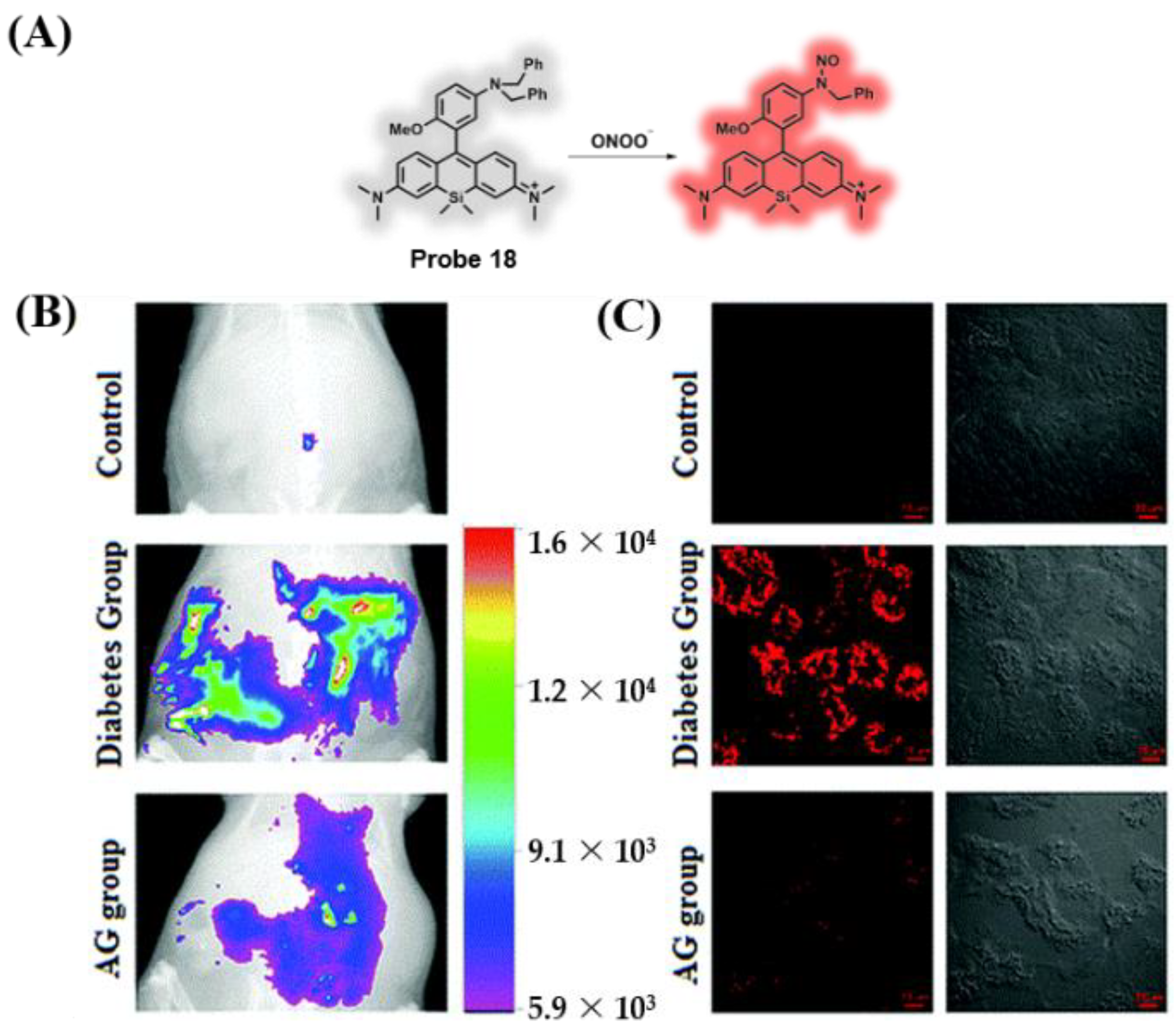
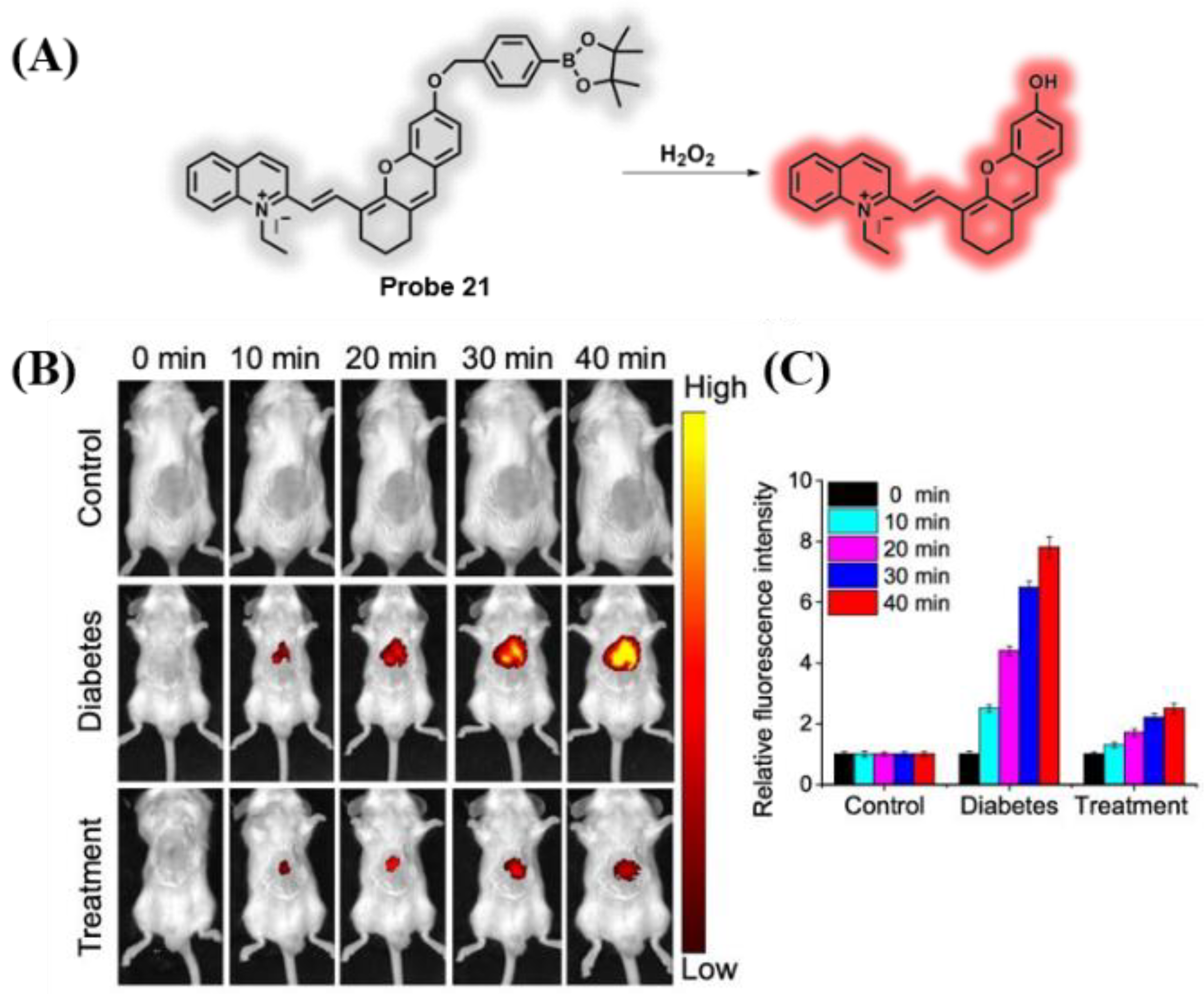
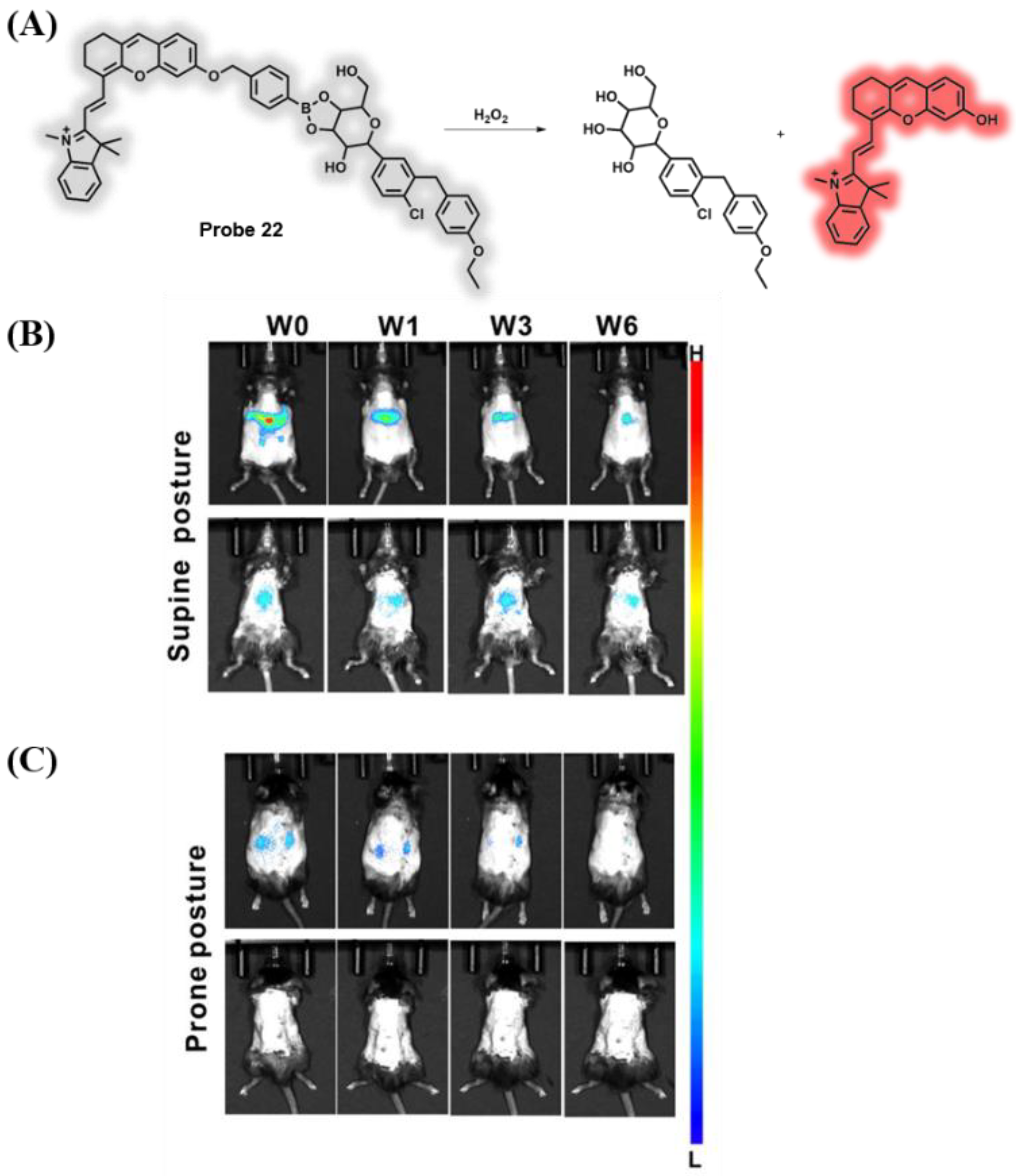
3.3. Probes Targeting ROS
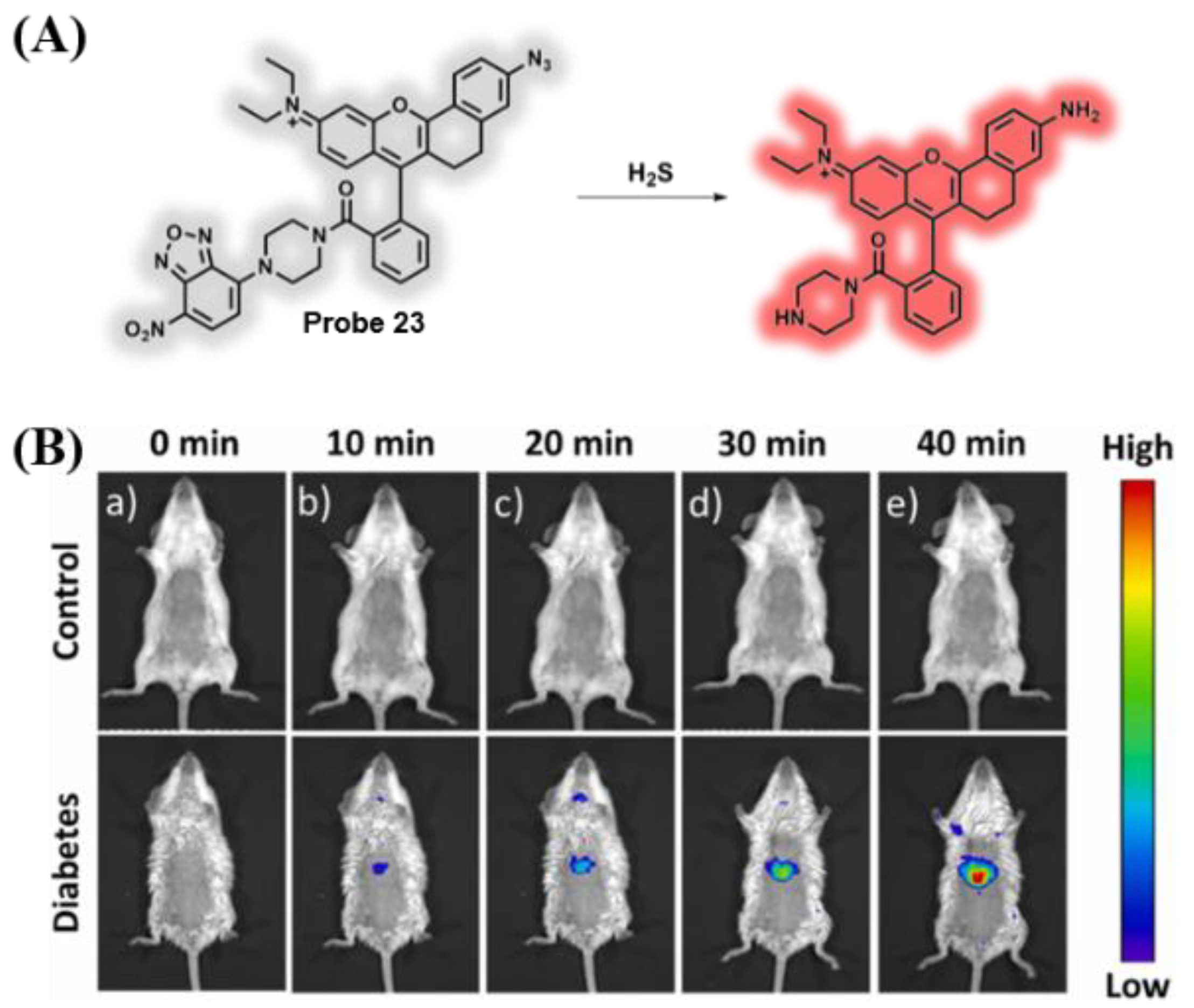
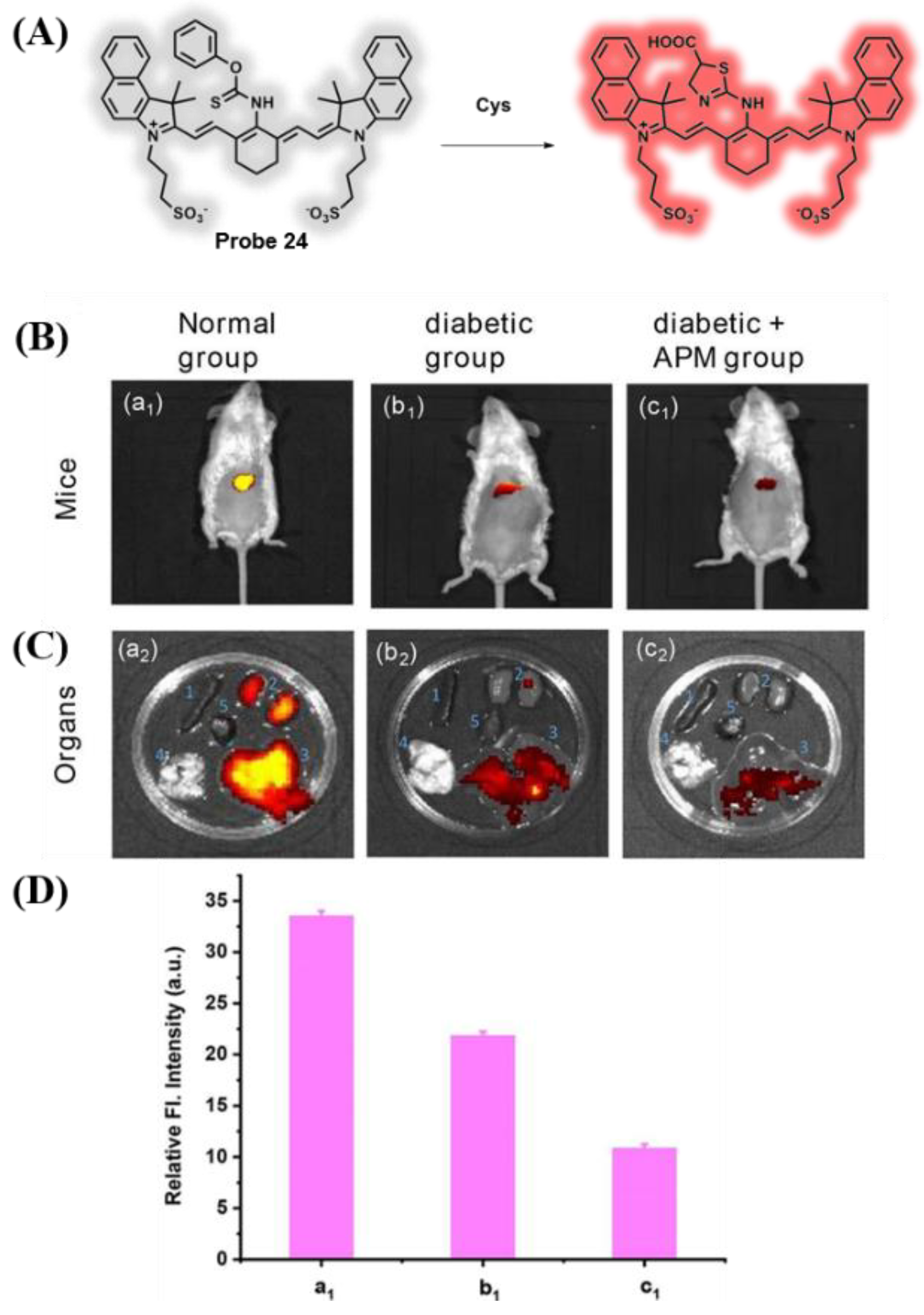
3.4. Probes Targeting H2S and Cys
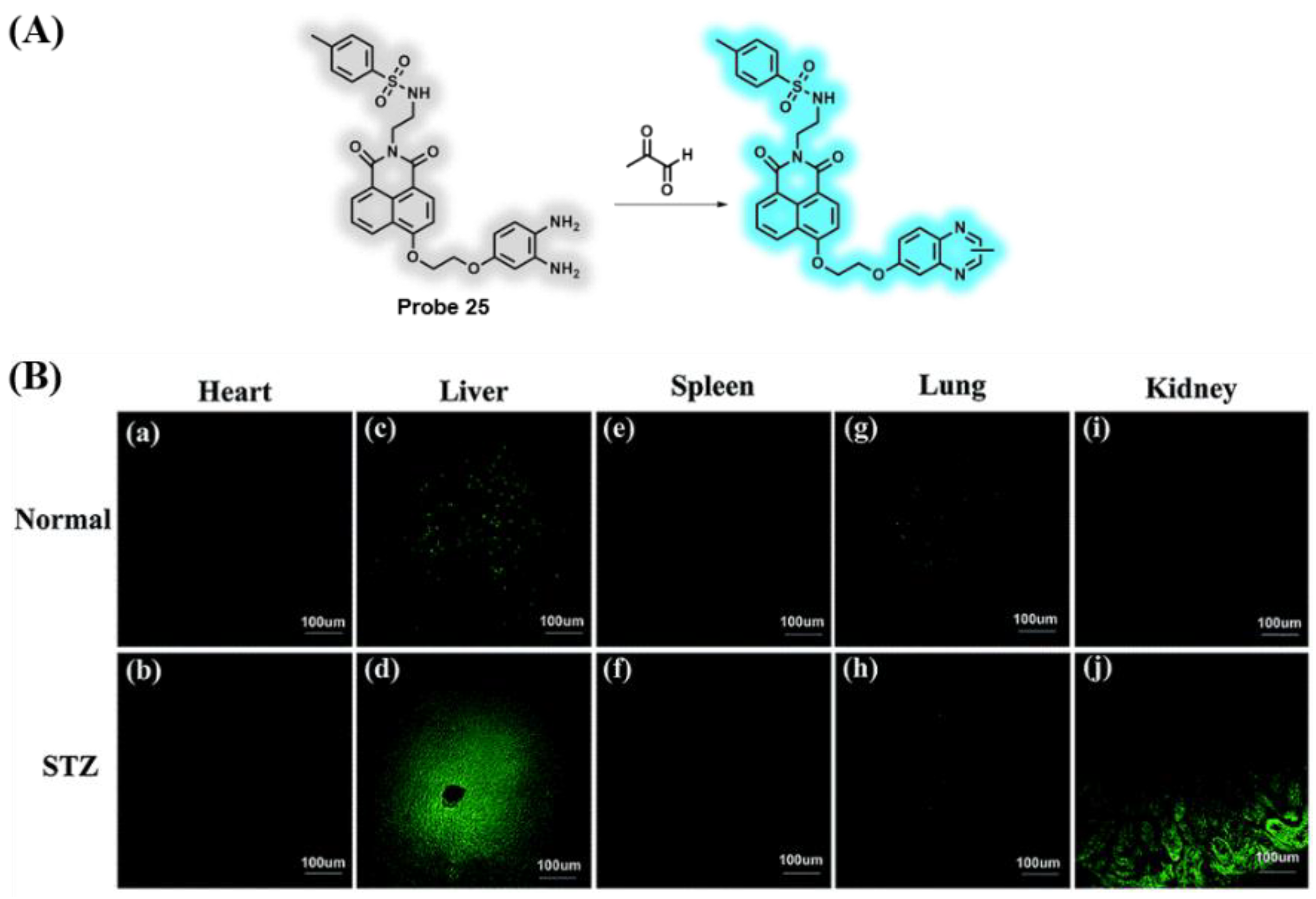
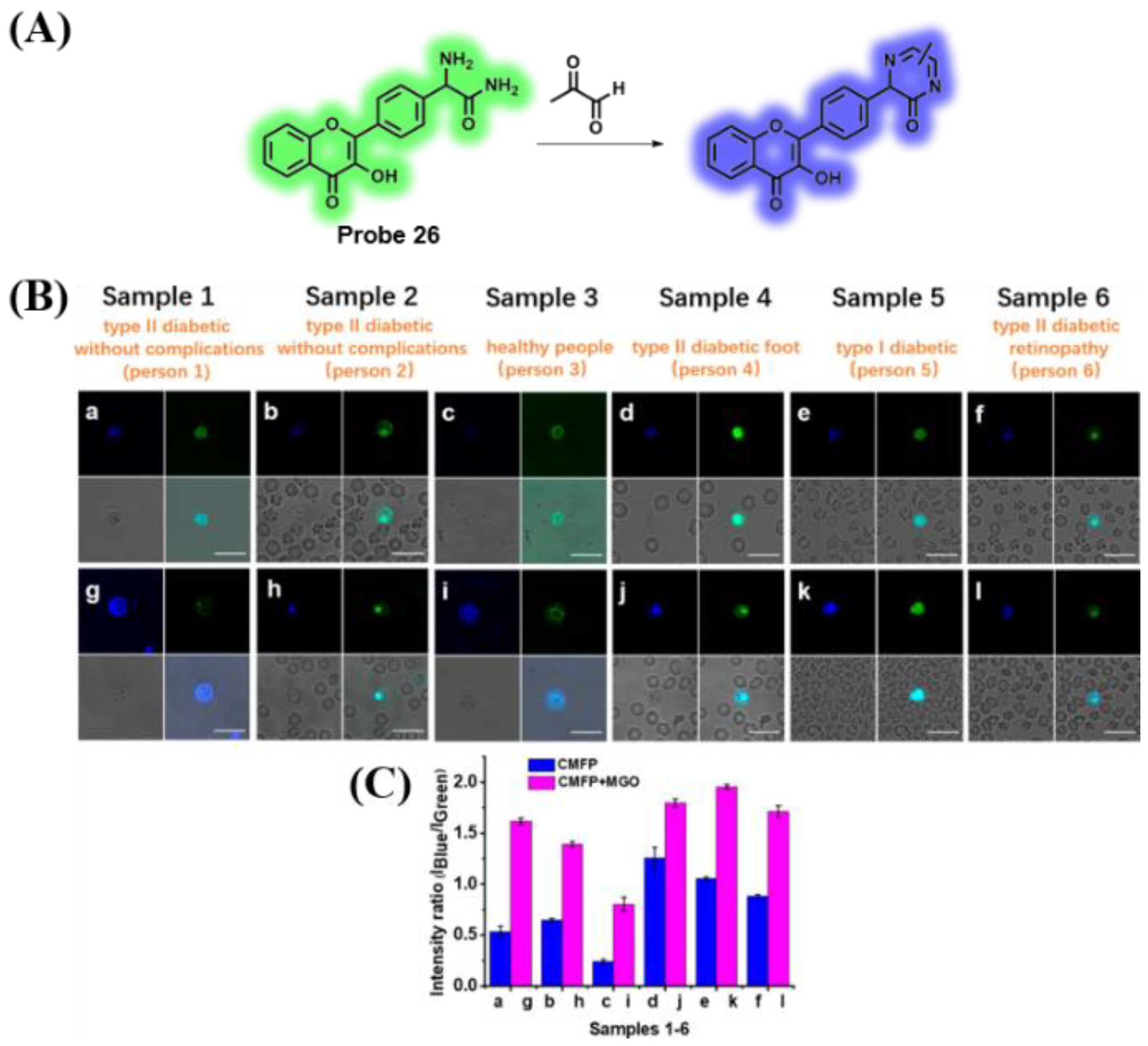
3.5. Probes Targeting Methylglyoxal (MGO)
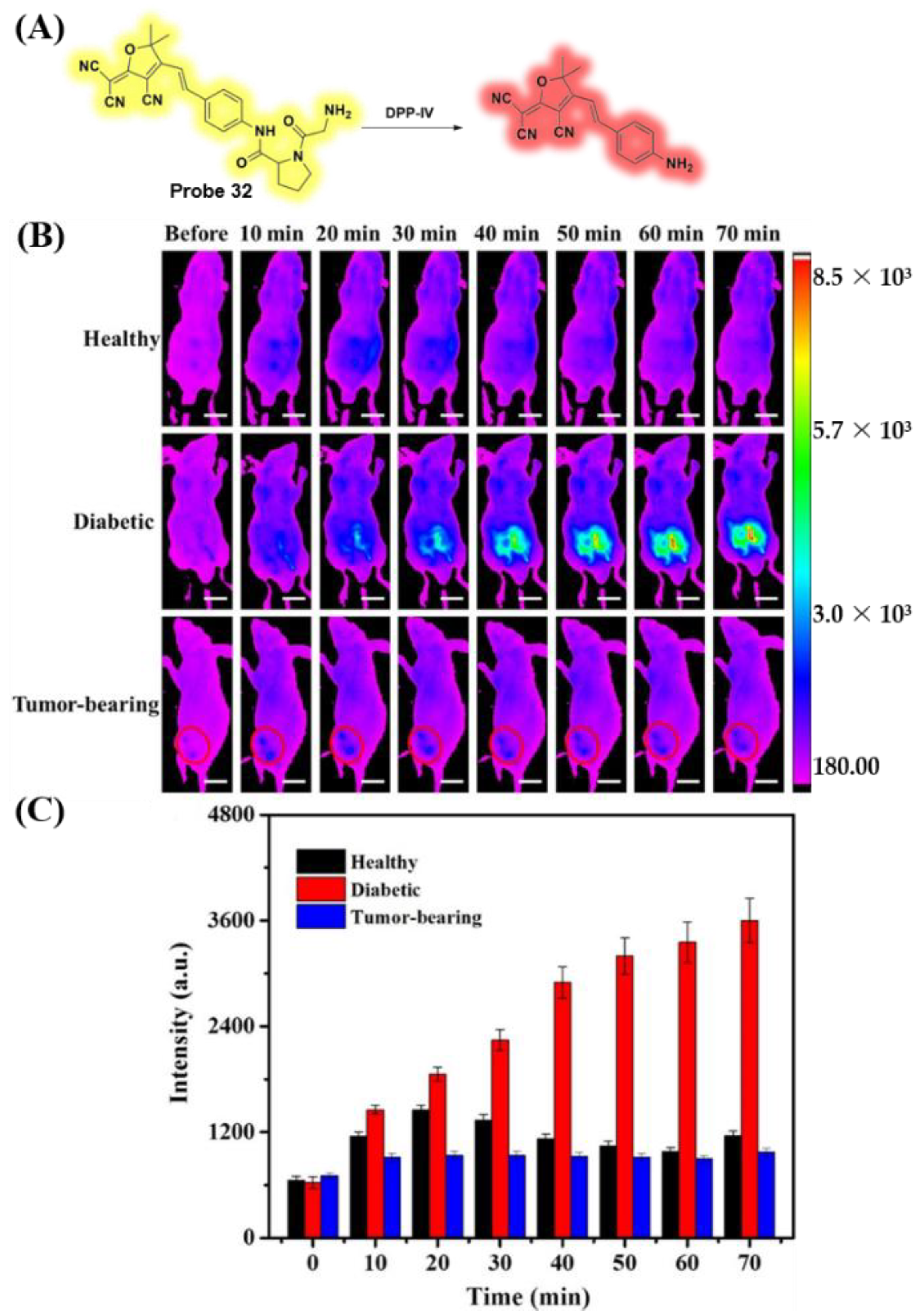
3.6. Probes Targeting Enzymes
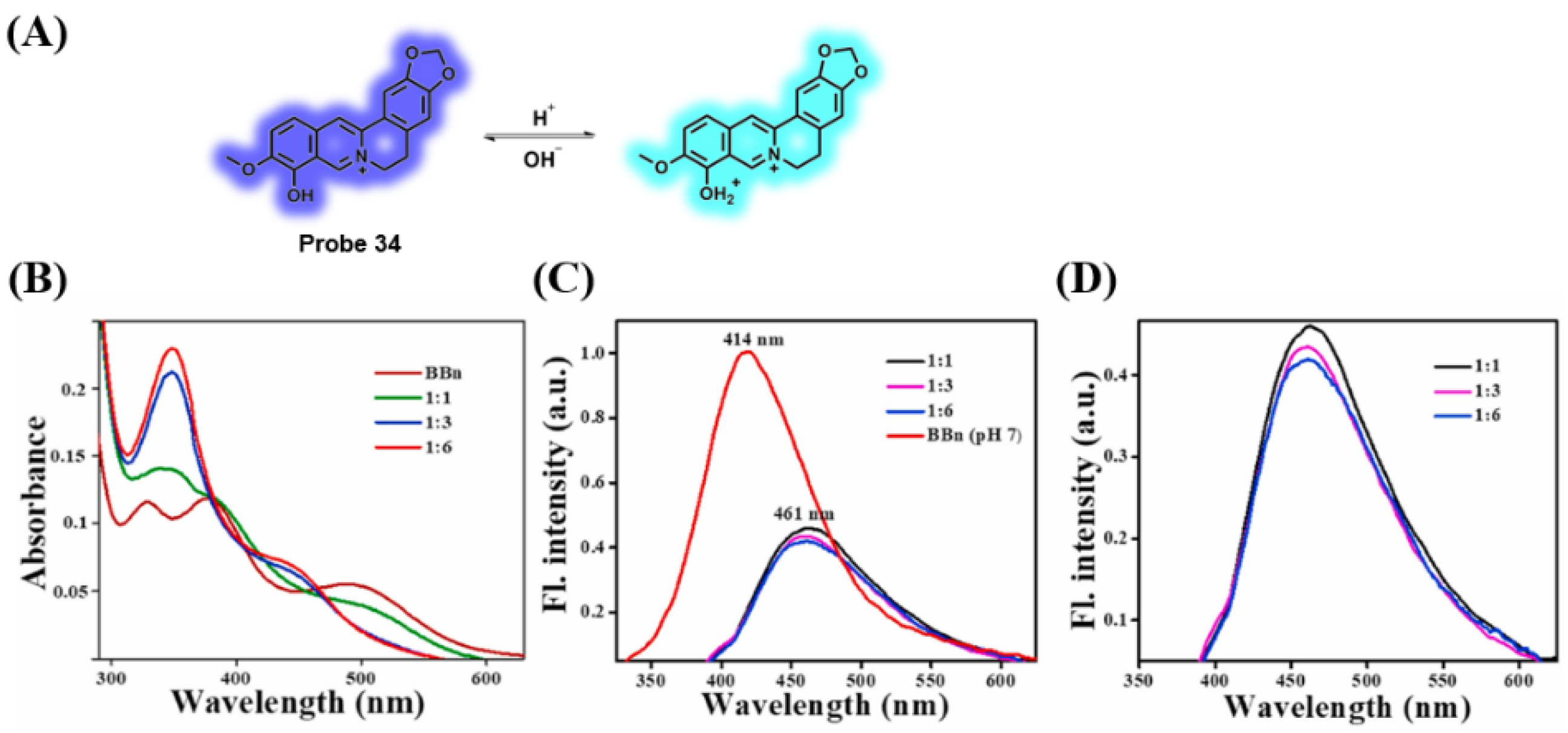
3.7. Probes Targeting pH Values
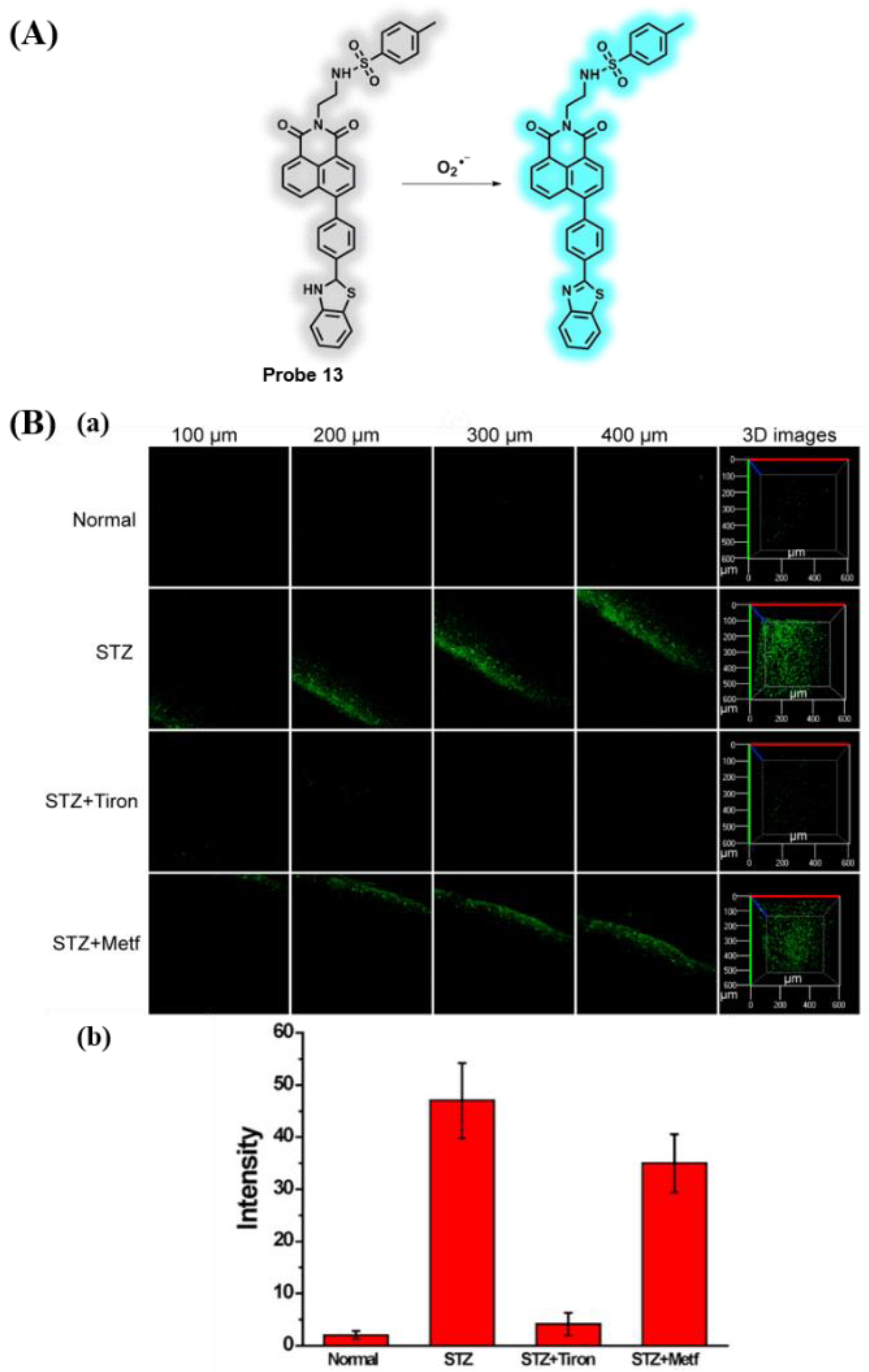
3.8. Probes Targeting O2•− and Polarity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vigliotta, G.; Miele, C.; Santopietro, S.; Portella, G.; Perfetti, A.; Maitan, M.A.; Cassese, A.; Oriente, F.; Trencia, A.; Fiory, F.; et al. Overexpression of the ped/pea-15 gene causes diabetes by impairing glucose-stimulated insulin secretion in addition to insulin action. Mol. Cell. Biol. 2004, 24, 5005–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for Type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. BBA Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef]
- Earle, K.; Walker, J.; Hill, C.; Viberti, G. Familial clustering of cardiovascular disease in patients with insulin-dependent diabetes and nephropathy. N. Engl. J. Med. 1992, 326, 673–677. [Google Scholar] [CrossRef]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef] [Green Version]
- Brufani, C.; Crino, A.; Fintini, D.; Patera, P.I.; Cappa, M.; Manco, M. Systematic review of metformin use in obese nondiabetic children and adolescents. Horm. Res. Paediatr. 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Ericson, U.; Hindy, G.; Drake, I.; Schulz, C.A.; Brunkwall, L.; Hellstrand, S.; Almgren, P.; Orho-Melander, M. Dietary and genetic risk scores and incidence of type 2 diabetes. Genes Nutr. 2018, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Wang, S.; Zhao, J.; Huang, W.; Li, J.; Xiao, Y.; Zhang, H.; Fu, Q.; Chen, Y.; Yang, T.; et al. Qingre Yiqi method along with oral hypoglycemic drugs in treating adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2021, 2021, 4395228. [Google Scholar] [CrossRef]
- Signorovitch, J.E.; Macaulay, D.; Diener, M.; Yan, Y.; Wu, E.Q.; Gruenberger, J.B.; Frier, B.M. Hypoglycaemia and accident risk in people with type 2 diabetes mellitus treated with non-insulin antidiabetes drugs. Diabetes Obes. Metab. 2013, 15, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in three α,β-dicarbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in advanced Maillard reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Sena, C.; Seica, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Tammineni, E.R.; Kraeva, N.; Figueroa, L.; Manno, C.; Ibarra, C.A.; Klip, A.; Riazi, S.; Rios, E. Intracellular calcium leak lowers glucose storage in human muscle, promoting hyperglycemia and diabetes. eLife 2020, 9, e53999. [Google Scholar] [CrossRef]
- Benninger, R.K.; Zhang, M.; Head, W.S.; Satin, L.S.; Piston, D.W. Gap junction coupling and calcium waves in the pancreatic islet. Biophys. J. 2008, 95, 5048–5061. [Google Scholar] [CrossRef] [Green Version]
- Syreeni, A.; Sandholm, N.; Cao, J.; Toppila, I.; Maahs, D.M.; Rewers, M.J.; Snell-Bergeon, J.K.; Costacou, T.; Orchard, T.J.; Caramori, M.L.; et al. Genetic determinants of glycated hemoglobin in type 1 diabetes. Diabetes 2019, 68, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Yudkin, J.S.; de Swiet, M. Fructosamine assay for gestational diabetes. Lancet 1983, 2, 1304. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Han, P.; Li, T. Glycated albumin and ratio of glycated albumin to glycated hemoglobin are good indicators of diabetic nephropathy in type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2017, 33, e2843. [Google Scholar] [CrossRef]
- Chen, J.; Stimpson, S.E.; Fernandez-Bueno, G.A.; Mathews, C.E. Mitochondrial reactive oxygen species and type 1 diabetes. Antioxid. Redox Signal. 2018, 29, 1361–1372. [Google Scholar] [CrossRef]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Reactive oxygen species: Sources, consequences and targeted therapy in type 2 diabetes. J. Drug Target. 2017, 25, 93–101. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.H. Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Lee, J.H.; Park, N.; Suh, M.; Chae, W.S.; Cao, J.; Peng, X.; Jung, H.; Kang, C.; et al. A self-calibrating bipartite viscosity sensor for mitochondria. J. Am. Chem. Soc. 2013, 135, 9181–9185. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Chen, S.; Tang, C.; Wang, G.; Du, J.; Jin, H. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J. Adv. Res. 2021, 27, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef] [Green Version]
- Jyoti; Mir, A.R.; Habib, S.; Siddiqui, S.S.; Ali, A.; Moinuddin. Neo-epitopes on methylglyoxal modified human serum albumin lead to aggressive autoimmune response in diabetes. Int. J. Biol. Macromol. 2016, 86, 799–809. [Google Scholar]
- Kirkpatrick, P. How DPP-IV takes a bite. Nat. Rev. Drug. Discov. 2003, 2, 92. [Google Scholar] [CrossRef]
- Hernandez, C.; Bogdanov, P.; Sola-Adell, C.; Sampedro, J.; Valeri, M.; Genis, X.; Simo-Servat, O.; Garcia-Ramirez, M.; Simo, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, Y.; Hamaguchi, M.; Nakanishi, N.; Ohbora, A.; Kojima, T.; Fukui, M. Urinary pH is a predictor of diabetes in men; a population based large scale cohort study. Diabetes Res. Clin. Pract. 2017, 130, 9–14. [Google Scholar] [CrossRef]
- Higashiura, Y.; Tanaka, M.; Furuhashi, M.; Koyama, M.; Ohnishi, H.; Numata, K.; Hisasue, T.; Hanawa, N.; Moniwa, N.; Miura, T. Low urine pH predicts new onset of diabetes mellitus during a 10-year period in men: BOREAS-DM1 study. J. Diabetes Investig. 2020, 11, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-T.; Kwon, N.; Wang, S.; Wang, B.; He, X.; Yoon, J.; Shen, J. Sulfur-based fluorescent probes for HOCl: Mechanisms, design, and applications. Coord. Chem. Rev. 2022, 450, 214232. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Wang, X.; Yang, F.; Xie, L.; Shen, J.; Brimble, M.A.; Xiao, Q.; Yao, S.Q. Fluorescent probes for bioimaging of potential biomarkers in Parkinson’s disease. Chem. Soc. Rev. 2021, 50, 1219–1250. [Google Scholar] [CrossRef]
- Hou, J.-T.; Yu, K.-K.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Wang, J.; Ren, W.X.; Wang, S.; Yu, X.-Q.; Kim, J.S. Fluorescent imaging of reactive oxygen and nitrogen species associated with pathophysiological processes. Chem 2020, 6, 832–866. [Google Scholar] [CrossRef]
- Han, H.H.; Tian, H.; Zang, Y.; Sedgwick, A.C.; Li, J.; Sessler, J.L.; He, X.P.; James, T.D. Small-molecule fluorescence-based probes for interrogating major organ diseases. Chem. Soc. Rev. 2021, 50, 9391–9429. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent probes for the visualization of cell viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef]
- Amels, P.; Elias, H.; Wannowius, K.-J. Kinetics and mechanism of the oxidation of dimethyl sulfide by hydroperoxides in aqueous medium study on the potential contribution of liquid-phase oxidation of dimethyl sulfide in the atmosphere. J. Chem. Soc. Faraday Trans. 1997, 93, 2537–2544. [Google Scholar] [CrossRef]
- Xing, P.; Gao, K.; Wang, B.; Gao, J.; Yan, H.; Wen, J.; Li, W.; Xu, Y.; Li, H.; Chen, J.; et al. HEPES is not suitable for fluorescence detection of HClO: A novel probe for HClO in absolute PBS. Chem. Commun. 2016, 52, 5064–5066. [Google Scholar] [CrossRef]
- Gadella, T.W.J. New near-infrared fluorescent probes and tools. Nat. Methods 2022, 19, 654–655. [Google Scholar] [CrossRef]
- Hou, J.-T.; Wang, B.; Zou, Y.; Fan, P.; Chang, X.; Cao, X.; Wang, S.; Yu, F. Molecular fluorescent probes for imaging and evaluation of hypochlorite fluctuations during diagnosis and therapy of osteoarthritis in cells and in a mouse model. ACS Sens. 2020, 5, 1949–1958. [Google Scholar] [CrossRef]
- Juvekar, V.; Park, S.J.; Yoon, J.; Kim, H.M. Recent progress in the two-photon fluorescent probes for metal ions. Coord. Chem. Rev. 2021, 427, 213574. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, H.; Wang, K.; Wang, G.; Liu, J.; James, T.D.; Zhang, H. mtDNA-Specific ultrasensitive near-infrared fluorescent probe enables the differentiation of healthy and apoptotic cells. Anal. Chem. 2022, 94, 7510–7519. [Google Scholar] [CrossRef] [PubMed]
- Usama, S.M.; Inagaki, F.; Kobayashi, H.; Schnermann, M.J. Norcyanine-carbamates are versatile near-infrared fluorogenic probes. J. Am. Chem. Soc. 2021, 143, 5674–5679. [Google Scholar] [CrossRef]
- Hou, J.-T.; Kim, H.S.; Duan, C.; Ji, M.S.; Wang, S.; Zeng, L.; Ren, W.X.; Kim, J.S. A ratiometric fluorescent probe for detecting hypochlorite in the endoplasmic reticulum. Chem. Commun. 2019, 55, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, Z.; Zhou, J.; Ma, Z. Simultaneous multi-signal quantification for highly precise serodiagnosis utilizing a rationally constructed platform. Nat. Commun. 2019, 10, 5361. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Zhou, K.; Wang, L.; Liu, K.; Lin, W. Construction of a ratiometric two-photon fluorescent probe to monitor the changes of mitochondrial viscosity. Sens. Actuators B Chem. 2018, 262, 452–459. [Google Scholar] [CrossRef]
- Krahmer, N.; Farese, R.V., Jr.; Walther, T.C. Balancing the fat: Lipid droplets and human disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Olofsson, S.O.; Andersson, L.; Haversen, L.; Olsson, C.; Myhre, S.; Rutberg, M.; Mobini, R.; Li, L.; Lu, E.; Boren, J.; et al. The formation of lipid droplets: Possible role in the development of insulin resistance/type 2 diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 215–218. [Google Scholar] [CrossRef]
- Ni, J.-Y.; Zhang, X.-Q.; Wang, M.-Y.; Yu, Q.; Sun, R.; Xu, Y.-J.; Song, Y.-L.; Ge, J.-F. Dicyanoisophorone derivatives with self-targeting abilities towards multiple organelles for fluorescent markers and viscosity detection. Sens. Actuators B Chem. 2022, 367, 132065. [Google Scholar] [CrossRef]
- Yin, J.; Kong, X.; Lin, W. Noninvasive cancer diagnosis in vivo based on a viscosity-activated near-infrared fluorescent probe. Anal. Chem. 2021, 93, 2072–2081. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Wang, S.; Zhang, Y.; Li, F.; Zan, Q.; Lu, W.; Shuang, S.; Dong, C. Real-time monitoring mitochondrial viscosity during mitophagy using a mitochondria-immobilized near-infrared aggregation-induced emission probe. Anal. Chem. 2021, 93, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Song, W.; Lu, Y.; Sun, Y.; Lin, W. Revealing the viscosity changes in lipid droplets during ferroptosis by the real-time and in situ near-infrared imaging. ACS Sens. 2021, 6, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, D.; Ye, Y.; Zhao, Y. Recent advances in multifunctional fluorescent probes for viscosity and analytes. Coord. Chem. Rev. 2022, 453, 214336. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Huang, H.; Qiu, K.; Zhang, C.; Chao, H.; Zhang, Q. A viscosity-sensitive iridium(iii) probe for lysosomal microviscosity quantification and blood viscosity detection in diabetic mice. Dalton Trans. 2019, 48, 3990–3997. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent advances of optical imaging in the second near-infrared window. Adv. Mater. 2018, 30, 1802394. [Google Scholar]
- Jing, L.; Sun, M.; Xu, P.; Yao, K.; Yang, J.; Wang, X.; Liu, H.; Sun, M.; Sun, Y.; Ni, R.; et al. Noninvasive in vivo imaging and monitoring of 3D-printed polycaprolactone scaffolds labeled with an NIR region II fluorescent dye. ACS Appl. Bio Mater. 2021, 4, 3189–3202. [Google Scholar] [CrossRef]
- Zhu, S.; Herraiz, S.; Yue, J.; Zhang, M.; Wan, H.; Yang, Q.; Ma, Z.; Wang, Y.; He, J.; Antaris, A.L.; et al. 3D NIR-II molecular imaging distinguishes targeted organs with high-performance NIR-II bioconjugates. Adv. Mater. 2018, 30, e1705799. [Google Scholar] [CrossRef]
- Wang, F.; Wan, H.; Ma, Z.; Zhong, Y.; Sun, Q.; Tian, Y.; Qu, L.; Du, H.; Zhang, M.; Li, L.; et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 2019, 16, 545–552. [Google Scholar] [CrossRef]
- Dou, K.; Huang, W.; Xiang, Y.; Li, S.; Liu, Z. Design of activatable NIR-II molecular probe for in vivo elucidation of disease-related viscosity variations. Anal. Chem. 2020, 92, 4177–4181. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Shen, W.; Chen, Y.; Yi, W.; Cai, C.; Zhu, L.; Zhu, Q. A highly sensitive red-emitting probe for the detection of viscosity changes in living cells, zebrafish, and human blood samples. J. Mater. Chem. B 2020, 8, 1310–1315. [Google Scholar] [CrossRef]
- Chen, B.; Mao, S.; Sun, Y.; Sun, L.; Ding, N.; Li, C.; Zhou, J. A mitochondria-targeted near-infrared fluorescent probe for imaging viscosity in living cells and a diabetic mice model. Chem. Commun. 2021, 57, 4376–4379. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ren, M.; Lin, W. Development of a novel NIR viscosity fluorescent probe for visualizing the kidneys in diabetic mice. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119627. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yin, J.; Liu, C.; Lin, W. NIR fluorescence imaging of lipid drops viscosity in liver organs of diabetic mice. Dyes Pigments 2021, 187, 109120. [Google Scholar] [CrossRef]
- Del Valle Batalla, F.; Lennon-Dumenil, A.M.; Yuseff, M.I. Tuning B cell responses to antigens by cell polarity and membrane trafficking. Mol. Immunol. 2018, 101, 140–145. [Google Scholar] [CrossRef]
- Mizukawa, B.; O’Brien, E.; Moreira, D.C.; Wunderlich, M.; Hochstetler, C.L.; Duan, X.; Liu, W.; Orr, E.; Grimes, H.L.; Mulloy, J.C.; et al. The cell polarity determinant CDC42 controls division symmetry to block leukemia cell differentiation. Blood 2017, 130, 1336–1346. [Google Scholar] [CrossRef] [Green Version]
- Scheler, B.; Schnepf, V.; Galgenmuller, C.; Ranf, S.; Huckelhoven, R. Barley disease susceptibility factor RACB acts in epidermal cell polarity and positioning of the nucleus. J. Exp. Bot. 2016, 67, 3263–3275. [Google Scholar] [CrossRef]
- Hu, J.; Yang, R.; Qin, H.; Sun, Y.; Qu, L.; Li, Z. Spying on the polarity dynamics during wound healing of zebrafish by using rationally designed carbon dots. Adv. Healthc. Mater. 2021, 10, e2002268. [Google Scholar] [CrossRef]
- Shuang, E.; Mao, Q.X.; Wang, J.H.; Chen, X.W. Carbon dots with tunable dual emissions: From the mechanism to the specific imaging of endoplasmic reticulum polarity. Nanoscale 2020, 12, 6852–6860. [Google Scholar] [CrossRef]
- Yin, J.; Quan, W.; Kong, X.; Liu, C.; Lu, B.; Lin, W. Utilizing a solvatochromic optical agent to monitor the polarity changes in dynamic liver injury progression. ACS Appl. Bio Mater. 2021, 4, 3630–3638. [Google Scholar] [CrossRef]
- Jiang, J.; Tian, X.; Xu, C.; Wang, S.; Feng, Y.; Chen, M.; Yu, H.; Zhu, M.; Meng, X. A two-photon fluorescent probe for real-time monitoring of autophagy by ultrasensitive detection of the change in lysosomal polarity. Chem. Commun. 2017, 53, 3645–3648. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, C.; Li, P.; Gao, W.; Zhang, W.; Zhang, W.; Tong, L.; Tang, B. Ratiometric photoacoustic imaging of endoplasmic reticulum polarity in injured liver tissues of diabetic mice. Chem. Sci. 2017, 8, 7025–7030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Zhang, X.; Cao, H.; Chen, G.; Huang, H.; Zhang, P.; Zhang, Q. A phosphorescent iridium probe for sensing polarity in the endoplasmic reticulum and in vivo. Dalton Trans. 2019, 48, 7728–7734. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiang, T.; Ren, L.; Cheng, F.; Hu, W.; Qu, R. Observation of macrophage autophagy in the healing of diabetic ulcers via a lysosome-targeting polarity-specific two-photon probe. RSC Adv. 2022, 12, 3654–3661. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Byrne, N.J.; Rajasekaran, N.S.; Abel, E.D.; Bugger, H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic. Biol. Med. 2021, 169, 317–342. [Google Scholar] [CrossRef]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High glucose augments ROS generation regulates mitochondrial dysfunction and apoptosis via stress signalling cascades in keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar]
- Matsuzaki, S.; Eyster, C.; Newhardt, M.F.; Giorgione, J.R.; Kinter, C.; Young, Z.T.; Kinter, M.; Humphries, K.M. Insulin signaling alters antioxidant capacity in the diabetic heart. Redox Biol. 2021, 47, 102140. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar]
- Makino, A.; Scott, B.T.; Dillmann, W.H. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010, 53, 1783–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, V.V.; Shakhristova, E.V.; Stepovaya, E.A.; Nosareva, O.L.; Fedorova, T.S.; Ryazantseva, N.V.; Novitsky, V.V. Effect of insulin, the glutathione system, and superoxide anion radical in modulation of lipolysis in adipocytes of rats with experimental diabetes. Biochem. Mosc. 2015, 80, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Liu, J.; Shu, M.; Ying, Y.; Yang, H. Detection strategies for superoxide anion: A review. Talanta 2022, 236, 122892. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, X.; Wu, C.; Wu, Y.; Li, P.; Guo, X.; Tang, B. A new endoplasmic reticulum-targeted two-photon fluorescent probe for imaging of superoxide anion in diabetic mice. Biosens. Bioelectron. 2017, 91, 449–455. [Google Scholar] [CrossRef]
- Song, W.; Dong, B.; Lu, Y.; Li, Z.; Zhang, W.; Lin, W. Two-photon fluorescent sensors for visual detection of abnormal superoxide anion in diabetes mice. Sens. Actuators B Chem. 2021, 332, 129537. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr. Opin. Pharmacol. 2006, 6, 136–141. [Google Scholar] [CrossRef]
- Suarez-Pinzon, W.L.; Szabo, C.; Rabinovitch, A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes 1997, 46, 907–911. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, X.; Lu, X.; Chen, Y.; Ju, J.; Wu, H.; Zhu, B.; Huang, S. An SMVT-targeting and peroxynitrite-activating fluorescent probe for head and neck cancer imaging and peroxynitrite detection. Sens. Actuators B Chem. 2021, 348, 130677. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Wang, Y.; Peng, C.; Hu, H.; Qiao, M.; Zhao, X.; Chen, D. Tumor-specific nitric oxide generator to amplify peroxynitrite based on highly penetrable nanoparticles for metastasis inhibition and enhanced cancer therapy. Biomaterials 2022, 283, 121448. [Google Scholar] [CrossRef]
- Li, W.; Feng, J.; Gao, C.; Wu, M.; Du, Q.; Tsoi, B.; Wang, Q.; Yang, D.; Shen, J. Nitration of Drp1 provokes mitophagy activation mediating neuronal injury in experimental autoimmune encephalomyelitis. Free Radic. Biol. Med. 2019, 143, 70–83. [Google Scholar] [CrossRef]
- Miao, J.; Huo, Y.; Liu, Q.; Li, Z.; Shi, H.; Shi, Y.; Guo, W. A new class of fast-response and highly selective fluorescent probes for visualizing peroxynitrite in live cells, subcellular organelles, and kidney tissue of diabetic rats. Biomaterials 2016, 107, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Huo, Y.; Shi, H.; Fang, J.; Wang, J.; Guo, W. A Si-rhodamine-based near-infrared fluorescent probe for visualizing endogenous peroxynitrite in living cells, tissues, and animals. J. Mater. Chem. B 2018, 6, 4466–4473. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Ramkumar, K.M. Role of ER stress inhibitors in the management of diabetes. Eur. J. Pharmacol. 2022, 922, 174893. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic opportunities for pancreatic beta-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Zhang, W.; Song, W.; Lin, W. A novel ER-targeted two-photon fluorescent probe for monitoring abnormal concentrations of HClO in diabetic mice. J. Mater. Chem. B 2021, 9, 7381–7385. [Google Scholar] [CrossRef]
- Zhang, R.; Lian, L.; Wang, B.; Zhu, L.; Ren, Y.; Shen, J.; Yu, X.Q.; Hou, J.-T. Observation of HOCl generation associated with diabetic cataract using a highly sensitive fluorescent probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121385. [Google Scholar] [CrossRef]
- Wang, W.X.; Jiang, W.L.; Mao, G.J.; Tan, M.; Fei, J.; Li, Y.; Li, C.Y. Monitoring the fluctuation of hydrogen peroxide in diabetes and its complications with a novel near-infrared fluorescent probe. Anal. Chem. 2021, 93, 3301–3307. [Google Scholar] [CrossRef]
- Yu, W.; Huang, J.; Lin, M.; Wei, G.; Yang, F.; Tang, Z.; Zeng, F.; Wu, S. Fluorophore-dapagliflozin dyad for detecting diabetic liver/kidney damages via fluorescent imaging and treating diabetes via inhibiting SGLT2. Anal. Chem. 2021, 93, 4647–4656. [Google Scholar] [CrossRef]
- Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef]
- Pichette, J.; Gagnon, J. Implications of hydrogen sulfide in glucose regulation: How H2S can alter glucose homeostasis through metabolic hormones. Oxid. Med. Cell. Longev. 2016, 2016, 3285074. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Banerjee, R. Regulation of the redox metabolome and thiol proteome by hydrogen sulfide. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, L.; Shen, J.; Jiang, J.; Yu, K.; Sun, R. Research progress in the visual sensors/sensing ensembles for L-Cysteine. Chin. J. Org. Chem. 2018, 38, 760–774. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Guo, Z.; Zhao, H.; Liu, L.; Wang, M.; Zhang, P.; Chen, G.; Li, X.; Wei, C. Monitoring the fluctuation of H2S in insulin-resistant HepG2 cells and diabetic mice with a dual-locked NIR fluorescent probe. Sens. Actuators B Chem. 2022, 353, 131141. [Google Scholar] [CrossRef]
- Yue, L.; Huang, H.; Song, W.; Lin, W. Research on mitochondrial oxidative stress accompanying the diabetic process under airborne particulate matter pollution by NIR fluorescence imaging of cysteine. Chem. Eng. J. 2022, 441, 135981. [Google Scholar] [CrossRef]
- Luengo, A.; Abbott, K.L.; Davidson, S.M.; Hosios, A.M.; Faubert, B.; Chan, S.H.; Freinkman, E.; Zacharias, L.G.; Mathews, T.P.; Clish, C.B.; et al. Reactive metabolite production is a targetable liability of glycolytic metabolism in lung cancer. Nat. Commun. 2019, 10, 5604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Methylglyoxal comes of AGE. Cell 2006, 124, 258–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, R.; Desai, K.; Wu, L. Upregulation of aldolase B and overproduction of methylglyoxal in vascular tissues from rats with metabolic syndrome. Cardiovasc. Res. 2011, 92, 494–503. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dicarbonyl intermediates in the maillard reaction. Ann. N. Y. Acad. Sci. 2005, 1043, 111–117. [Google Scholar] [CrossRef]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 2010, 59, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, B.; Dinarvand, R.; Mohammadpour, H.; Kamarul, T.; Sharifi, A.M. Dual l-carnosine/Aloe vera nanophytosomes with synergistically enhanced protective effects against methylglyoxal-induced angiogenesis impairment. Mol. Pharm. 2021, 18, 3302–3325. [Google Scholar] [CrossRef]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal metabolism and aging-related disease: Moving from correlation toward causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fan, J.; Zhang, J.; Du, J.; Peng, X. Visualization of methylglyoxal in living cells and diabetic mice model with a 1,8-naphthalimide-based two-photon fluorescent probe. Chem. Sci. 2018, 9, 6758–6764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xu, Y.; Rao, L.; Yang, C.; Yuan, H.; Gao, T.; Chen, X.; Sun, H.; Xian, M.; Liu, C.; et al. Ratiometric fluorescent probe for monitoring endogenous methylglyoxal in living cells and diabetic blood samples. Anal. Chem. 2019, 91, 5646–5653. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Q.; Song, X.; Wang, C.; Wang, X.; Ma, S.; Wang, X.; Feng, Y.; Meng, X.; Liu, X.; et al. Fluorophore-promoted facile deprotonation and exocyclic five-membered ring cyclization for selective and dynamic tracking of labile glyoxals. Anal. Chem. 2020, 92, 13829–13838. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.K.; Dawlaty, M.M.; Verma, A.; Maitra, A. Roles and regulations of TET enzymes in solid tumors. Trends Cancer 2021, 7, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, R.; Kwon, N.; Ma, H.; Yoon, J. Activatable fluorescent probes for in situ imaging of enzymes. Chem. Soc. Rev. 2022, 51, 450–463. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Li, X.; Ma, H. Recognition moieties of small molecular fluorescent probes for bioimaging of enzymes. Acc. Chem. Res. 2019, 52, 1892–1904. [Google Scholar] [CrossRef]
- Singh, H.; Tiwari, K.; Tiwari, R.; Pramanik, S.K.; Das, A. Small molecule as fluorescent probes for monitoring intracellular enzymatic transformations. Chem. Rev. 2019, 119, 11718–11760. [Google Scholar] [CrossRef]
- Wang, W.X.; Jiang, W.L.; Guo, H.; Li, Y.; Li, C.Y. Real-time imaging of alkaline phosphatase activity of diabetes in mice via a near-infrared fluorescent probe. Chem. Commun. 2021, 57, 480–483. [Google Scholar] [CrossRef]
- Guo, X.; Mu, S.; Li, J.; Zhang, Y.; Liu, X.; Zhang, H.; Gao, H. Fabrication of a water-soluble near-infrared fluorescent probe for selective detection and imaging of dipeptidyl peptidase IV in biological systems. J. Mater. Chem. B 2020, 8, 767–775. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Qu, Y.; Yang, Y.; Cao, T.; Cao, Y.; Iqbal, A.; Qin, W.; Liu, Y. Long-wavelength ratiometric fluorescent probe for the early diagnosis of diabetes. Anal. Chem. 2021, 93, 11461–11469. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Braun-Falco, O. The effect of detergents on skin pH and its consequences. Clin. Dermatol. 1996, 14, 23–27. [Google Scholar] [CrossRef]
- Ehlers, C.; Ivens, U.; Møller, M.; Senderovitz, T.; Serup, J. Technology, females have lower skin surface pH than men: A study on the influence of gender, forearm site variation, right/left difference and time of the day on the skin surface pH. Skin Res. Technol. 2001, 7, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52. [Google Scholar]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Kasiewicz, L.N.; Whitehead, K.A. Recent advances in biomaterials for the treatment of diabetic foot ulcers. Biomater. Sci. 2017, 5, 1962–1975. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 41. [Google Scholar] [CrossRef]
- Nanda, S.; Longo, S.; Bhatt, S.P.; Pamula, J.; Sharma, S.G.; Dale, T.H. Stress cardiomyopathy—A unique presentation of diabetic ketoacidosis. Ann. Clin. Biochem. 2009, 46, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Mai, H.; Wang, Y.; Li, S.; Jia, R.; Li, S.; Peng, Q.; Xie, Y.; Hu, X.; Wu, S. A pH-sensitive near-infrared fluorescent probe with alkaline pKa for chronic wound monitoring in diabetic mice. Chem. Commun. 2019, 55, 7374–7377. [Google Scholar] [CrossRef]
- Meenu, M.T.; Cherian, A.R.; Sherin, D.R.; Nair, A.R.; Manojkumar, T.K.; Radhakrishnan, K.V.; Varghese, A. A protoberberine alkaloid based ratiometric pH-responsive probe for the detection of diabetic ketoacidosis. Dyes Pigments 2021, 194, 109636. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Li, S.; Zhang, X.; Guo, Z.; Hu, J.; Shao, X.; Song, N.; Zhao, Y.; Li, H.; et al. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol. 2020, 32, 101514. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.W.; Zhu, H.T.; Chen, K.L.; Dong, X.; Wei, J.; Qiu, C.; Xue, J.H. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 2013, 12, 158. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Deng, Z.; Zhang, J.; Yang, C.; Liu, J.; Han, W.; Ye, P.; Si, Y.; Chen, G. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J. Transl. Med. 2019, 17, 251. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Wu, C.; Li, P.; Tang, B. Simultaneous fluorescence visualization of endoplasmic reticulum superoxide anion and polarity in myocardial cells and tissue. Anal. Chem. 2018, 90, 6081–6088. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, T.-T.; Li, Y.; Niu, H. Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy. Chemosensors 2022, 10, 280. https://doi.org/10.3390/chemosensors10070280
Jia T-T, Li Y, Niu H. Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy. Chemosensors. 2022; 10(7):280. https://doi.org/10.3390/chemosensors10070280
Chicago/Turabian StyleJia, Tong-Tong, Yashan Li, and Huawei Niu. 2022. "Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy" Chemosensors 10, no. 7: 280. https://doi.org/10.3390/chemosensors10070280
APA StyleJia, T.-T., Li, Y., & Niu, H. (2022). Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy. Chemosensors, 10(7), 280. https://doi.org/10.3390/chemosensors10070280





