Copper-Induced Fluorescence Quenching in a Bis[2-(2′-hydroxyphenyl)benzoxazole]pyridinium Derivative for Quantification of Cu2+ in Solution
Abstract
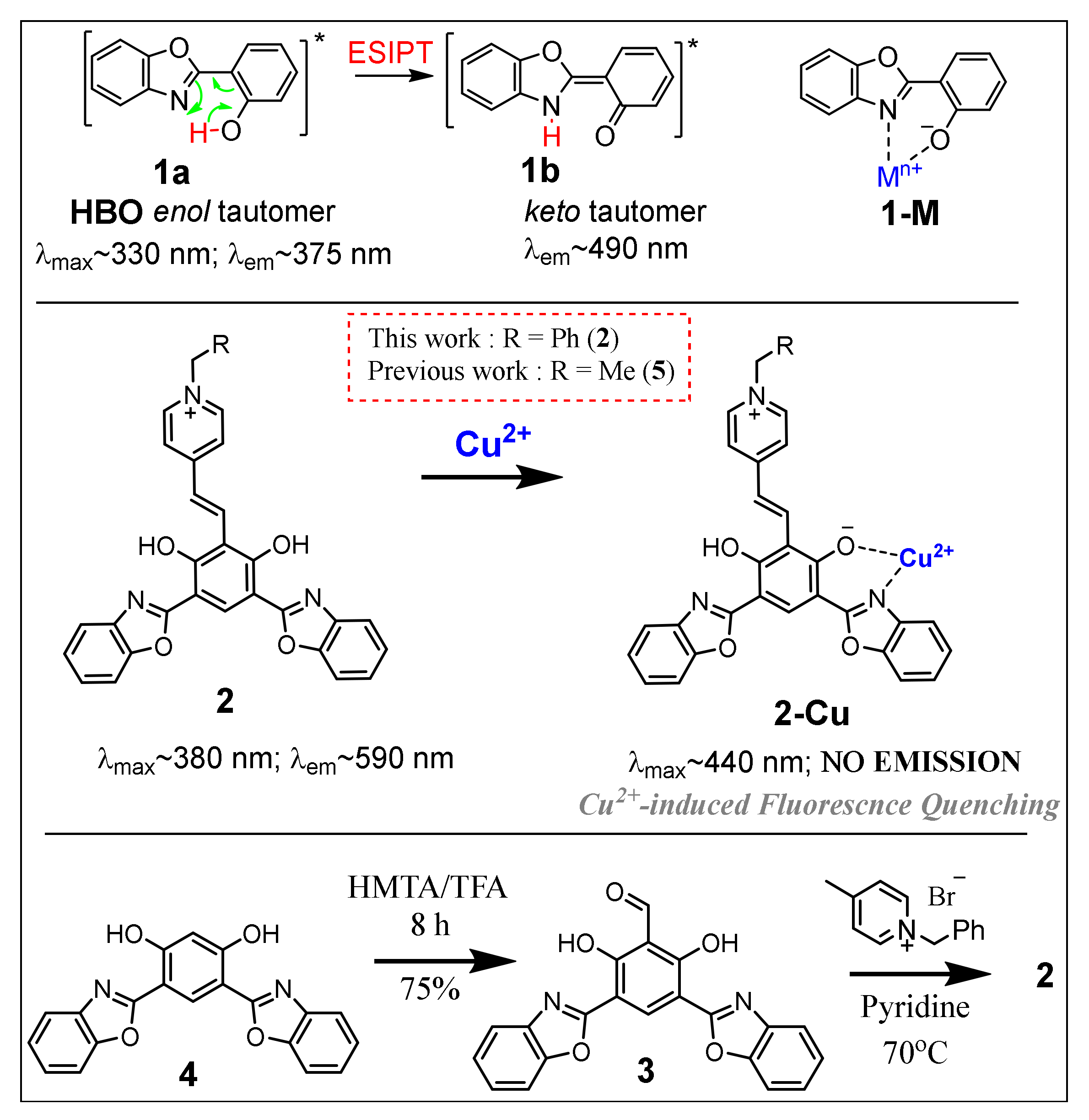
:1. Introduction
2. Materials and Methods
2.1. Synthesis of (E)-4-(3,5-Bis(benzo[d]oxazol-2-yl)-2,6-dihydroxystyryl)-1-benzylpyridin-1-ium Iodide (2)
2.2. Fluorescence Quantum Yield Calculation
2.3. Spectroscopic Titrations and Calculations
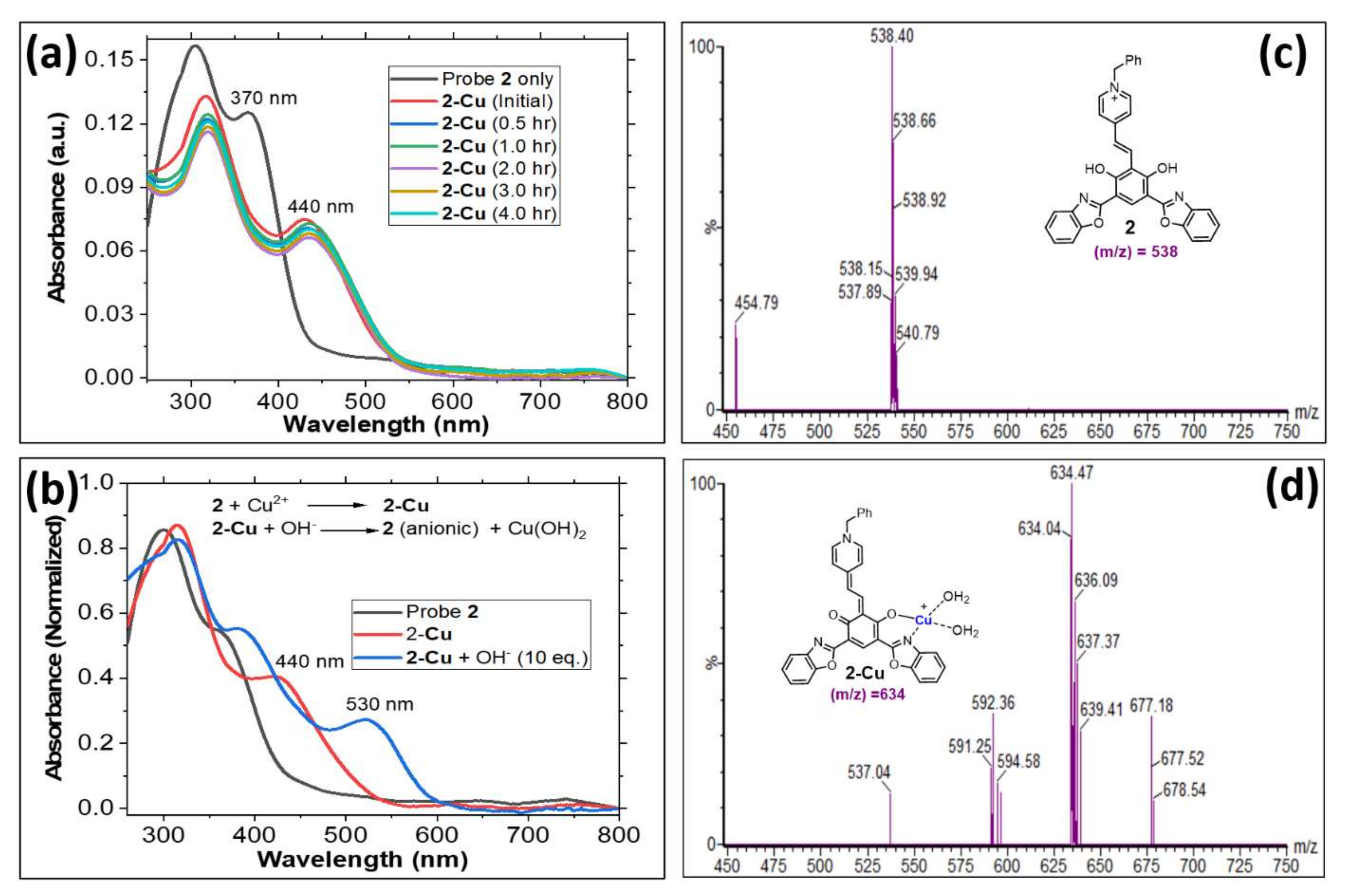
3. Results and Discussion
3.1. Optical Properties of Probe 2
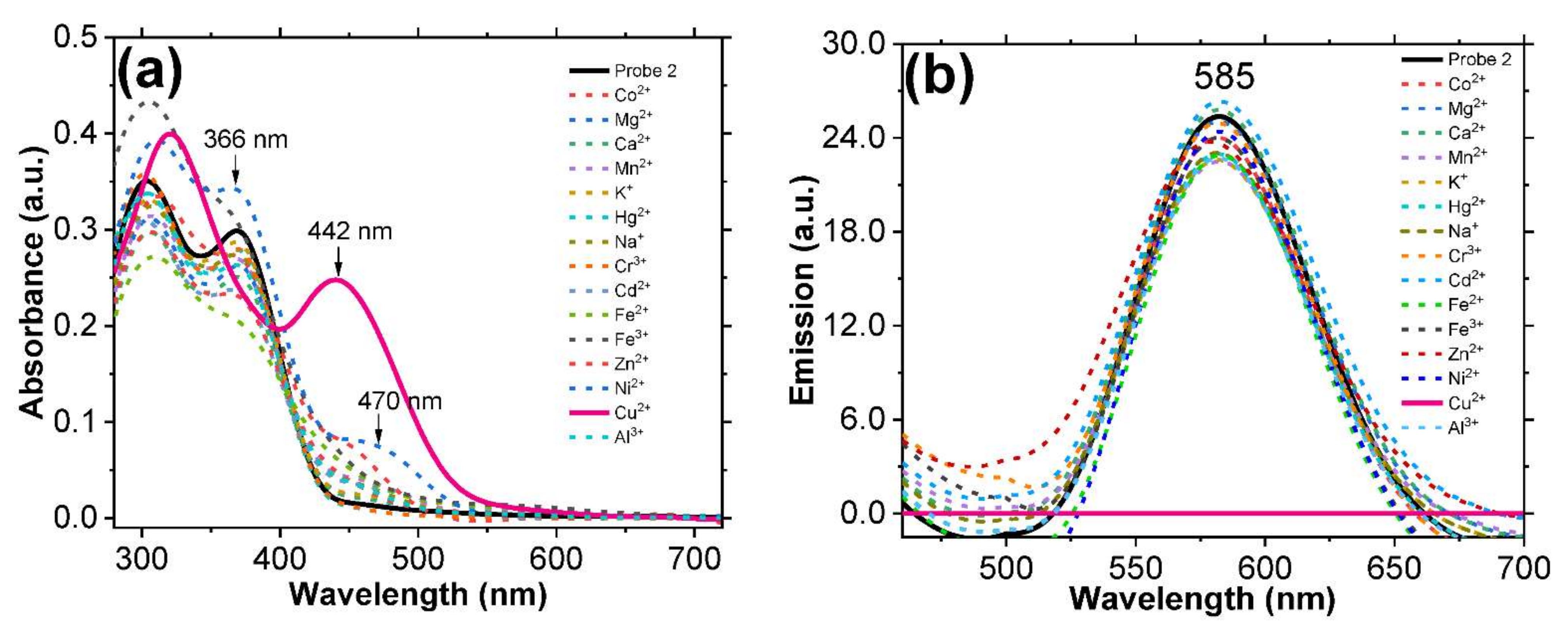
3.2. Evaluating Cation Sensing Ability of Probe 2 by Spectroscopy
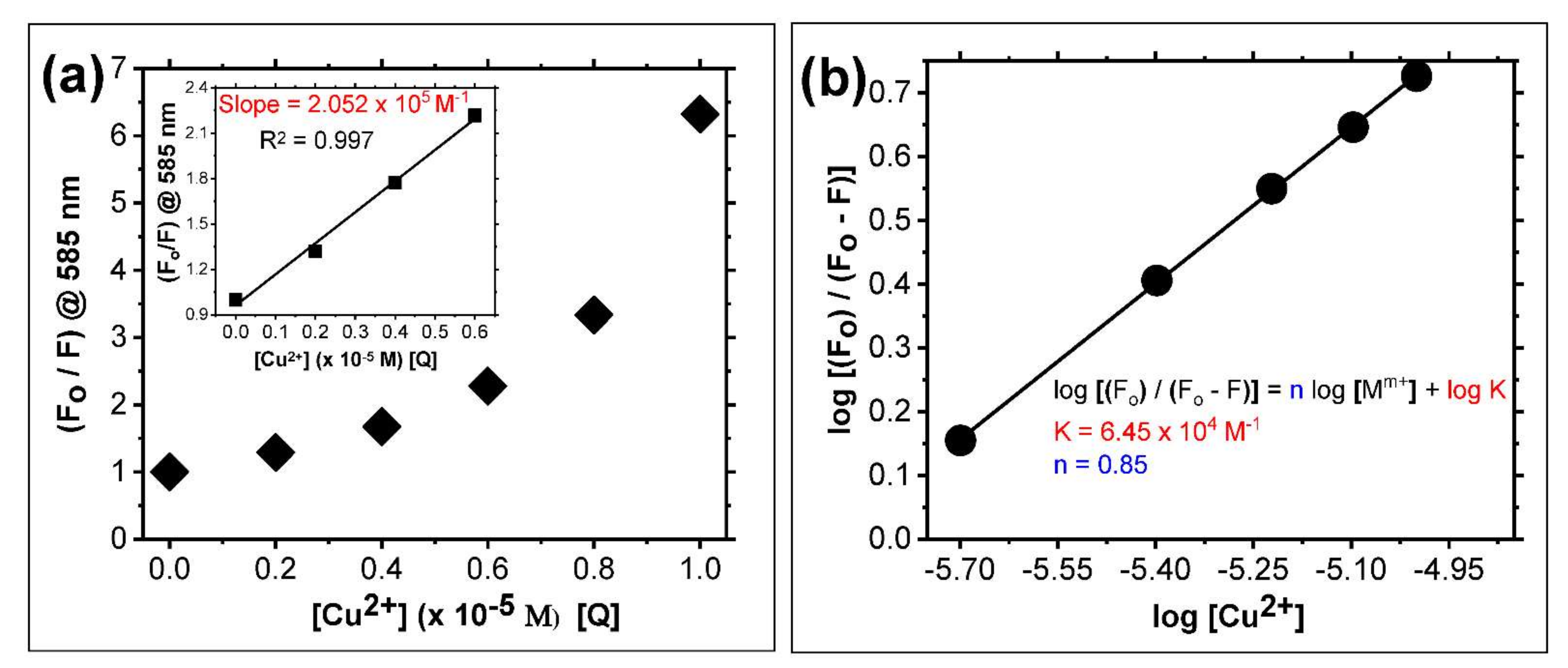
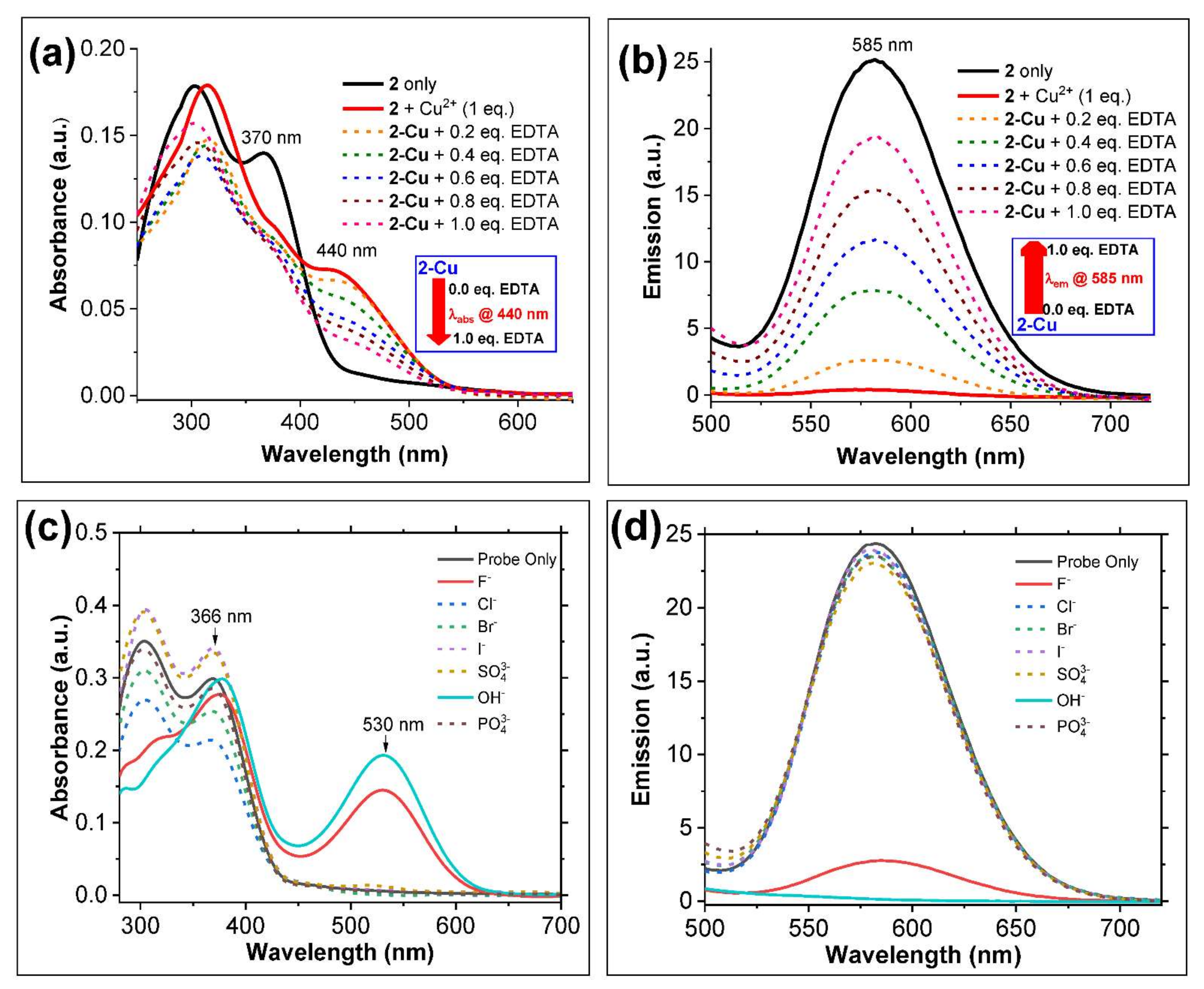
3.3. Study of Cu2+ Binding Properties of Probe 2 by Spectroscopic Titrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taki, M.; Wolford, J.L.; O’Halloran, T. V Emission Ratiometric Imaging of Intracellular Zinc: Design of a Benzoxazole Fluorescent Sensor and Its Application in Two-Photon Microscopy. J. Am. Chem. Soc. 2004, 126, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, S.; You, Y.; Baek, K.H.; Ohkubo, K.; Cho, J.; Fukuzumi, S.; Shin, I.; Park, S.Y.; Nam, W. Fluorescent Zinc Sensor with Minimized Proton-Induced Interferences: Photophysical Mechanism for Fluorescence Turn-on Response and Detection of Endogenous Free Zinc Ions. Inorg. Chem. 2012, 51, 8760–8774. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Bertman, K.A.; Plescia, C.B.; Stahelin, R.V.; Pang, Y. Structural Effect on the Cellular Selectivity of an NIR-Emitting Cyanine Probe: From Lysosome to Simultaneous Nucleus and Mitochondria Selectivity with Potential for Monitoring Mitochondria Dysfunction in Cells. ACS Appl. Bio Mater. 2019, 2, 5174–5181. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Leray, I. Design Principles of Fluorescent Molecular Sensors for Cation Recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Mizukami, S.; Nagano, T.; Urano, Y.; Odani, A.; Kikuchi, K. A Fluorescent Anion Sensor That Works in Neutral Aqueous Solution for Bioanalytical Application. J. Am. Chem. Soc. 2002, 124, 3920–3925. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical PH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef]
- Li, C.; Liu, S. Responsive Nanogel-Based Dual Fluorescent Sensors for Temperature and Hg2+ Ions with Enhanced Detection Sensitivity. J. Mater. Chem. 2010, 20, 10716–10723. [Google Scholar] [CrossRef]
- Khan, Z.G.; Patil, P.O. A Comprehensive Review on Carbon Dots and Graphene Quantum Dots Based Fluorescent Sensor for Biothiols. Microchem. J. 2020, 157, 105011. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Li, Y.; Ramanah, A.; Owitipana, D.N.; Wijesinghe, K.J.; Pang, Y. Albumin-Induced Large Fluorescence Turn ON in 4-(Diphenylamino)Benzothiazolium Dyes for Clinical Applications in Protein Detection. Sens. Actuators B Chem. 2022, 368, 132199. [Google Scholar] [CrossRef]
- Hu, Q.; Yao, B.; Owyong, T.C.; Prashanth, S.; Wang, C.; Zhang, X.; Wong, W.W.H.; Tang, Y.; Hong, Y. Detection of Urinary Albumin Using a “Turn-on” Fluorescent Probe with Aggregation-Induced Emission Characteristics. Chem. Asian J. 2021, 16, 1245–1252. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Bertman, K.A.; McDonald, L.; Alexander, N.; Dahal, D.; Baumann, H.J.; Salmon, C.R.; Tessier, C.; Wesdemiotis, C.; Konopka, M. Synthesis of Highly Selective Lysosome Markers by Coupling 2-(2′-Hydroxyphenyl) Benzothiazole (HBT) with Benzothiazolium Cyanine (Cy): The Impact of Substituents towards Selectivity and Optical Properties. J. Mater. Chem. B 2019, 7, 7502–7514. [Google Scholar] [CrossRef]
- Chen, W.-H.; Pang, Y. Excited-State Intramolecular Proton Transfer in 2-(2′,6′-Dihydroxyphenyl)Benzoxazole: Effect of Dual Hydrogen Bonding on the Optical Properties. Tetrahedron Lett. 2010, 51, 1914–1918. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, W. Excited-State Intramolecular Proton Transfer in 2-(2′-Hydroxyphenyl)Benzoxazole Derivatives. In Hydrogen Bonding Transfer Excited State; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 747–760. [Google Scholar]
- Abeywickrama, C.S.; Pang, Y. Synthesis of Fused 2-(2′-Hydroxyphenyl) Benzoxazole Derivatives: The Impact of Meta-/Para-Substitution on Fluorescence and Zinc Binding. Tetrahedron Lett. 2016, 57, 3518–3522. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Pang, Y. Fused Bis [2-(2′-Hydroxyphenyl) Benzoxazole] Derivatives for Improved Fluoride Sensing: The Impact of Regiochemistry and Competitive Hydrogen Bonding. Tetrahedron Lett. 2017, 58, 1627–1632. [Google Scholar] [CrossRef]
- Qinghui, C.; Medvetz, D.A.; Yi, P. A Polymeric Colorimetric Sensor with Excited-State Intramolecular Proton Transfer for Anionic Species. Chem. Mater. 2007, 19, 6421–6429. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Dou, B.; Wright, B.; Wang, J.; Pang, Y. Zn2+ Binding-Enabled Excited State Intramolecular Proton Transfer: A Step toward New near-Infrared Fluorescent Probes for Imaging Applications. Adv. Healthc. Mater. 2012, 1, 485–492. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Pang, Y. Synthesis of a Bis [2-(2′-Hydroxyphenyl) Benzoxazole] Pyridinium Derivative: The Fluoride-Induced Large Spectral Shift for Ratiometric Response. New J. Chem. 2021, 45, 9102–9108. [Google Scholar] [CrossRef]
- Council, N.R. Health Effects of Excess Copper. In Copper in Drinking Water; National Academies Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically Diverse Small Molecule Fluorescent Chemosensors for Copper Ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Golbedaghi, R.; Justino, L.L.G.; Ooshall, F.; Jamehbozorgi, S.; Abdolmaleki, M.; Fausto, R. A New Schiff Base Ligand as a Fluorescence Probe for Cu (II) Detection in Semi-Aqueous Solution: Synthesis, Characterization, Fluorescence and Mechanistic Insight. Inorg. Chim. Acta 2021, 528, 120623. [Google Scholar] [CrossRef]
- Warrier, S.B.; Kharkar, P.S. A Coumarin Based Chemosensor for Selective Determination of Cu (II) Ions Based on Fluorescence Quenching. J. Lumin. 2018, 199, 407–415. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Mao, S.; Aderinto, S.O.; Shi, X.; Shen, K.; Wu, H. A New, Highly Potent 1, 8-naphthalimide-based Fluorescence Turn-off Chemosensor Capable of Detecting Cu (II) Ions in Real-world Water Samples. Color. Technol. 2018, 134, 230–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Wang, J.; Jia, L.; Yang, R.; Guo, X. A 4,5-Quinolimide-Based Fluorescent Sensor for Sequential Detection of Cu2+ and Cysteine in Water and Living Cells with Application in a Memorized Device. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118030. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Di, J.; Li, L.; Tu, Y.; Yan, J. Copper Ion Detection with Improved Sensitivity through Catalytic Quenching of Gold Nanocluster Fluorescence. Talanta 2018, 187, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, H.; Chen, S.; Huang, Y.; Zhu, X.; Li, H.; Zhang, Y.; Yao, S. A Turn-on Red-Emitting Fluorescent Probe for Determination of Copper (II) Ions in Food Samples and Living Zebrafish. Food Chem. 2021, 343, 128513. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, X.; Wei, P.; Gao, Y.; Li, Q. Highly Selective Fluorescent Probe for the Detection of Copper (II) and Its Application in Live Cell Imaging. J. Anal. Methods Chem. 2019, 2019, 8130767. [Google Scholar] [CrossRef]
- Makowska, J.; Żamojć, K.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L. Copper (II) Complexation by Fragment of Central Part of FBP28 Protein from Mus Musculus. Biophys. Chem. 2018, 241, 55–60. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhou, L.; He, H.; Yin, J.; Gao, Q.; Wei, J.; Duan, C.; Peng, X. A Novel Rhodamine B-Based “off-on’’fluorescent Sensor for Selective Recognition of Copper (II) Ions. Talanta 2018, 184, 143–148. [Google Scholar] [CrossRef]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper (II) Ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Lakowicz, J.R. Quenching of Fluorescence. In Principles of Fluorescence Spectroscopy; Springer Science: Berlin/Heidelberg, Germany, 1983; pp. 257–301. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Fraiji, L.K.; Hayes, D.M.; Werner, T.C. Static and Dynamic Fluorescence Quenching Experiments for the Physical Chemistry Laboratory. J. Chem. Educ. 1992, 69, 424. [Google Scholar] [CrossRef]
- Marton, A.; Clark, C.C.; Srinivasan, R.; Freundlich, R.E.; Narducci Sarjeant, A.A.; Meyer, G.J. Static and Dynamic Quenching of Ru (II) Polypyridyl Excited States by Iodide. Inorg. Chem. 2006, 45, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Keizer, J. Nonlinear Fluorescence Quenching and the Origin of Positive Curvature in Stern-Volmer Plots. J. Am. Chem. Soc. 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Parui, R.; Garai, R.; Chanu, M.A.; Iyer, P.K. Dual “Static and Dynamic” Fluorescence Quenching Mechanisms Based Detection of TNT via a Cationic Conjugated Polymer. ACS Meas. Sci. Au 2021, 2, 23–30. [Google Scholar] [CrossRef]
- Tikhonova, T.N.; Shirshin, E.A.; Budylin, G.S.; Fadeev, V.V.; Petrova, G.P. Assessment of the Europium (III) Binding Sites on Albumin Using Fluorescence Spectroscopy. J. Phys. Chem. B 2014, 118, 6626–6633. [Google Scholar] [CrossRef]
- Da Silva, J.C.G.; Machado, A.; Pinto, M.S. Study of the Complexation of Cu (II) by Fulvic Acids Extracted from a Sewage Sludge and Its Compost. Fresenius. J. Anal. Chem. 1997, 357, 950–957. [Google Scholar] [CrossRef]



| Solvent | Hexane | DCM | CHCl3 | ACN | DMSO | EtOH | MeOH | Water |
|---|---|---|---|---|---|---|---|---|
| λabs (nm) | 390 | 386 | 383 | 369 | 382 | 372 | 370 | 366 |
| λem (nm) | 560 | 582 | 568 | 585 | 515 | 586 | 590 | 592 |
| ∆λ (nm) | 170 | 196 | 185 | 216 | 133 | 213 | 220 | 226 |
| φfl | <0.001 | 0.050 | 0.057 | 0.072 | 0.016 | 0.064 | 0.052 | <0.002 |
| ε (M−1 cm−1) | 3385 | 35,600 | 28,003 | 30,773 | 35,461 | 29,795 | 27,757 | 3908 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajapakshe, B.U.; Li, Y.; Corbin, B.; Wijesinghe, K.J.; Pang, Y.; Abeywickrama, C.S. Copper-Induced Fluorescence Quenching in a Bis[2-(2′-hydroxyphenyl)benzoxazole]pyridinium Derivative for Quantification of Cu2+ in Solution. Chemosensors 2022, 10, 382. https://doi.org/10.3390/chemosensors10100382
Rajapakshe BU, Li Y, Corbin B, Wijesinghe KJ, Pang Y, Abeywickrama CS. Copper-Induced Fluorescence Quenching in a Bis[2-(2′-hydroxyphenyl)benzoxazole]pyridinium Derivative for Quantification of Cu2+ in Solution. Chemosensors. 2022; 10(10):382. https://doi.org/10.3390/chemosensors10100382
Chicago/Turabian StyleRajapakshe, Buddhima U., Yonghao Li, Brian Corbin, Kaveesha J. Wijesinghe, Yi Pang, and Chathura S. Abeywickrama. 2022. "Copper-Induced Fluorescence Quenching in a Bis[2-(2′-hydroxyphenyl)benzoxazole]pyridinium Derivative for Quantification of Cu2+ in Solution" Chemosensors 10, no. 10: 382. https://doi.org/10.3390/chemosensors10100382
APA StyleRajapakshe, B. U., Li, Y., Corbin, B., Wijesinghe, K. J., Pang, Y., & Abeywickrama, C. S. (2022). Copper-Induced Fluorescence Quenching in a Bis[2-(2′-hydroxyphenyl)benzoxazole]pyridinium Derivative for Quantification of Cu2+ in Solution. Chemosensors, 10(10), 382. https://doi.org/10.3390/chemosensors10100382





