Abstract
Trabecular meshwork (TM) is the main channel of aqueous humor (AH) outflow and the crucial tissue responsible for intraocular pressure (IOP) regulation. The aberrant fibrotic activity of human TM (HTM) cells is thought to be partially responsible for the increased resistance to AH outflow and elevated IOP. This study aimed to identify the TM cell fibrotic activity biomarker and illustrate the mechanisms of fibrotic activity regulation in HTM cells. We used TGFβ2-treated HTM cells and detected the changes in the cytoskeletal structure, the Yes-associated protein (YAP) and its transcriptional co-activator with PDZ-binding domain (TAZ) activation, and the expression levels of the fibrosis-related proteins Collagen I and α-SMA in HTM cells by immunofluorescence staining or western bolt analyses. The expression of YAP was inhibited using siRNA transfection. The results showed that the expression levels of YAP/TAZ and the fibrosis-related proteins Collagen I and α-SMA in HTM cells were elevated under TGF-β2 treatment, which was correlated with the structural change of the cellular F-actin cytoskeleton. Furthermore, the inhibition of YAP decreased the expression of connective tissue growth factor (CTGF), Collagen I, and α-SMA in HTM cells. These findings demonstrate that YAP/TAZ are potential biomarkers in evaluating the TM cell fibrotic activity, and it could sense cytoskeletal structure cues and regulate the fibrotic activity of TM cells.
1. Introduction
Glaucoma is the leading irreversible eye disease around the world, making it one of the major health issues of global concern [1]. Pathological IOP elevation due to a reduction in the AH outflow rate is a major cause of glaucomatous optic neuropathy and can lead to irreversible blindness [2]. Reducing IOP by decreasing the resistance to AH outflow and increasing the rate of AH outflow is the most common treatment for glaucoma. TM is the main channel for AH outflow [3], and it is sensitive to mechanical forces [4]. Therefore, the study of the mechanotransduction pathway in this tissue could provide new therapeutic strategies to reduce AH outflow resistance and regulate IOP. However, it is still poorly understood how TM responds to mechanical cues.
TGF-β2 is known to induce actin cytoskeletal remodeling and extracellular matrix (ECM) deposition in various tissues [5,6]. The anomalous deposition of ECM proteins, increased matrix stiffness, and the activation of myofibroblasts are characteristic of cellular fibrosis [7,8]. Abnormal fibrotic activity in HTM cells is thought to be partly responsible for AH outflow resistance increase and IOP elevation [9]. The overexpression of TGF-β2 in the mouse eye can lead to IOP elevation [10]. Previous studies have shown that the Hippo pathway plays a crucial role in mediating the effects of mechanical stimulation on cell behavior, either from internal or external sources [11,12]. YAP and TAZ are two important transcriptional co-activators in the Hippo signaling pathway, both expressed in HTM tissue [13]. The actin cytoskeleton is known to be highly sensitive to the mechanical environment in which the cells are exposed, and cells respond to that by modifying the structure and tension of their stress fibers [14,15]. In addition, studies have shown that the polymerization of F-actin and the formation of stress fibers promote the activity of YAP/TAZ [15]. Studies on Drosophila and mammals have revealed that the Hippo pathway and YAP/TAZ may indirectly perceive and respond to cellular mechanical environment changes by monitoring the actin cytoskeleton [16]. YAP/TAZ have different localizations in cells and performs different functions. When YAP and TAZ are translocated from the cytoplasm into the nucleus, they form a transcriptional regulatory complex with a TEA domain family member (TEAD) to regulate the transcription of the CTGF. The CTGF is a group of cytokines widely involved in fibrosis in various tissues, and its expression level is significantly increased in the AH of glaucoma patients compared to normal people [17]. However, the intrinsic relationship between YAP/TAZ activation and cytoskeletal changes and their role in regulating fibrotic activity remain unclear in TGFβ2-treated HTM cells. We hypothesized that cytoskeletal changes are the dominant input cues for YAP/TAZ activation. YAP/TAZ transcriptional activity elevation is associated with TM cell dysfunction. It has been found that the YAP/TAZ nuclear localization was increased in the glaucomatous HTM cells compared to the normal HTM cells [18]. This study aimed to investigate the regulatory effect of YAP/TAZ on the fibrotic activity of HTM cells and their potential as biomarkers for fibrotic activity.
2. Materials and Methods
2.1. Cell Culture and Treatment
HTM cells were purchased from ScienCell Research Laboratories. The cells were cultured in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F12, 11330032, Gibco) supplemented with 10% fetal bovine serum (FBS, 10091148, Gibco) and 1% penicillin/streptomycin (SV30010, Hyclone). The cells were cultured at 37 °C with 5% CO2. When the TM cells were treated with dexamethasone (DEX), myocilin was significantly upregulated, a characteristic that is not present in neighboring cells. Therefore, the DEX induction of myocilin is the most common and reliable method currently used to identify TM cells [19]. The cultured HTM cells were stimulated with 1 μM DEX (HY-14648, MedChemExpress) for 72 h. The myocilin (MYOC) expression level was assessed by western blot. The cells of passages 3–5 were used in this study. The HTM cells were treated with 5 ng/mL TGF-β2 (HY-P7119, MedChemExpress) for 48 h, and before treatment, the cells were maintained in a serum-free culture medium for 24 h.
2.2. Western Bolt Analysis
The HTM cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors on ice and then centrifuged at 140,000 rpm for 20 min at 4 °C to obtain proteins. The BCA protein assay was used to measure the total protein concentration. Subsequently, the proteins were loaded and ran on denatured 4–15% gradient polyacrylamide ready-made gels (P0519S, Beyotime). Then, the protein was transferred to PVDF membranes. The membranes were blocked with 5% skimmed milk for 1 h and further incubated at 4 °C overnight with the following primary antibodies: FN ((1:1000, MA1116, BOSTER), MMP3 (1:1000, 17873-1-AP, ProteinTech), myocilin (1:800, sc-137233, Santa Cruz), Collagen I (1:1000, ab260043, Abcam), α-smooth muscle actin (α-SMA, 1:10,000, ab124964, Abcam), YAP (1:1000, 14074, CST), TAZ (1:1000, 83669, CST), CTGF (1:1000, 23936-1-AP, ProteinTech), F-actin (1:1000, bs-1571R, Bioss), GAPDH (1:2000, 10494-1-AP, ProteinTech), and β-actin (1:2000, 20536-1-AP, ProteinTech). The membranes were washed three times with Tris-buffered saline with Tween Solution (TBST) and incubated with the horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000, 111-035-003, Jackson ImmunoResearch Laboratories) or anti-mouse IgG (1:2000, 7076, CST) for 1 h at room temperature. The bands were then visualized and imaged with a Bio-Rad imaging system. The data were analyzed by Gel-Pro Analyzer.
2.3. Cell Staining
The HTM cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.3% Triton X-100 for 20 min, followed by blocking in 5% bovine serum albumin for 1 h at room temperature. The fixed cells were incubated at 4 °C overnight with primary antibodies against YAP/TAZ (1:100, 8418, CST). Then, the cells were washed and incubated with anti-rabbit IgG (Alexa Fluor 594 Conjugate, 8889, CST) for 2 h at room temperature. After washing, the cells were incubated with a mounting buffer containing DAPI for 5 min. Cytoskeleton staining was performed with Alexa Fluor-488 phalloidin (1:20, 8878, CST). Images were obtained by a Nikon fluorescence microscope.
2.4. Cell siRNA Transfection
The siRNA of YAP (siYAP) was supplied by Hanbio Biotechnology Co., Ltd. The sequences were as follows: siYAP sense, 5′-GGACTAAGCATGAGCAGCTACAGTG-3′; siCtrl sense, 5′-TTCTCCGAACGTGTCACGTAA-3′. ThesiRNA transfection was performed with lentivirus at 20 multiplicities of infection. The medium was replaced 24 h after transfection. Then, the cells were incubated at 37 °C for 48 h. The transfection efficiency was determined by western blot analysis.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS software. Two-group comparisons were assessed using an unpaired t-test. All the data were presented as the mean ± S.E.M. for at least three independent experiments. p < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of HTM Cells
We characterized the HTM cells as previously described [19,20,21]. The cell morphology is shown in Figure 1A. In addition, the HTM cells expressed MMP3 and FN at passage 2 (P2) and passage 4 (P4) as shown in Figure 1B. After treatment with DEX, the expression level of myocilin (p < 0.05) increased significantly compared to that of the control cells (Figure 1C,D).
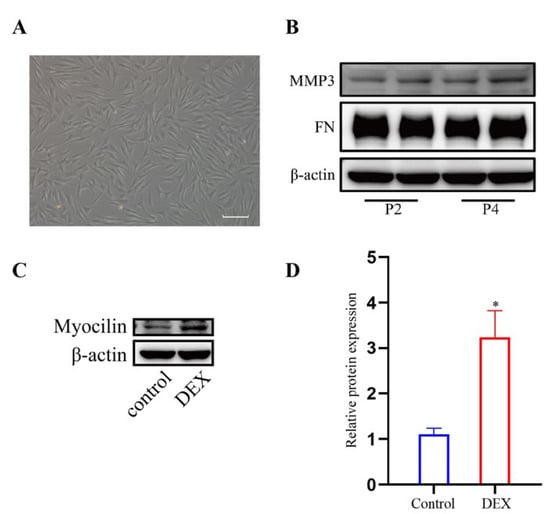
Figure 1.
Characterization of HTM cells. (A) Phase-contrast microscopic images of HTM cells. (B) The band of MMP3 and FN in HTM cells at passage 2(P2) and passage 4(P4). (C,D) DEX treatment (1 μM for 72 h) increased myocilin expression in HTM cells, which was indicated by (C) the bands of myocilin and (D) quantification analysis of the myocilin expression level. * p < 0.05.
3.2. TGF-β2 Treatment Induced Cytoskeleton Alteration and Fibrotic Activity in HTM Cells
To explore the effect of TGF-β2 treatment on the cytoskeleton of HTM cells, the cytoskeletal structure was detected by phalloidin fluorescence staining. The cytoskeleton deformed from a long spindle shape to an interlaced and complex actin network. The stress fibers of the control cells were mostly arranged regularly in one direction, while those of the TGFβ2-treated cells showed disorderly distribution (Figure 2A). However, the expression level of F-actin in the TGFβ2-treated cells was not obviously different from that in the control cells (Figure 2B,C). We performed western blot analysis to detect the expression levels of Collagen I, a major component of ECM [22], and α-SMA, a marker protein of myofibroblast activation [23,24]. TGF-β2 treatment significantly promoted the expression levels of Collagen I (p < 0.05) and α-SMA (p < 0.05) compared to the control cells (Figure 3A,B), which indicated an increase in fibrotic activity in the HTM cells under TGF-β2 treatment.
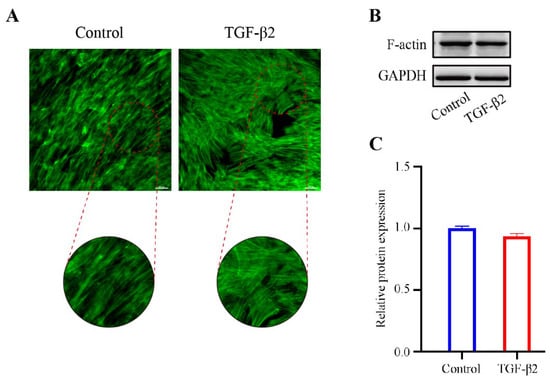
Figure 2.
The effect of TGF-β2 treatment on the cytoskeleton actin stress fibers in HTM cells. (A) The TGFβ2-induced changes in the cytoskeletal structure were evaluated by phalloidin fluorescence stating. (B) The bands of F-actin and (C) quantification analysis of F-actin expression levels in HTM cells treated with or without TGF-β2 (5 ng/mL for 48 h). Scale bar: 100 μm.
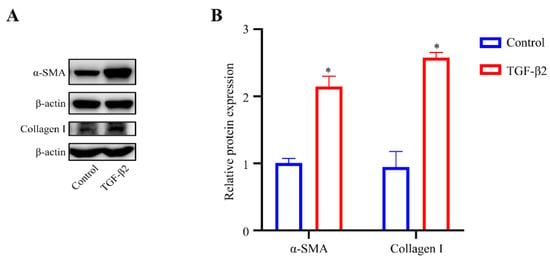
Figure 3.
TGF-β2 treatment induced the fibrotic activity of HTM cells. (A) Serum-starved HTM cells (24 h) treated with TGF-β2 (5 ng/mL for 48 h) presented a significant increase in the levels of α-SMA and Collagen I compared with the control cells. (B) Quantification analyses of α-SMA and Collagen I expression levels. * p < 0.05.
3.3. TGF-β2 Treatment Induced YAP/TAZ Activation
YAP and TAZ are known to play an essential role in sensing mechanical cues [25]. In order to explore the role of YAP/TAZ in the TGFβ2-induced changes of the cytoskeleton in HTM cells, 24 h serum-free HTM cells were treated with TGF-β2 (5 ng/mL) for 48 h, and the expression levels of YAP and TAZ were evaluated by western blot analysis. Compared with the control cells, the expression levels of YAP (p < 0.05) and TAZ (p < 0.05) were both upregulated in the TGFβ2-treated cells (Figure 4A,B), indicating the activation of YAP and TAZ. Additionally, immunofluorescence staining for YAP/TAZ showed that the TGFβ2-treated cells exhibited increased nuclear localization compared to the control cells (Figure 4C,D).
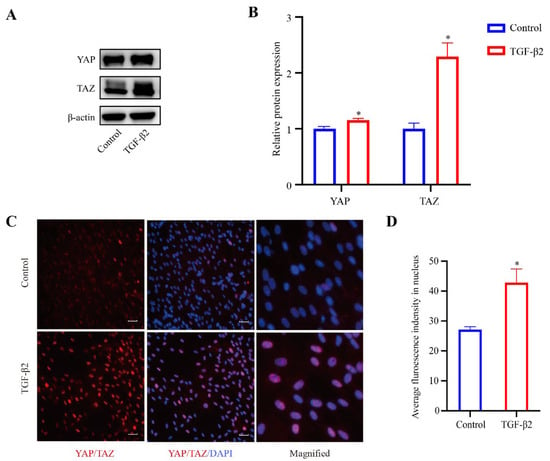
Figure 4.
TGF-β2 treatment induced the activation of YAP/TAZ. (A) Serum-starved HTM cells (24 h) treated with TGF-β2 (5 ng/mL for 48 h) presented increased expression levels of YAP and TAZ compared with control cells. (B) Quantification analyses of YAP and TAZ expression levels. * p < 0.05. (C) Stimulation of HTM cells with TGF-β2 led to increased localization of YAP/TAZ proteins in the nucleus compared with control cells. (D) Quantification immunofluorescence analysis of YAP/TAZ in the nucleus. * p < 0.05. Scale bar: 100 μm.
3.4. YAP Was Required for the Fibrotic Activity of HTM Cells
YAP and TAZ are transcriptional co-activators acting downstream of the Hippo pathway. YAP/TAZ nuclear translocation promotes the expression of CTGF and is associated with ECM production and wound healing [15,26,27]. To determine the relative importance of YAP in the fibrotic activity in HTM cells, lentivirus was used to inhibit the YAP expression level, and then western blot analysis was performed to assess the lentivirus transfection efficiency of YAP and to evaluate the expression levels of fibrosis-related proteins in the cultured HTM cells. As shown in Figure 5, the expression level of YAP (p < 0.05) in the HTM cells was efficiently decreased after siYAP treatment compared to the control cells. Furthermore, the HTM cells treated with siYAP showed significantly decreased expression levels of CTGF (p < 0.05), Collagen I (p < 0.05), and α-SMA (p < 0.05) compared to the control cells.
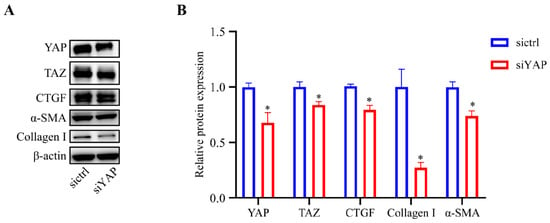
Figure 5.
Inhibition of YAP decreased the expression of CTGF, Collagen I, and α-SMA. (A) Cells treated with siYAP showed significantly decreased expression levels of YAP, TAZ, CTGF, α-SMA, and Collagen I compared with control cells based on western blot analysis. (B) Quantification analyses of YAP, TAZ, CTGF, α-SMA, and Collagen I expression levels. * p < 0.05.
4. Discussion
Our results showed that HTM cells treated with TGF-β2 presented fibrosis-like manifestations with the overexpression of collagen I and α-SMA. Collagen I is a major component of ECM [22], while α-SMA is known to be a biomarker of fibroblast activation [23,24]. ECM deposition and myofibroblasts activation are the typical features of fibrotic activity [8]. The fibroblast is a highly contractile cell that further stiffens ECM, and that secretes and activates fibrogenic growth factors [28]. Previous studies have shown that the expression levels of the CTGF protein and mRNA were markedly increased in HTM cells after treatment with TGF-β2 [29,30,31]. At the same time, we found that TGF-β2 induced the actin cytoskeleton to form an interlaced and complex actin network, with the cytoskeletal structure changing from a long spindle to an expanded shape with extended pseudopods. YAP/TAZ activity in a cell could be dictated by the cytoskeletal structure [26]. Therefore, we examined whether YAP/TAZ were activated in HTM cells after treatment with TGF-β2. The expression levels of YAP and TAZ in the TGFβ2-treated HTM cells were significantly upregulated compared with those in the control cells, suggesting that the activity of YAP/TAZ were elevated. The immunostaining results revealed that TGF-β2 induced the nuclear translocation of YAP/TAZ, further confirming YAP/TAZ activation. As two transcriptional co-activators, the functions of YAP and TAZ overlap largely. Nonetheless, compared with TAZ knockdown, YAP knockdown has a greater impact on the cell physiology (e.g., cell size, proliferation, migration) [32]. Moreover, the behaviors of YAP knockdown cells are more similar to those of YAP/TAZ double knockdown cells [32]. Therefore, YAP was inhibited in this study, which reduced the expression levels of collagen I and α-SMA, suggesting that YAP may be essential for TGFβ2-induced fibrotic activity in HTM cells.
YAP/TAZ are considered as a sensor of the structural and mechanical characteristics of the cellular microenvironment [33]. It was found that YAP/TAZ were localized in the nucleus and had transcriptional activity in the cells cultured on a stiff ECM, whereas YAP/TAZ were excluded from the nucleus and the function was inhibited in the cells cultured on a soft ECM [34]. Another study has revealed that YAP and TAZ were prominently expressed in fibrotic lung tissue, and the activation of fibroblast YAP/TAZ promoted the fibrotic response in vivo [35]. Cells sense and respond to mechanical signals through mechanotransduction [36,37], and the cytoskeleton is vital for these mechanoresponses [15,34,38]. In response to mechanical forces, such as forces generated from the cell–ECM and cell–cell, the cells remodeled the actin cytoskeleton [33], and the cytoskeleton is a key regulator of YAP/TAZ signalings [16,33]. Our findings showed that the actin cytoskeleton structure was associated with increased nuclear YAP/TAZ. Similar results have been found in various cell studies. The actin cytoskeleton remodeling involves YAP/TAZ nuclear localization and activation, which is induced by shear stresses in endothelial cells [39]. The arrangement of the cytoskeleton in dental pulp stem cells cultured on scaffolds with different surface morphologies was related to the nuclear localization of YAP [40]. In vitro cultured skin keratinocytes were shown to induce the nuclear translocation of YAP/TAZ through the mechanical forces of integrin adhesion and the actin cytoskeleton, thereby inducing cell proliferation [41]. It needs to be clarified that the cytoskeletal tension affects YAP/TAZ nuclear localization and activity [15]. The linker of the nucleoskeleton and cytoskeleton (LINC) complex and the integrin adaptor protein talin are critically relevant for intracellular mechanical connections among the different cytoskeletal structures [42]. The LINC complex connects the nucleus to the stress fibers mechanically [43], whereas talin unfolding causes the formation of stress fibers [44]. The LINC complex and talin unfolding could mechanically connect the cytoskeleton and the nucleoskeleton, enabling forces to access the nucleus and drive YAP translocation and activation [42].
It has been suggested that the actin cytoskeleton can influence the mechanics and shape of the nucleus through the Nesprin and SUN domain-containing protein complexes, thus inducing nuclear deformation and increasing nuclear pore complex (NPC) permeability to promote YAP/TAZ nuclear translocation [45]. Furthermore, YAP/TAZ transcriptional activation was inhibited when Rho was inhibited or the actin cytoskeleton was disrupted [15]. RhoA activation increased the expression of YAP/TAZ and cell stiffness in HTM cells [46]. These results indicate that the RhoA/ROCK pathway may have an important role in YAP/TAZ activation regulated by the cytoskeleton. Researchers had found that the overexpression of F-actin resulted in increased YAP/TAZ in the nucleus, while the loss of F-actin resulted in the accumulation of YAP/TAZ in the cytoplasm [16]. However, the results of our research showed that the expression level of F-actin in the TGFβ2-treated HTM was not obviously different from that in the control cells, suggesting that the activation of YAP in TGFβ2-treated HTM cells is due to cytoskeletal rearrangement rather than F-actin expression level changes. The sensitivity of YAP/TAZ to the actin cytoskeleton is not only due to mechanical regulation but is also mediated by G-protein-coupled receptor signaling. G-protein-coupled receptor signaling regulates Hippo signaling by upregulating F-actin via Rho-GTPase [47,48]. In summary, we propose several possible molecular pathways for the cytoskeletal regulation of YAP/TAZ in TM cell fibrosis activity, but they need to be identified in future studies.
YAP/TAZ not only respond to mechanical cues but also act as mechanical signal mediators [34]. A previous study has demonstrated that YAP/TAZ activation in TM cells causes cytoskeletal remodeling and affects the elasticity of TM cells [13]. Compared with RhoA activator treatment, the stiffness of HTM cells treated with both the Rho activator and siYAP decreased, indicating that YAP activity affects cellular mechanical properties [46]. In HTM cells, DEX treatment promoted the formation of cross-linked actin networks (CLANs) in the actin cytoskeleton. However, siYAP and siTAZ cotransfection attenuated the DEX-induced CLANs formation [49]. We considered that, due to the wide variety of mechanosensory proteins, the transduction of mechanosensing into biochemical processes may activate lots of signaling pathways, which may interact to generate functional responses. Subsequent cellular responses may trigger additional mechanosensory signals, leading to a second round of responses [36]. Since DEX and TGF-β2 are the two most commonly used agents to induce TM cell fibrosis, we hypothesize that the inhibition of YAP would also attenuate the TGFβ2-induced cytoskeletal structure changes in HTM cells.
This study demonstrated that YAP regulates the fibrotic activity of HTM cells by sensing changes in cytoskeletal cues and can be considered as a potential biomarker in evaluating TM fibrotic activity. These findings not only improve our understanding of the TM response to intracellular mechanics cues but also provide new ideas for reducing AH resistance and treating glaucoma. One limitation of this research is that the effect of YAP in regulating the cytoskeletal structure was not explored. The potential effect of the interaction between YAP and the cytoskeleton on the fibrotic activity in TM cells needs to be further investigated. The other is that this study only detected the effects of YAP knockdown on HTM cell fibrotic activity. Although YAP and TAZ are functionally similar, they are not identical [50]. We should knockdown TAZ itself and in combination with YAP to investigate the corresponding effects in future studies.
Author Contributions
Conceptualization, S.L. and Z.L. (Zhicheng Liu, zcliu@ccmu.edu.cn); Methodology, S.H. and Z.L. (Zhicheng Liu, zcliu1994@126.com); Investigation, S.H. and Z.L. (Zhicheng Liu, zcliu1994@126.com); Funding acquisition, Z.L. (Zhicheng Liu, zcliu@ccmu.edu.cn); Formal analysis, S.H., X.Q., L.L., H.Z., and S.L.; Validation, S.H.; Writing—original draft, S.H.; Writing—review & editing, S.H., S.L., and Z.L. (Zhicheng Liu, zcliu@ccmu.edu.cn). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Natural Science Foundation of China (grant no. 31570952).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the following reasons. The fetal bovine serum and secondary antibodies (anti-mouse IgG and anti-rabbit IgG) used in this study are commercial products. The human trabecular meshwork cells used in this study were purchased from ScienCell Research Laboratories and we have received the ethical statement provided by ScienCell.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors (lishanshan@ccmu.edu.cn or zcliu@ccmu.edu.cn) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Sihota, R.; Angmo, D.; Ramaswamy, D.; Dada, T. Simplifying “target” intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J. Ophthalmol. 2018, 66, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, R.F.; Sumida, G.M.; Stamer, W.D. Cyclic mechanical stress and trabecular meshwork cell contractility. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3826–3832. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.H.; Clark, A.F. TGFbeta2-induced changes in human trabecular meshwork: Implications for intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschner, A.; Strat, A.N.; Yablonski, J.; Yoo, H.; Bagué, T.; Li, H.; Zhao, J.; Bollinger, K.E.; Herberg, S.; Ganapathy, P.S.; et al. Mechanosensitive channel inhibition attenuates TGFβ2-induced actin cytoskeletal remodeling and reactivity in mouse optic nerve head astrocytes. Exp. Eye Res. 2021, 212, 108791. [Google Scholar] [CrossRef]
- Pouw, A.E.; Greiner, M.A.; Coussa, R.G.; Jiao, C.; Han, I.C.; Skeie, J.M.; Fingert, J.H.; Mullins, R.F.; Sohn, E.H. Cell-Matrix Interactions in the Eye: From Cornea to Choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef]
- Honjo, M.; Igarashi, N.; Nishida, J.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kano, K.; Aoki, J.; Aihara, M. Role of the Autotaxin-LPA Pathway in Dexamethasone-Induced Fibrotic Responses and Extracellular Matrix Production in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Shepard, A.R.; Millar, J.C.; Pang, I.H.; Jacobson, N.; Wang, W.H.; Clark, A.F. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2067–2076. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Qiu, Y.; Lin, K.C.; Kumar, A.; Placone, J.K.; Fang, C.; Wang, K.C.; Lu, S.; Pan, M.; Hong, A.W.; et al. RAP2 mediates mechanoresponses of the Hippo pathway. Nature 2018, 560, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Morgan, J.T.; Dreier, B.; Reilly, C.M.; Thomasy, S.M.; Wood, J.A.; Ly, I.; Tuyen, B.C.; Hughbanks, M.; Murphy, C.J.; et al. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Investig. Ophthalmol. Vis. Sci. 2013, 54, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Perez-Gonzalez, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.T.; Osterwald, A.; Ruf, I.; Hunziker, D.; Mattei, P.; Challa, P.; Vann, R.; Ullmer, C.; Rao, P.V. Role of the Autotaxin-Lysophosphatidic acid axis in glaucoma, aqueous humor drainage and fibrogenic activity. BBA Mol. Basis Dis. 2020, 1866, 165560. [Google Scholar] [CrossRef]
- Li, H.; Raghunathan, V.; Stamer, W.D.; Ganapathy, P.S.; Herberg, S. Extracellular Matrix Stiffness and TGFβ2 Regulate YAP/TAZ Activity in Human Trabecular Meshwork Cells. Front. Cell Dev. Biol. 2022, 10, 844342. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Zhu, W.; Gramlich, O.W.; Laboissonniere, L.; Jain, A.; Sheffield, V.C.; Trimarchi, J.M.; Tucker, B.A.; Kuehn, M.H. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 3492–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisling, A.; Lust, R.M.; Katwa, L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.H.; Chang, Y.; Reed, N.I.; Sheppard, D. Alpha-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFbeta activation or collagen production across multiple models of organ fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, 824–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Wang, K.C.; Yeh, Y.T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.L.; Li, Y.S.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef] [Green Version]
- Walraven, M.; Hinz, B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol. 2018, 71–72, 205–224. [Google Scholar] [CrossRef]
- Yemanyi, F.; Vranka, J.; Raghunathan, V.K. Glucocorticoid-induced cell-derived matrix modulates transforming growth factor β2 signaling in human tr;abecular meshwork cells. Sci. Rep. 2020, 10, 15641. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Yu, A.H.; Welge-Lüssen, U.; Tamm, E.R. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Rao, V.R.; Lautz, J.D.; Kaja, S.; Foecking, E.M.; Lukács, E.; Stubbs, E.B., Jr. Mitochondrial-Targeted Antioxidants Attenuate TGF-β2 Signaling in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis Sci. 2019, 60, 3613–3624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L.; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, 344–357. [Google Scholar] [CrossRef] [Green Version]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.; Hong, H.; Lee, S.H.; Lee, J.K.; Jung, E.; Kim, J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016, 35, 462–478. [Google Scholar] [CrossRef]
- Janota, C.S.; Calero-Cuenca, F.J.; Gomes, E.R. The role of the cell nucleus in mechanotransduction. Curr. Opin. Cell Biol. 2020, 63, 204–211. [Google Scholar] [CrossRef]
- Dupont, S. Regulation of YAP/TAZ Activity by Mechanical Cues: An Experimental Overview. Methods Mol. Biol. 2019, 1893, 183–202. [Google Scholar]
- Du, Y.; Montoya, C.; Orrego, S.; Wei, X.; Ling, J.; Lelkes, P.I.; Yang, M. Topographic cues of a novel bilayered scaffold modulate dental pulp stem cells differentiation by regulating YAP signalling through cytoskeleton adjustments. Cell Prolif. 2019, 52, e12676. [Google Scholar] [CrossRef]
- Elbediwy, A.; Thompson, B.J. Evolution of mechanotransduction via YAP/TAZ in animal epithelia. Curr. Opin. Cell Biol. 2018, 51, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Rico-Lastres, P.; Le Roux, A.L.; Shanahan, C.M. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jiang, G.; Cai, Y.; Monkley, S.J.; Critchley, D.R.; Sheetz, M.P. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008, 10, 1062–1068. [Google Scholar] [CrossRef] [Green Version]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Qian, X.; Li, L.; Zhang, H.; Liu, Z. RhoA/ROCK-YAP/TAZ Axis Regulates the Fibrotic Activity in Dexamethasone-Treated Human Trabecular Meshwork Cells. Front. Mol. Biosci. 2021, 8, 728932. [Google Scholar] [CrossRef]
- Miller, E.; Yang, J.; DeRan, M.; Wu, C.; Su, A.I.; Bonamy, G.M.; Liu, J.; Peters, E.C.; Wu, X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012, 19, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.S.; Yu, F.X.; Gong, R.; Brown, J.H.; Guan, K.L. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012, 26, 2138–2143. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Wang, H.; Wang, X.; Sun, M.; Deng, S.; Wang, Y. YAP and TAZ mediate steroid-induced alterations in the trabecular meshwork cytoskeleton in human trabecular meshwork cells. Int. J. Mol. Med. 2018, 41, 164–172. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).