Effects of Calcination Temperature on CO-Sensing Mechanism for NiO-Based Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Synthesis and Layer Deposition
2.2. Materials Characterization
2.2.1. Structural Investigations
2.2.2. Morphological Investigations
2.2.3. Surface Chemistry Investigations
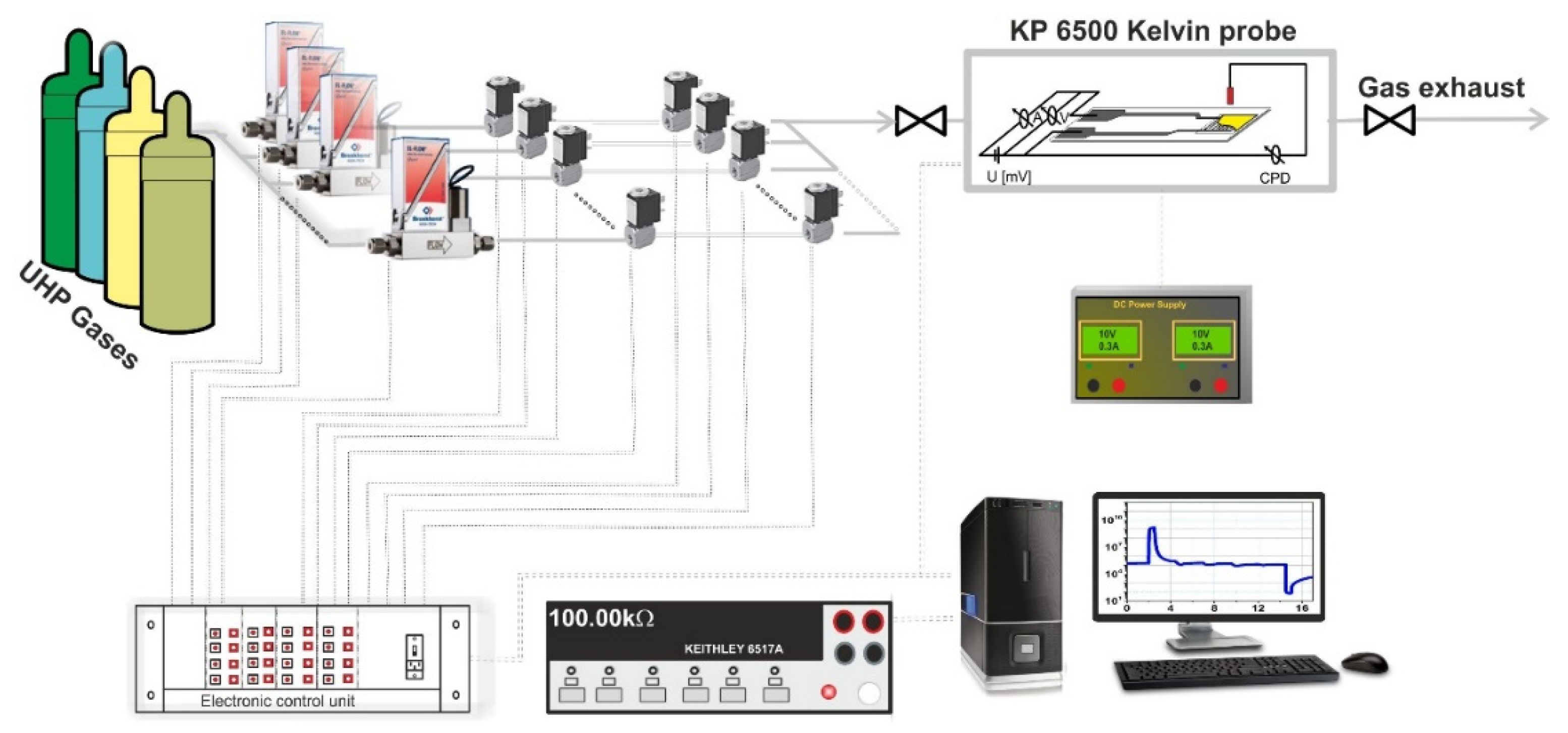
2.2.4. Gas-Sensing Investigations and Experimental Chronology
3. Results
3.1. XRD Results
3.2. Analytical TEM Results
3.3. X-ray Photoelectron Spectroscopy (XPS) Results
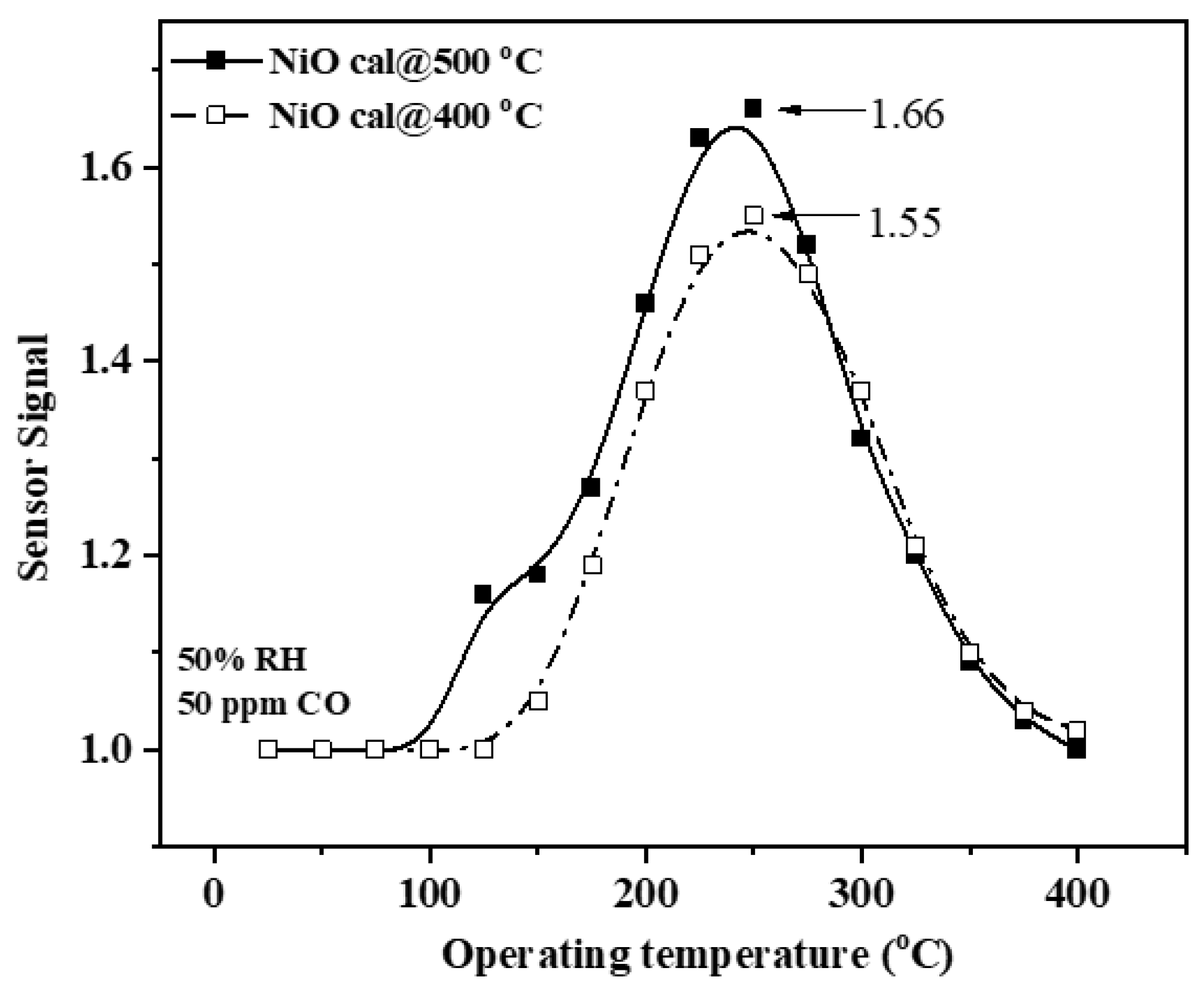
3.4. Gas-Sensing Results
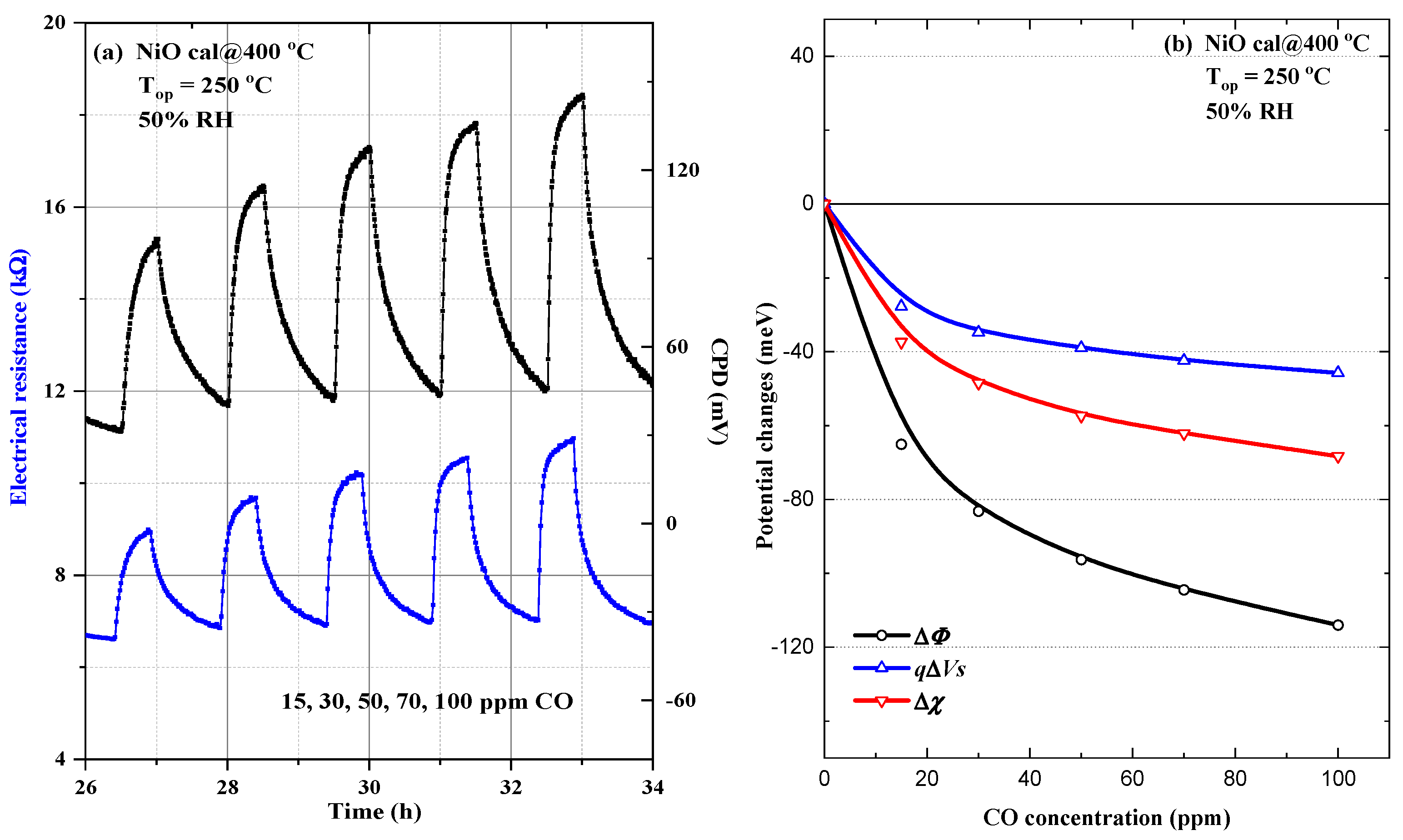
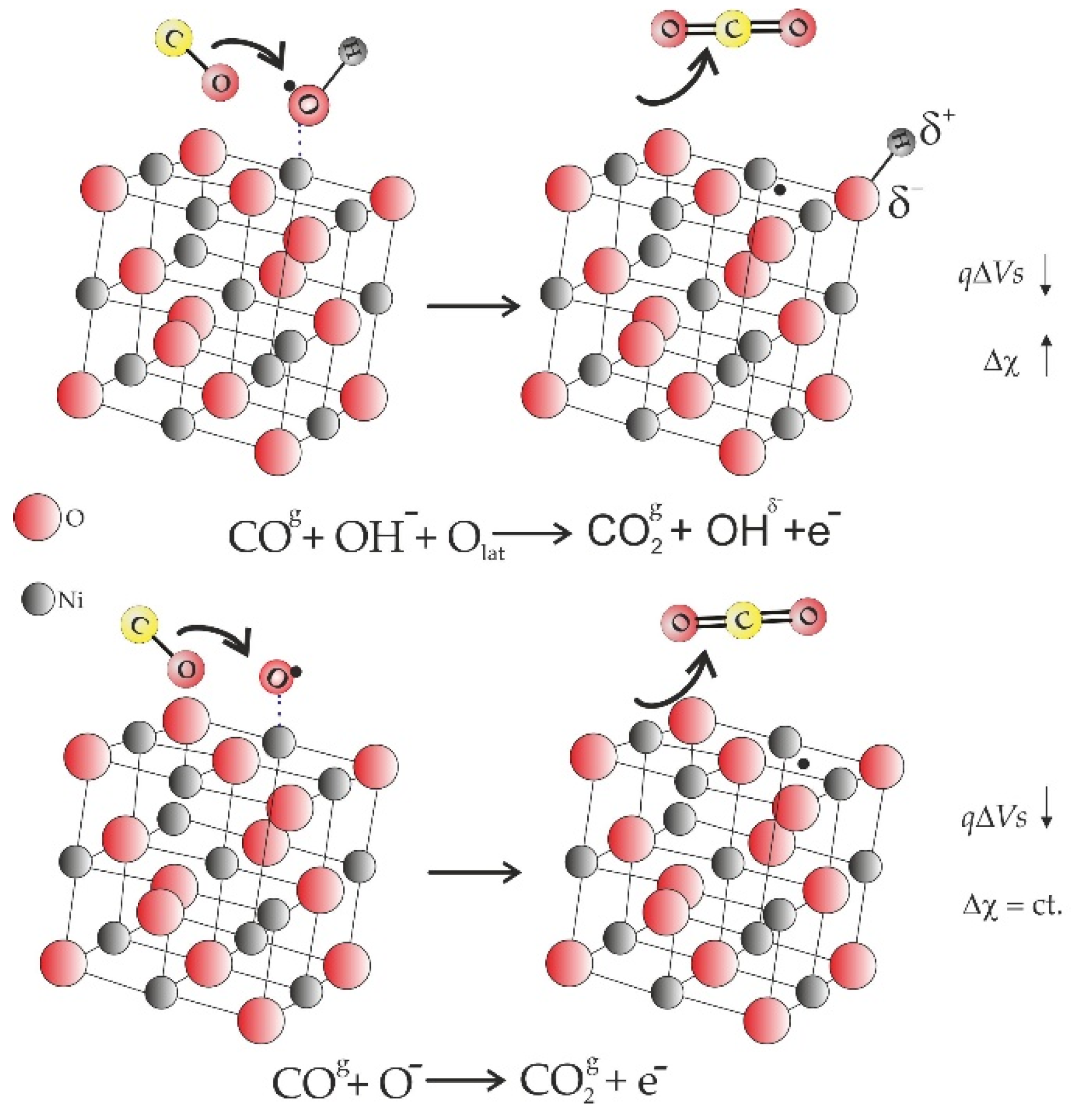
3.4.1. Gas-Sensing Mechanism towards CO Exposure under 50% RH for NiO Calcined at 400 °C
3.4.2. Gas-Sensing Mechanism towards CO Exposure under 50% RH for NiO Calcined at 500 °C
4. Conclusions
- -
- For NiO calcined at 400 °C, CO prefers a direct interaction with the surface hydroxyl groups;
- -
- For NiO calcined at 500 °C, a two-step reaction mechanism is possible above and below 15 ppm of CO, namely: with the surface hydroxyl groups (up to 15 ppm CO) followed by CO–oxygen interaction above 15 ppm CO.
Author Contributions
Funding
Conflicts of Interest
References
- Dey, S.; Mehta, N.S. Oxidation of carbon monoxide over various nickel oxide catalysts in different conditions: A review. Chem. Eng. J. Adv. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Khodmanee, S.; Amnuaylojaroen, T. Impact of Biomass Burning on Ozone, Carbon Monoxide, and Nitrogen Dioxide in Northern Thailand. Front. Environ. Sci. 2021, 9, 27. [Google Scholar] [CrossRef]
- Savin, M.; Mihailescu, C.-M.; Moldovan, C.; Grigoroiu, A.; Ion, I.; Ion, A.C. Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review. Molecules 2022, 27, 821. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Simion, C.E.; Stanoiu, A.; Taylor, A.; Guldin, S.; Covington, J.A.; Carmalt, C.J.; Blackman, C.S. Humidity-Tolerant Ultrathin NiO Gas-Sensing Films. ACS Sens. 2020, 5, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.-C.; Cao, X.-R.; Xu, S.-L.; Yang, J.-H.; Babar, N.; He, Z.-J.; Zhang, Q.-Y. Synthesis of hollow spherical nickel oxide and its gas-sensing properties. Rare Met. 2021, 40, 1622–1631. [Google Scholar] [CrossRef]
- Ayyala, S.K.; Covington, J.A. Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection. Chemosensors 2021, 9, 247. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Sayed, M.H.; Patil, V.L.; Boshta, M.; Patil, P.S. Gas sensing performance of sprayed NiO thin films towards NO2 gas. J. Alloy. Compd. 2021, 855, 160908. [Google Scholar] [CrossRef]
- Hotovy, I.; Rehacek, V.; Kemeny, M.; Ondrejka, P.; Kostic, I.; Mikolasek, M.; Spiess, L. Preparation and gas-sensing properties of very thin sputtered NiO films. J. Electr. Eng. 2021, 72, 61–65. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloys Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Egbo, K.O.; Liu, C.P.; Ekuma, C.E.; Yu, K.M. Vacancy defects induced changes in the electronic and optical properties of NiO studied by spectroscopic ellipsometry and first-principles calculations. J. Appl. Phys. 2020, 128, 135705. [Google Scholar] [CrossRef]
- Juang, F.-R.; Wang, W.-Y. Ethanol Gas Sensors with Nanocomposite of Nickel Oxide and Tungsten Oxide. IEEE Sens. J. 2021, 21, 19740–19752. [Google Scholar] [CrossRef]
- Jian, J.-C.; Chang, Y.-C.; Chang, S.-P.; Chang, S.-J. High Response of Ethanol Gas Sensor Based on NiO-Doped Apple Pectin by the Solution Process. Coatings 2021, 11, 1073. [Google Scholar] [CrossRef]
- Bartolome, J.; Taeno, M.; Casado-Martinez, R.; Maestre, D.; Cremades, A. Ethanol gas sensing mechanisms of p-type NiO at room temperature. Appl. Surf. Sci. 2022, 579, 152134. [Google Scholar] [CrossRef]
- Susman, M.D.; Pham, H.N.; Zhao, X.; West, D.H.; Chinta, S.; Bollini, P.; Datye, A.K.; Rimer, J.D. Synthesis of NiO Crystals Exposing Stable High-Index Faces. Angew. Chem. 2020, 59, 15119–15123. [Google Scholar] [CrossRef]
- Su, D.; Ford, M.; Wang, G. Mesoporous NiO crystals with dominantly expose {110} reactive facets for ultrafast lithium storage. Sci. Rep. 2012, 2, 924. [Google Scholar] [CrossRef] [Green Version]
- Poulain, R.; Klein, A.; Proost, J. Electrocatalytic Properties of (100)-, (110)-, and (111)-Oriented NiO Thin Films toward the Oxygen Evolution Reaction. J. Phys. Chem. C 2018, 122, 22252–22263. [Google Scholar] [CrossRef]
- Hermawan, A.; Hanindriyo, A.; Hongo, K.; Maezono, R.; Yin, S. Impact of Surface Faceting on Gas Sensing Selectivity of NiO: Revealing the Adsorption Sites of Organic Vapors on the {111} Facet. J. Phys. Chem. C 2022. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, Q.; Liu, Z.; Zhang, X.; Zhang, J.; Lei, S.; Li, Y.; Mu, J.; Yang, E.-C. NiO nanobelts with exposed {110} crystal planes as an efficient electrocatalyst for the oxygen evolution reaction. Phys. Chem. Chem. Phys. 2022, 24, 6087–6092. [Google Scholar] [CrossRef]
- Cappus, D.; Xu, C.; Ehrlich, D.; Dillmann, B.; Ventrice, C.A.; Shamery, K.A.; Kuhlenbeck, H.; Freund, H.-J. Hydroxil groups on oxide surface: NiO(100), NiO(111) and Cr2O3(111). Chem. Phys. 1993, 177, 533–546. [Google Scholar] [CrossRef]
- Kitakatsu, N.; Maurice, V.; Hinnen, C.; Marcus, P. Surface hydroxylation and local structure of NiO thin films formed on Ni(111). Surf. Sci. 1998, 407, 36–58. [Google Scholar] [CrossRef]
- Zhao, W.; Bajdich, M.; Carey, S.; Vojvodic, A.; Nørskov, J.K.; Campbell, C.T. Water Dissociative Adsorption on NiO(111): Energetics and Structure of the Hydroxylated Surface. ACS Catal. 2016, 6, 7377–7384. [Google Scholar] [CrossRef]
- Manjunatha, C.; Khosla, A.; Krishna, R.H.; Kumar, P.V.; Chakraborty, S.; Krishna, M. Recent Advances in the Synthesis, Characterization of Pure and Doped NiO Nanostructures for Gas Sensor Applications. ECS Trans. 2022, 107, 3725. [Google Scholar] [CrossRef]
- Hussain, N.A.; Abbas, L.Y.; Latif, L.A. Preparation of Nickel Oxide Microparticles by Pulsed Laser Ablation and Application to Gas Sensors. Eng. Technol. J. 2021, 39, 1011–1018. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined chemoresistive and in situ FTIR spectroscopy study of nanoporous NiO films for light-activated nitrogen dioxide and acetone gas sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]
- Kampara, R.K.; Sonia, T.; Balamurugan, D.; Jeyaprakash, B.G. Formaldehyde vapour sensing property of electrospun NiO nanograins. Front. Mater. Sci. 2021, 15, 416–430. [Google Scholar] [CrossRef]
- Lekshmi, M.S.; Suja, K.J. Acetone gas sensing at room temperature using metal oxide semiconductor nanomaterial based gas sensor. AIP Conf. Proc. 2020, 2222, 020013. [Google Scholar] [CrossRef]
- Simion, C.E.; Ghica, C.; Mihalcea, C.G.; Ghica, D.; Mercioniu, I.; Somacescu, S.; Florea, O.G.; Stanoiu, A. Insights about CO Gas-Sensing Mechanism with NiO-Based Gas Sensors-The Influence of Humidity. Chemosensors 2021, 9, 244. [Google Scholar] [CrossRef]
- Ghica, D.; Vlaicu, I.D.; Stefan, M.; Maraloiu, V.A.; Joita, A.C.; Ghica, C. Tailoring the Dopant Distribution in ZnO:Mn Nanocrystals. Sci. Rep. 2019, 9, 6894. [Google Scholar] [CrossRef] [Green Version]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide based gas sensing mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Ling, T.; Zhu, J.; Yu, H.; Xie, L. Size Effect on Crystal Morphology of Faceted Face-Centered Cubic Fe Nanoparticles. J. Phys. Chem. C 2009, 113, 9450–9453. [Google Scholar] [CrossRef]
- Kaumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database 20, Version 4.1. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 8 May 2022).
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1979. [Google Scholar]
- Khawaja, E.E.; Salim, M.A.; Khan, M.A.; Al-Adel, F.F.; Khattak, G.D.; Hussain, Z.J. XPS, auger, electrical and optical studies of vanadium phosphate glasses doped with nickel oxide. J. Non-Cryst. Solids 1989, 110, 33–43. [Google Scholar] [CrossRef]
- Lian, K.K.; Kirk, D.W.; Thorpe, S.J. Investigation of a “Two-State” Tafel Phenomenon for the Oxygen Evolution Reaction on an Amorphous Ni-Co Alloy. J. Electrochem. Soc. 1995, 142, 3704. [Google Scholar] [CrossRef]
- Venezia, A.M.; Bertoncello, R.; Deganello, G. X-ray photoelectron spectroscopy investigation of pumice-supported nickel catalysts. Surf. Interface Anal. 1995, 23, 239–247. [Google Scholar] [CrossRef]
- Matienzo, J.; Yin, L.I.; Grim, S.O.; Swartz, W.E. X-ray photoelectron spectroscopy of nickel compounds. Inorg. Chem. 1973, 12, 2762–2769. [Google Scholar] [CrossRef]
- Ding, H.; Meng, M.; Lin, Z.; Duan, C.; Zhang, Q.; He, M. Performance and characterization of MnOx-NiO catalysts for removing formaldehyde at room temperature. J. Phys. Conf. Ser. 2021, 1976, 012059. [Google Scholar] [CrossRef]
- Somacescu, S.; Florea, M.; Osiceanu, P.; Calderon-Moreno, J.M.; Ghica, C.; Serra, J.M. Ni-doped (CeO2-δ)-YSZ mesoarchitectured with nanocrystalline framework: The effect of thermal treatment on structure, surface chemistry and catalytic properties in the partial oxidation of methane (CPOM). J. Nanopart. Res. 2015, 17, 426. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Ortiz, J.; Gautier, J.L. Characterization of the spinel-related oxides Ni(x)Co(3-x)O4 (x = 0.3, 1.3, 1.8) prepared by spray pyrolysis at 350 degrees C. Appl. Surf. Sci. 2004, 227, 175–186. [Google Scholar] [CrossRef]
- Hermawan, A.; Hanindriyo, A.; Ramadhan, E.R.; Asakura, Y.; Hasegawa, T.; Hongo, K.; Inada, M.; Maezono, R.; Yin, S. Octahedral morphology of NiO with (111) facet synthesized from the transformation of NiOHCl for the NOx detection and degradation: Experiment and DFT calculation. Inorg. Chem. Front. 2020, 7, 3431–3442. [Google Scholar] [CrossRef]
- Kim, K.S.; Winograd, N. X-ray photoelectron spectroscopic studies of nickel-oxygen surface using oxygen and argon ion-bombardament. Surf. Sci. 1974, 43, 625–643. [Google Scholar] [CrossRef]
- Koziej, D.; Barsan, N.; Weimar, U.; Szuber, J.; Shimanoe, K.; Yamazoe, N. Water-oxygen interplay on tin dioxide surface: Implication on gas sensing. Chem. Phys. Lett 2005, 410, 321–323. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. Review: Influence of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sci. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Sato, H.; Minami, T.; Takata, S.; Yamada, T. Transparent conducting p-type NiO thin films prepared by magnetron sputtering. Thin Solid Film. 1993, 236, 27–31. [Google Scholar] [CrossRef]
- Bielanski, A.; Najbar, M. Adsorption Species of Oxygen on the Surfaces of Transition Metal Oxides. J. Catal. 1972, 25, 398–406. [Google Scholar] [CrossRef]
- De Rosa, B.; Dufour, L.-C.; Nowotny, J. Nickel monoxide-oxygen interaction between 20 and 1000 °C and its impact on the nickel monoxide reduction mechanism. React. Solids 1987, 4, 53–72. [Google Scholar] [CrossRef]
- Heiland, G.; Kohl, D.; Seiyama, T. (Eds.) Chemical Sensor Technology; Kodansha: Tokyo, Japan, 1988; Volume 1, pp. 15–38. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanoiu, A.; Ghica, C.; Mihalcea, C.G.; Ghica, D.; Somacescu, S.; Florea, O.G.; Simion, C.E. Effects of Calcination Temperature on CO-Sensing Mechanism for NiO-Based Gas Sensors. Chemosensors 2022, 10, 191. https://doi.org/10.3390/chemosensors10050191
Stanoiu A, Ghica C, Mihalcea CG, Ghica D, Somacescu S, Florea OG, Simion CE. Effects of Calcination Temperature on CO-Sensing Mechanism for NiO-Based Gas Sensors. Chemosensors. 2022; 10(5):191. https://doi.org/10.3390/chemosensors10050191
Chicago/Turabian StyleStanoiu, Adelina, Corneliu Ghica, Catalina G. Mihalcea, Daniela Ghica, Simona Somacescu, Ovidiu G. Florea, and Cristian E. Simion. 2022. "Effects of Calcination Temperature on CO-Sensing Mechanism for NiO-Based Gas Sensors" Chemosensors 10, no. 5: 191. https://doi.org/10.3390/chemosensors10050191
APA StyleStanoiu, A., Ghica, C., Mihalcea, C. G., Ghica, D., Somacescu, S., Florea, O. G., & Simion, C. E. (2022). Effects of Calcination Temperature on CO-Sensing Mechanism for NiO-Based Gas Sensors. Chemosensors, 10(5), 191. https://doi.org/10.3390/chemosensors10050191







