Plasmonic Sensing of Glucose Based on Gold–Silver Core–Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
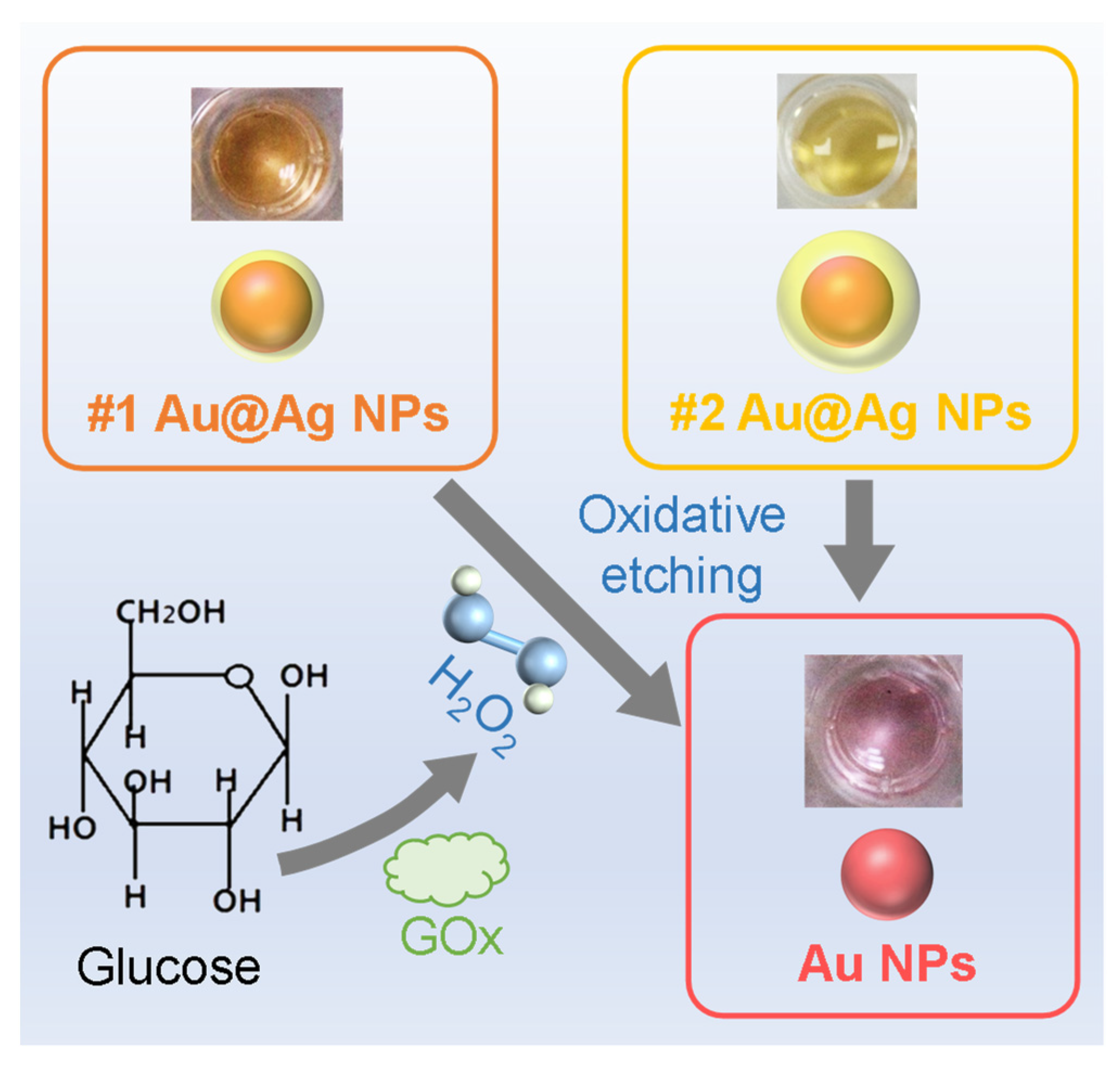
3.1. Design and Preparation of the Au@Ag NPs
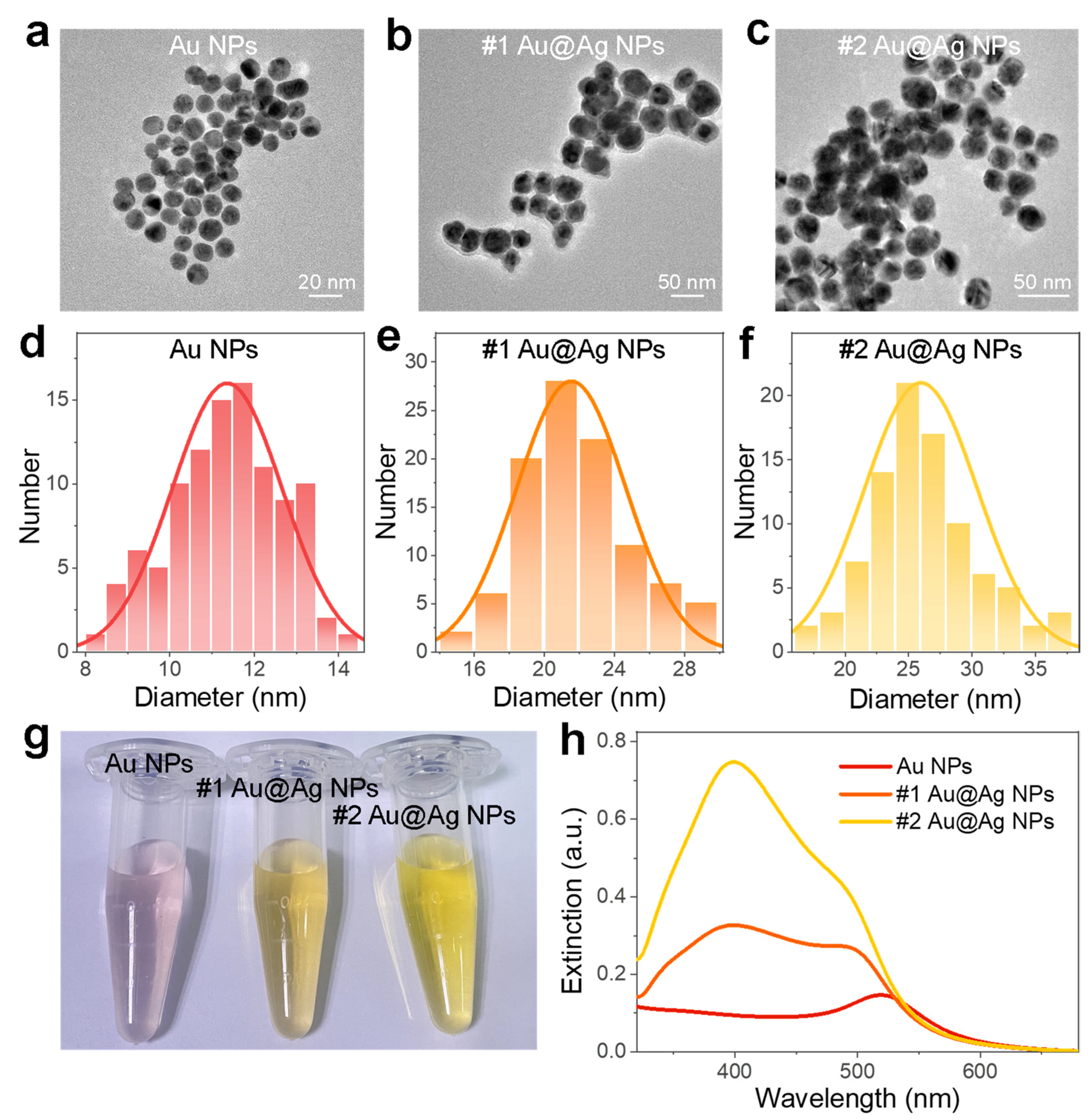
3.2. Characterizations of the Au@Ag NPs
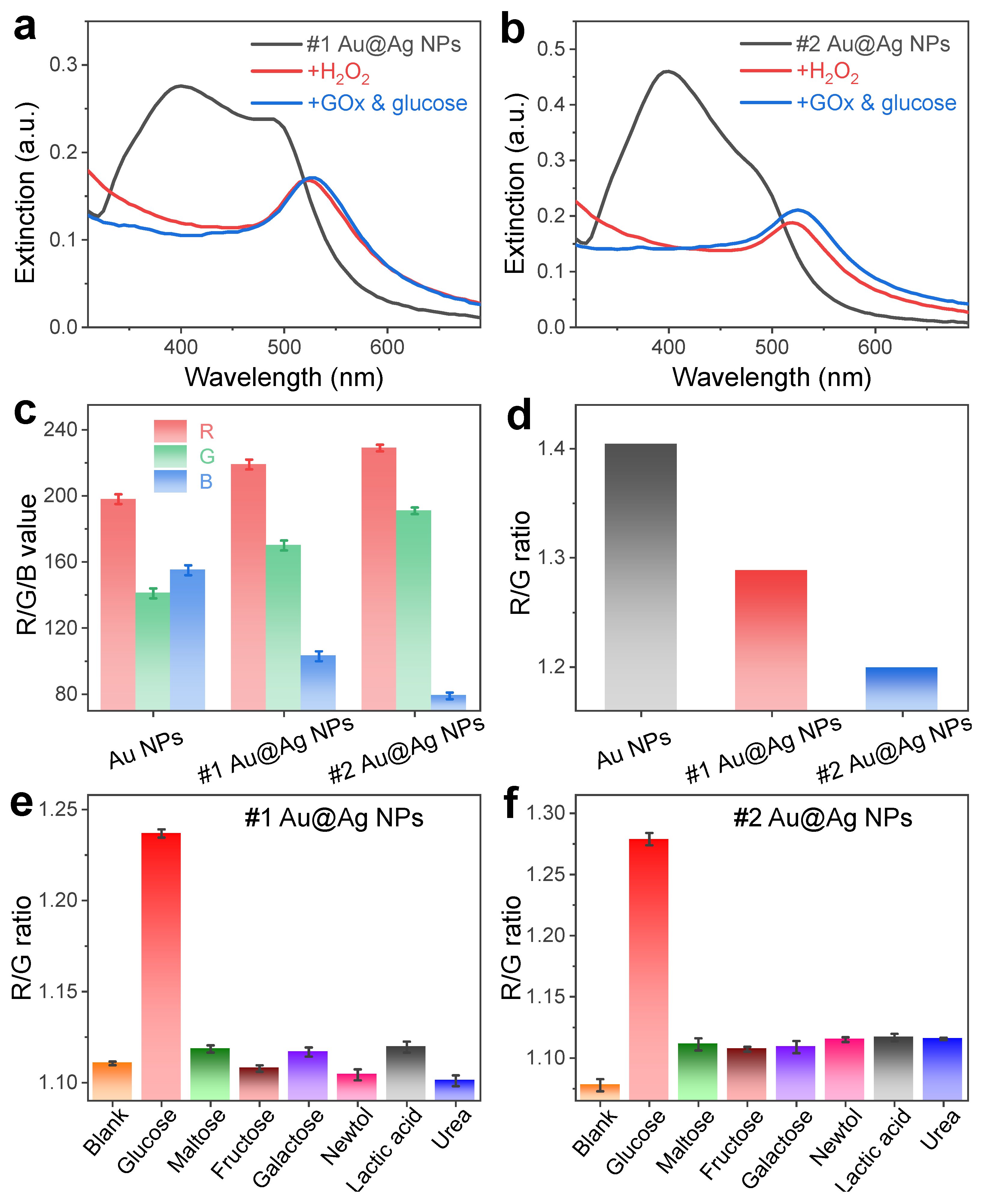
3.3. Colorimetric Changes during the Sensing of H2O2 and Glucose
3.4. Optimization of the Sensing Systems
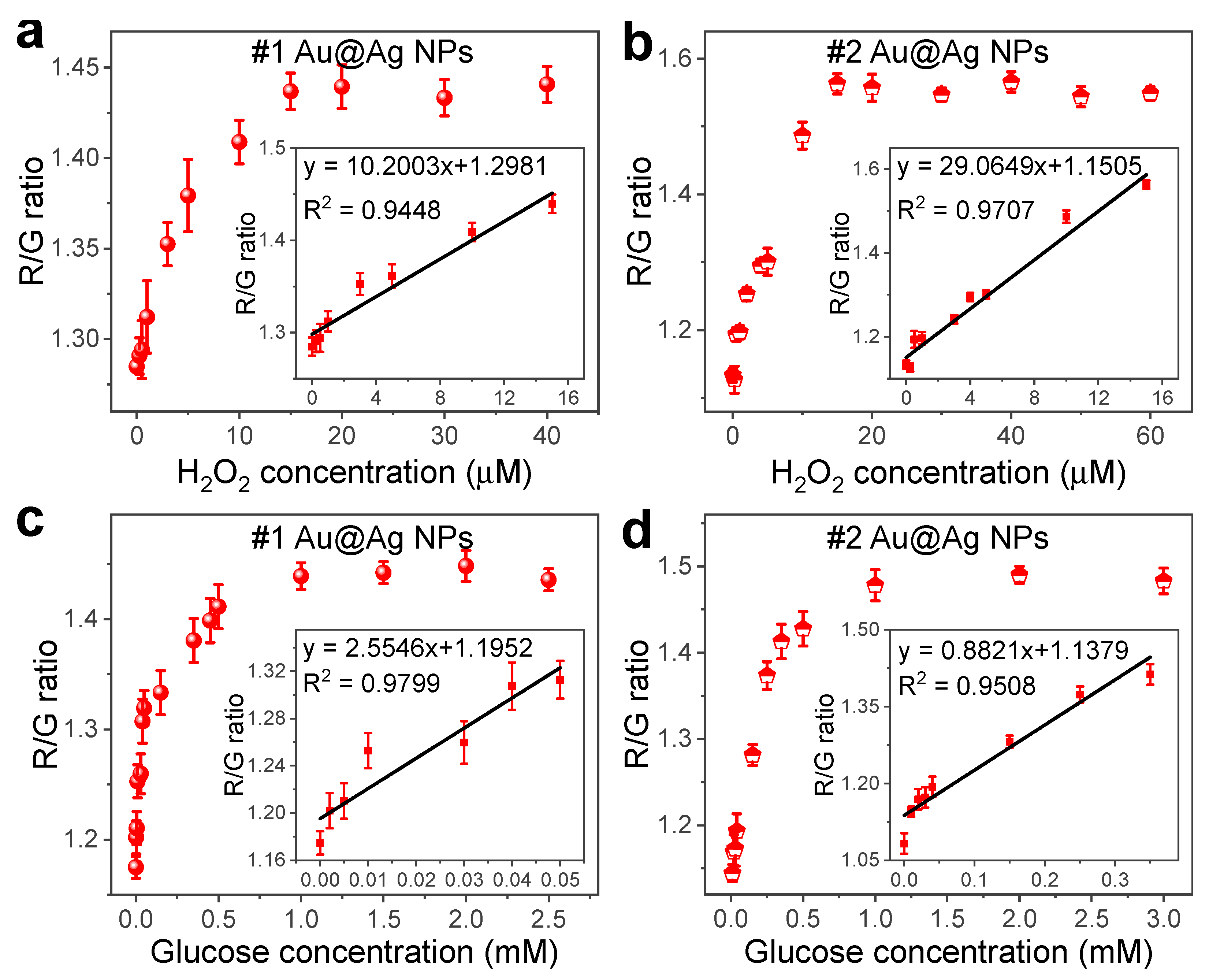
3.5. Sensitive Detection of H2O2 and Glucose
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, X.; Xu, F.; Zhang, L.; Ren, G.; Sheng, H.; Wang, J.; Wang, K.; Yu, L.; Wang, J.; Li, G.; et al. Simple, skin-attachable, and multifunctional colorimetric sweat sensor. ACS Sens. 2022, 7, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.T.; An, H.; Hong, Y.C.; Lee, S.C.; Le, T.N.; Le, X.A.; Kwak, H.S.; Lee, Y.; Jeong, Y.; Park, J.; et al. Ultrarapid, size-controlled, high-crystalline plasma-mediated synthesis of ceria nanoparticles for reagent-free colorimetric glucose test strips. Sens. Actuators B Chem. 2020, 320, 128404. [Google Scholar] [CrossRef]
- Liu, X.G.; Huang, D.L.; Lai, C.; Qin, L.; Zeng, G.M.; Xu, P.; Li, B.S.; Yi, H.; Zhang, M.M. Peroxidase-like activity of smart nanomaterials and their advanced application in colorimetric glucose biosensors. Small 2019, 15, e1900133. [Google Scholar] [CrossRef] [PubMed]
- Vaquer, A.; Baron, E.; de la Rica, R. Detection of low glucose levels in sweat with colorimetric wearable biosensors. Analyst 2021, 146, 3273–3279. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, skin-integrated multifunctional microfluidic systems for accurate colorimetric analysis of sweat biomarkers and temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Wang, L.; Yue, X.; Sun, Q.; Zhang, L.; Ren, G.; Lu, G.; Yu, H.; Huang, W. Flexible organic electrochemical transistors for chemical and biological sensing. Nano Res. 2021, 15, 2433–2464. [Google Scholar] [CrossRef]
- Ghaffari, R.; Choi, J.; Raj, M.S.; Chen, S.; Lee, S.P.; Reeder, J.T.; Aranyosi, A.J.; Leech, A.; Li, W.; Schon, S.; et al. Soft wearable systems for colorimetric and electrochemical analysis of biofluids. Adv. Funct. Mater. 2020, 30, 1907269. [Google Scholar] [CrossRef]
- Khan, M.S.; Misra, S.K.; Wang, Z.; Daza, E.; Schwartz-Duval, A.S.; Kus, J.M.; Pan, D. Paper-based analytical biosensor chip designed from Graphene-Nanoplatelet-Amphiphilic-diblock-co-Polymer composite for cortisol detection in human saliva. Anal. Chem. 2017, 89, 2107–2115. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Cho, Y.S.; Kim, Y.; Tae, J.H.; No, T.I.; Shim, J.S.; Jeong, Y.; Kang, S.H.; Lee, K.H. Electrical cartridge sensor enables reliable and direct identification of microRNAs in urine of patients. ACS Sens. 2021, 6, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Lee, H.; Kim, J.; Lee, M.; Choi, H.J.; Hyeon, T.; Kim, D. Multifunctional Wearable System that Integrates Sweat-Based Sensing and Vital-Sign Monitoring to Estimate Pre-/Post-Exercise Glucose Levels. Adv. Funct. Mater. 2018, 28, 1805754. [Google Scholar] [CrossRef]
- Guo, X.; Zong, L.; Jiao, Y.; Han, Y.; Zhang, X.; Xu, J.; Li, L.; Zhang, C.W.; Liu, Z.; Ju, Q.; et al. Signal-Enhanced Detection of Multiplexed Cardiac Biomarkers by a Paper-Based Fluorogenic Immunodevice Integrated with Zinc Oxide Nanowires. Anal. Chem. 2019, 91, 9300–9307. [Google Scholar] [CrossRef] [PubMed]
- Yokus, M.A.; Daniele, M.A. Integrated non-invasive biochemical and biophysical sensing systems for health and performance monitoring: A systems perspective. Biosens. Bioelectron. 2021, 184, 113249. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Li, L.; Ghattamaneni, R.; Ahn, C.H.; Nyein, H.Y.Y.; Tai, L.; Javey, A. Glove-based sensors for multimodal monitoring of natural sweat. Sci. Adv. 2020, 6, eabb8308. [Google Scholar] [CrossRef]
- Yeung, K.K.; Huang, T.; Hua, Y.; Zhang, K.; Yuen, M.M.F.; Gao, Z. Recent Advances in Electrochemical Sensors for Wearable Sweat Monitoring: A Review. IEEE Sens. J. 2021, 21, 14522–14539. [Google Scholar] [CrossRef]
- Sekine, Y.; Kim, S.B.; Zhang, Y.; Bandodkar, A.J.; Xu, S.; Choi, J.; Irie, M.; Ray, T.R.; Kohli, P.; Kozai, N.; et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip 2018, 18, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric Fluorescent Nanohybrid for Noninvasive and Visual Monitoring of Sweat Glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef]
- Jiao, Y.; Du, C.; Zong, L.; Guo, X.; Han, Y.; Zhang, X.; Li, L.; Zhang, C.; Ju, Q.; Liu, J.; et al. 3D vertical-flow paper-based device for simultaneous detection of multiple cancer biomarkers by fluorescent immunoassay. Sens. Actuators B Chem. 2020, 306, 127239. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, J.; Wang, F.; Kong, D. Stretchable and superwettable colorimetric sensing patch for epidermal collection and analysis of sweat. ACS Sens. 2021, 6, 2261–2269. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Qin, Y. Electrochemical sensors. Anal. Chem. 2006, 78, 3965–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bau, L.; Tecilla, P.; Mancin, F. Sensing with fluorescent nanoparticles. Nanoscale 2011, 3, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hwang, E.; Zhang, J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors 2018, 18, 1440. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Z.; Huang, X.; Lu, G.; Huang, W. Single-molecule mapping of catalytic reactions on heterostructures. Nano Today 2020, 34, 100957. [Google Scholar] [CrossRef]
- Li, X.; Wen, F.; Creran, B.; Jeong, Y.; Zhang, X.; Rotello, V.M. Colorimetric Protein Sensing Using Catalytically Amplified Sensor Arrays. Small 2012, 8, 3589–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.L.; Anslyn, E.V. A colorimetric sensing ensemble for heparin. J. Am. Chem. Soc. 2002, 124, 9014–9015. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guan, M.; Wang, J.; Sheng, H.; Chen, Y.; Liang, Y.; Peng, Q.; Lu, G. Plasmon-mediated photochemical transformation of inorganic nanocrystals. Appl. Mater. Today 2021, 24, 101125. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Lu, G. Recent developments of flexible and transparent SERS substrates. J. Mater. Chem. C 2020, 8, 3956–3969. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Sheng, H.; Wang, J.; Zhang, C.; Chen, Y.; Huang, W.; Lu, G. Molecular coadsorption of p-hydroxythiophenol on silver nanoparticles boosts the plasmon-mediated decarboxylation reaction. ACS Catal. 2022, 12, 2938–2946. [Google Scholar] [CrossRef]
- Nocerino, V.; Miranda, B.; Tramontano, C.; Chianese, G.; Dardano, P.; Rea, I.; De Stefano, L. Plasmonic Nanosensors: Design, Fabrication, and Applications in Biomedicine. Chemosensors 2022, 10, 150. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Wong, C.L.; Olivo, M. Surface Plasmon Resonance Imaging Sensors: A Review. Plasmonics 2014, 9, 809–824. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Sheng, H.; Wang, J.; Wang, J.; He, S.; Guan, M.; Chen, Y.; Lu, G. Monitoring the thiol/thiophenol molecule-modulated plasmon-mediated silver oxidation with dark-field optical microscopy. Chem. Eur. J. 2022, 28, e202103709. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Sheng, H.; Wang, J.; Zhu, Y.; Yu, L.; Wang, J.; Peng, Q.; Lu, G. Molecular cocatalyst of p-mercaptophenylboronic acid boosts the plasmon-mediated reduction of p-nitrothiophenol. ACS Appl. Mater. Interfaces 2022, 14, 38302–38310. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Zhu, Y.; Yue, X.; Liang, Y.; Sheng, H.; Wang, J.; Zhang, C.; Peng, Q.; Lu, G. Molecular cocatalyst-induced enhancement of the plasmon-mediated coupling of p-nitrothiophenols at the silver nanoparticle–graphene oxide interface. ACS Appl. Nano Mater. 2021, 4, 10976–10984. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Liang, Y.; Zhu, Y.; Li, Z.; Huang, X.; Lu, G. Modulating the plasmon-mediated oxidation of p-aminothiophenol with asymmetrically grafted thiol molecules. J. Phys. Chem. Lett. 2020, 11, 7650–7656. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Lu, G.; Li, H.; Zhang, H. Gold-nanoparticle-embedded polydimethylsiloxane elastomers for highly sensitive Raman detection. Small 2012, 8, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, H.; Zhang, H. Nanoparticle-coated PDMS elastomers for enhancement of Raman scattering. Chem. Commun. 2011, 47, 8560–8562. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Zhu, X.-Y.; Jiao, H.-J.; Dong, Y.-M.; Wu, X.-M.; Li, Z.-J. “Oxidative etching-aggregation” of silver nanoparticles by melamine and electron acceptors: An innovative route toward ultrasensitive and versatile functional colorimetric sensors. Anal. Chim. Acta 2012, 747, 92–98. [Google Scholar] [CrossRef]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yue, X.; Zhang, Y.; Zhu, C.; Kang, X.; Yu, H.-D.; Lu, G. Plasmonic Sensing of Glucose Based on Gold–Silver Core–Shell Nanoparticles. Chemosensors 2022, 10, 404. https://doi.org/10.3390/chemosensors10100404
Wang J, Yue X, Zhang Y, Zhu C, Kang X, Yu H-D, Lu G. Plasmonic Sensing of Glucose Based on Gold–Silver Core–Shell Nanoparticles. Chemosensors. 2022; 10(10):404. https://doi.org/10.3390/chemosensors10100404
Chicago/Turabian StyleWang, Junjie, Xiaoping Yue, Yulong Zhang, Chengcheng Zhu, Xing Kang, Hai-Dong Yu, and Gang Lu. 2022. "Plasmonic Sensing of Glucose Based on Gold–Silver Core–Shell Nanoparticles" Chemosensors 10, no. 10: 404. https://doi.org/10.3390/chemosensors10100404
APA StyleWang, J., Yue, X., Zhang, Y., Zhu, C., Kang, X., Yu, H.-D., & Lu, G. (2022). Plasmonic Sensing of Glucose Based on Gold–Silver Core–Shell Nanoparticles. Chemosensors, 10(10), 404. https://doi.org/10.3390/chemosensors10100404





