A Turn-Off Fluorescent Strategy for Calprotectin Detection Based on the Inhibitory Effect of Calprotectin upon the Activity of Zn(Ⅱ)-Dependent DNAzyme
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Procedure of the Assay
3. Results and Discussion
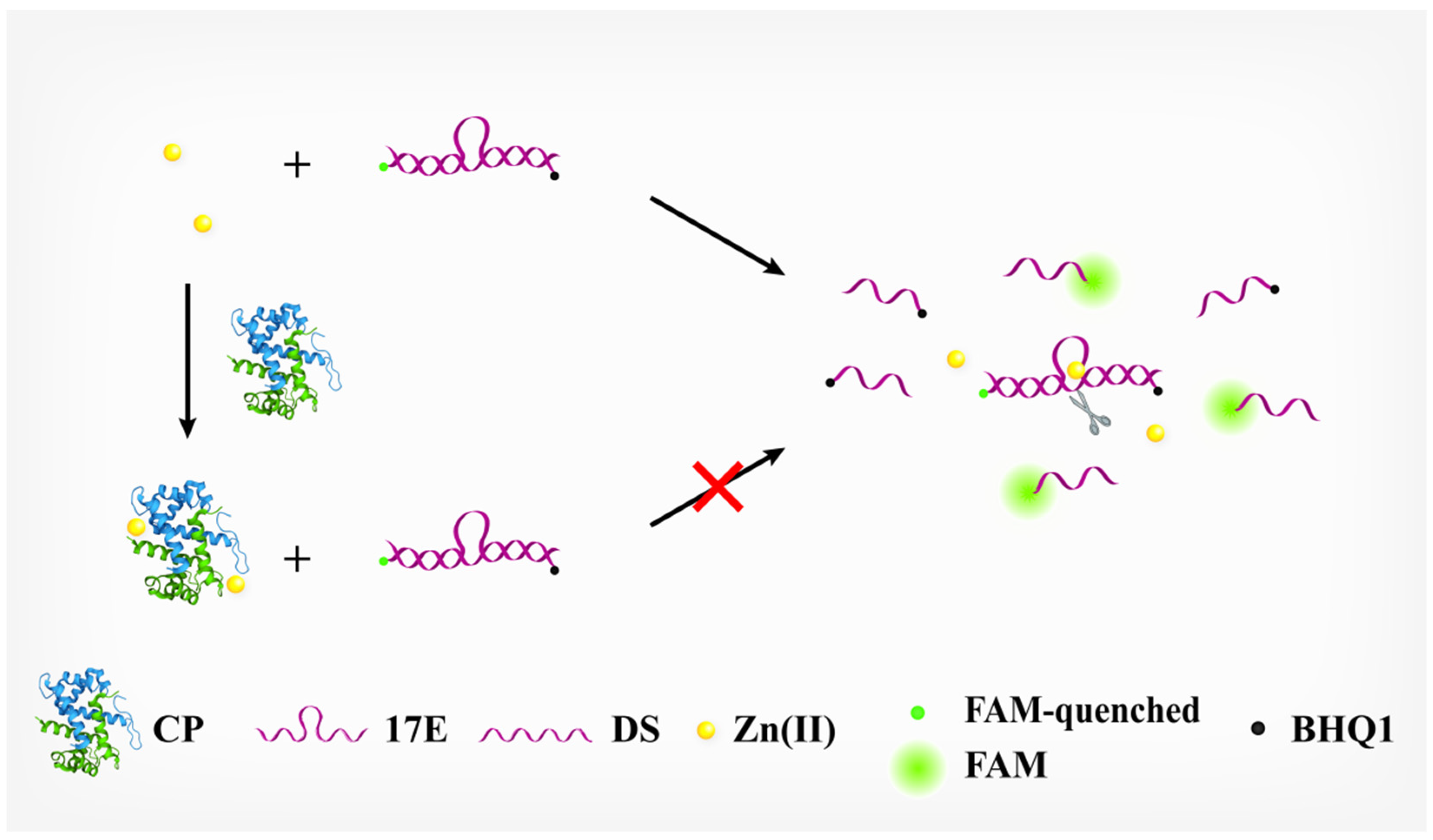
3.1. Principle of the Assay
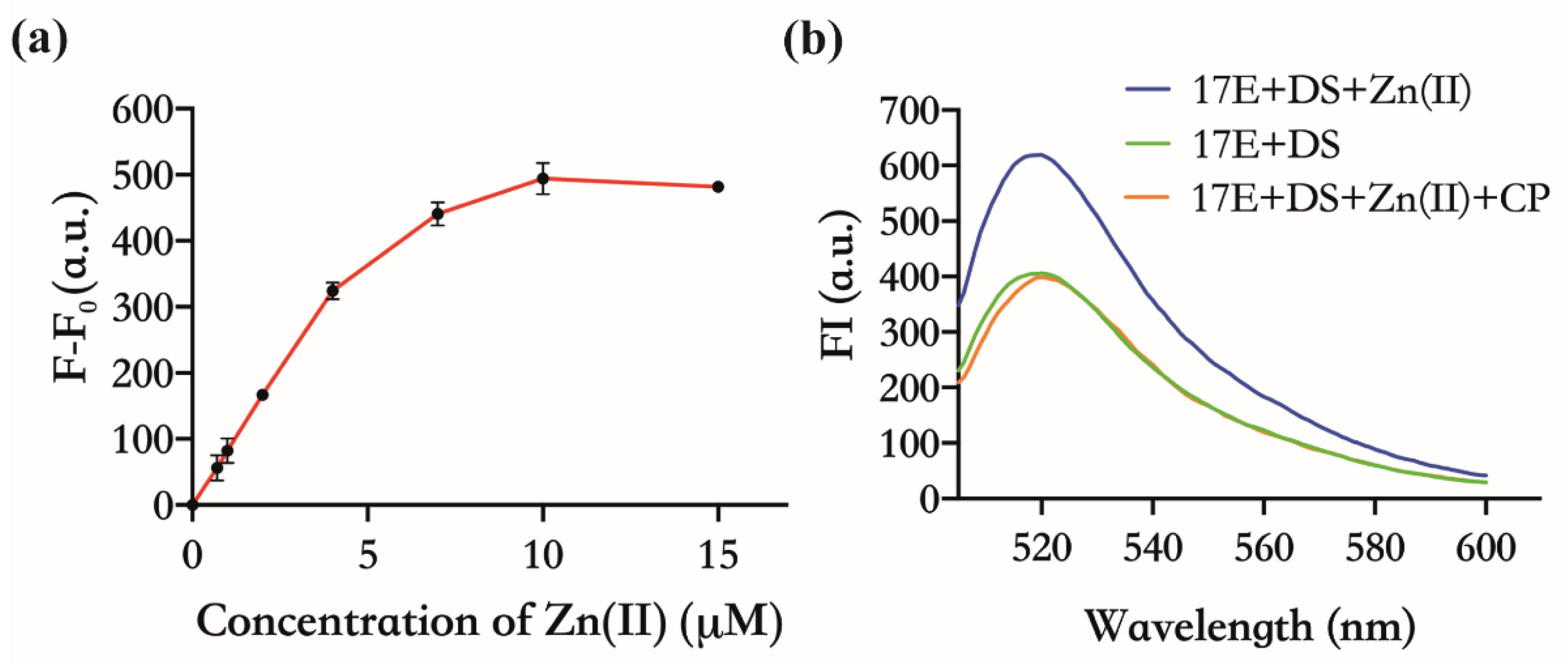
3.2. Verifying the Inhibitory Effect of CP upon the Activity of 17E DNAzyme
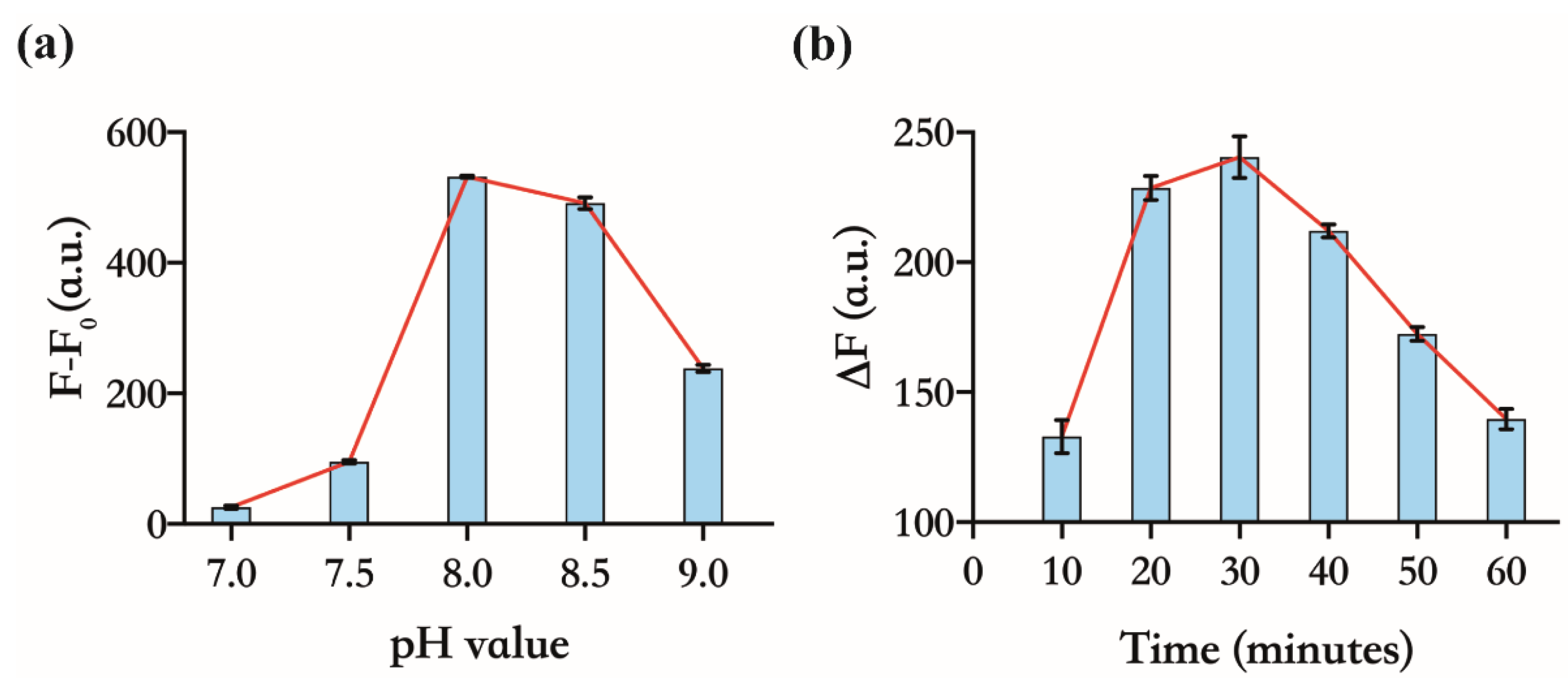
3.3. Optimizing the Experimental Conditions of the Assay
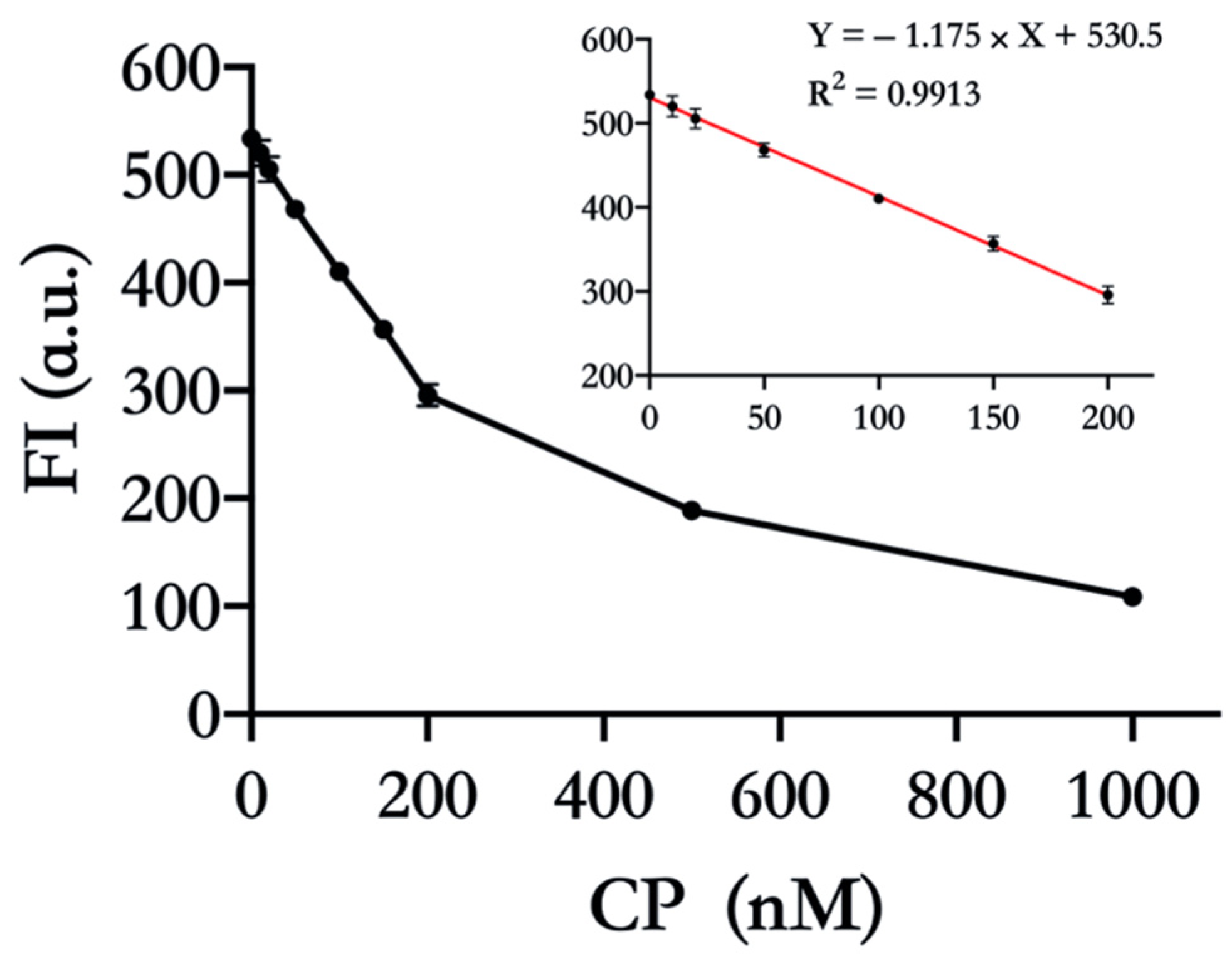
3.4. Quantitative Detection of CP using 17E DNAyzme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chams, S.; Badran, R.; Sayegh, S.E.; Chams, N.; Shams, A.; Inaya, H.H. Inflammatory bowel disease: Looking beyond the tract. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Gao, X.; Hu, N.; Huang, M.; Ran, Z.; Liu, Z.; Zhong, J.; Zou, D.; Wu, X.; et al. Current diagnosis and management of Crohn’s disease in China: Results from a multicenter prospective disease registry. BMC Gastroenterol. 2019, 19, 145. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal Calprotectin is a Surrogate Marker for Endoscopic Lesions in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef]
- Rokkas, T.; Portincasa, P.; Koutroubakis, I.E. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: A diagnostic accuracy meta-analysis. J. Gastrointest. Liver Dis. 2018, 27, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Havelka, A.; Sejersen, K.; Venge, P.; Pauksens, K.; Larsson, A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci. Rep. 2020, 10, 4208. [Google Scholar] [CrossRef] [PubMed]
- Grützner, N.; Heilmann, R.M.; Suchodolski, J.S.; Steiner, J.M.; Holzenburg, A. Cold-microwave enhanced enzyme-linked immunosorbent assays--a path to high-throughput clinical diagnostics. Anal. Biochem. 2014, 457, 65–73. [Google Scholar] [CrossRef]
- Hu, R.; Liu, T.; Zhang, X.B.; Yang, Y.; Chen, T.; Wu, C.; Liu, Y.; Zhu, G.; Huan, S.; Fu, T.; et al. DLISA: A DNAzyme-Based ELISA for Protein Enzyme-Free Immunoassay of Multiple Analytes. Anal. Chem. 2015, 87, 7746–7753. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Argosf, P. Engineering protein thermal stability: Sequence statistics point to residue substitutions in α-helices. J. Mol. Biol. 1989, 206, 397–406. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- McGhee, C.E.; Loh, K.Y.; Lu, Y. DNAzyme sensors for detection of metal ions in the environment and imaging them in living cells. Curr. Opin. Biotechnol. 2017, 45, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Lake, R.J.; Yang, Z.; Zhang, J.; Lu, Y. DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions. Acc. Chem. Res. 2019, 52, 3275–3286. [Google Scholar] [CrossRef]
- Leukert, N.; Sorg, C.; Roth, J. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14). Biol. Chem. 2005, 386, 429–434. [Google Scholar] [CrossRef]
- Pröpper, C.; Huang, X.; Roth, J.; Sorg, C.; Nacken, W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J. Biol. Chem. 1999, 274, 183–188. [Google Scholar] [CrossRef]
- Nakashige, T.G.; Stephan, J.R.; Cunden, L.S.; Brophy, M.B.; Wommack, A.J.; Keegan, B.C.; Shearer, J.M.; Nolan, E.M. The Hexahistidine Motif of Host-Defense Protein Human Calprotectin Contributes to Zinc Withholding and Its Functional Versatility. J. Am. Chem. Soc. 2016, 138, 12243–12251. [Google Scholar] [CrossRef] [PubMed]
- Silvers, R.; Stephan, J.R.; Griffin, R.G.; Nolan, E.M. Molecular Basis of Ca(II)-Induced Tetramerization and Transition-Metal Sequestration in Human Calprotectin. J. Am. Chem. Soc. 2021, 143, 18073–18090. [Google Scholar] [CrossRef] [PubMed]
- Damo, S.M.; Kehl-Fie, T.E.; Sugitani, N.; Holt, M.E.; Rathi, S.; Murphy, W.J.; Zhang, Y.; Betz, C.; Hench, L.; Fritz, G.; et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 3841–3846. [Google Scholar] [CrossRef]
- Moon, W.J.; Yang, Y.; Liu, J. Zn2+-Dependent DNAzymes: From Solution Chemistry to Analytical, Materials and Therapeutic Applications. ChemBioChem 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Kwon, A.H.; Lu, Y. In vitro selection and characterization of a highly efficient Zn(II)-dependent RNA-cleaving deoxyribozyme. Nucleic Acids Res. 2000, 28, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Brophy, M.B.; Hayden, J.A.; Nolan, E.M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 2012, 134, 18089–18100. [Google Scholar] [CrossRef] [PubMed]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Lozoya Angulo, M.E.; de Las Heras Gómez, I.; Martinez Villanueva, M.; Noguera Velasco, J.A.; Avilés Plaza, F. Faecal calprotectin, an useful marker in discriminating between inflammatory bowel disease and functional gastrointestinal disorders. Gastroenterol. Y Hepatol. 2017, 40, 125–131. [Google Scholar] [CrossRef]
| Name | Sequence (5′–3′) |
|---|---|
| 17E DNAzyme (17E) | CATCTCTTCTCCGAGCCGGTCGAAATAGTGAGT |
| DNAzyme substrate (DS) | 6-FAM-ACTCACTATrAGGAAGAGATG-BHQ1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, J.; Zhou, W.; Zhou, D.; Fang, Y.; Shen, X.; Zhu, C. A Turn-Off Fluorescent Strategy for Calprotectin Detection Based on the Inhibitory Effect of Calprotectin upon the Activity of Zn(Ⅱ)-Dependent DNAzyme. Chemosensors 2022, 10, 495. https://doi.org/10.3390/chemosensors10120495
Si J, Zhou W, Zhou D, Fang Y, Shen X, Zhu C. A Turn-Off Fluorescent Strategy for Calprotectin Detection Based on the Inhibitory Effect of Calprotectin upon the Activity of Zn(Ⅱ)-Dependent DNAzyme. Chemosensors. 2022; 10(12):495. https://doi.org/10.3390/chemosensors10120495
Chicago/Turabian StyleSi, Jingyi, Wei Zhou, Da Zhou, Ying Fang, Xizhong Shen, and Changfeng Zhu. 2022. "A Turn-Off Fluorescent Strategy for Calprotectin Detection Based on the Inhibitory Effect of Calprotectin upon the Activity of Zn(Ⅱ)-Dependent DNAzyme" Chemosensors 10, no. 12: 495. https://doi.org/10.3390/chemosensors10120495
APA StyleSi, J., Zhou, W., Zhou, D., Fang, Y., Shen, X., & Zhu, C. (2022). A Turn-Off Fluorescent Strategy for Calprotectin Detection Based on the Inhibitory Effect of Calprotectin upon the Activity of Zn(Ⅱ)-Dependent DNAzyme. Chemosensors, 10(12), 495. https://doi.org/10.3390/chemosensors10120495







