Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication & Characterization
2.2. Gas Sensor Measurement
3. Results and Discussions
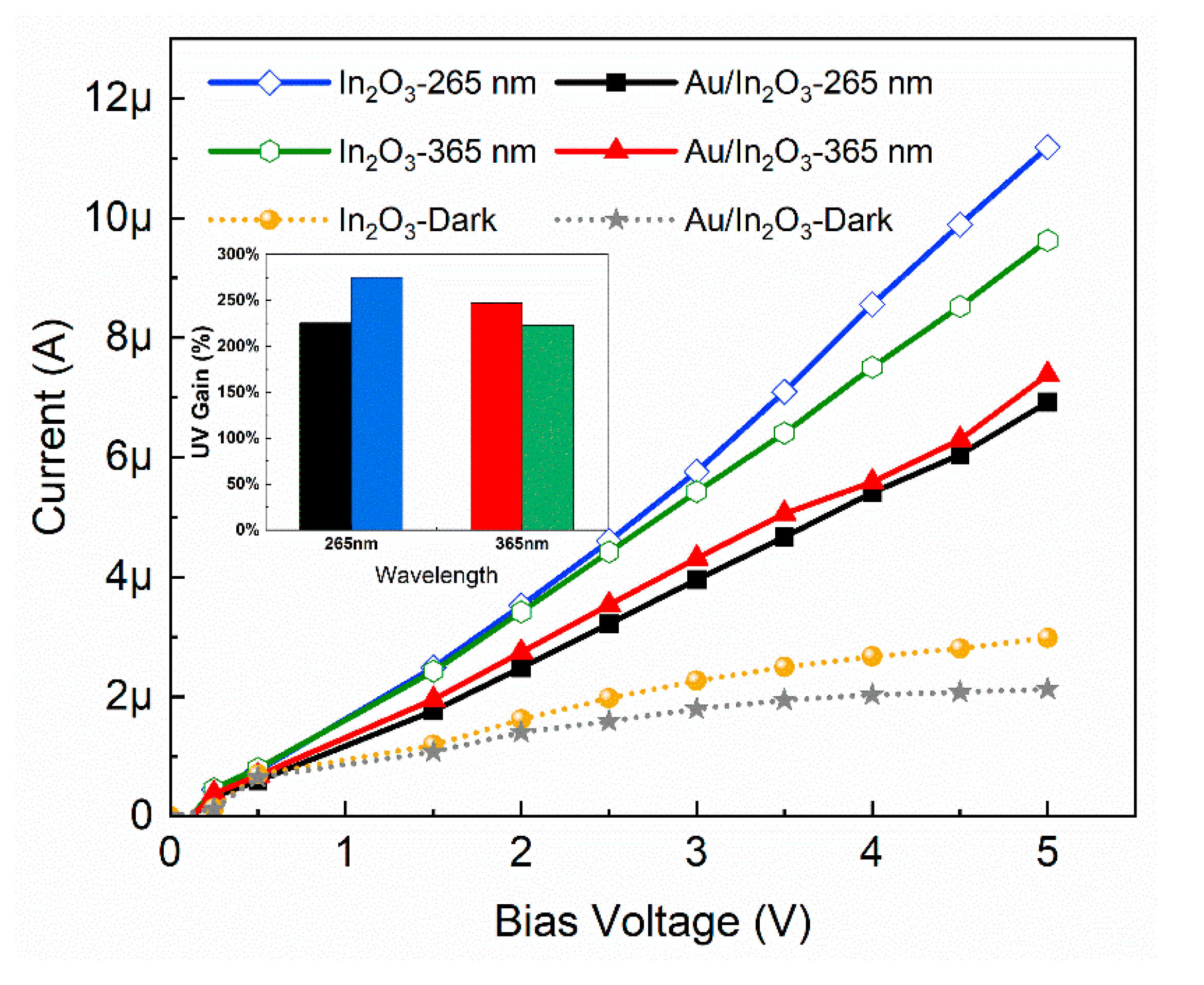
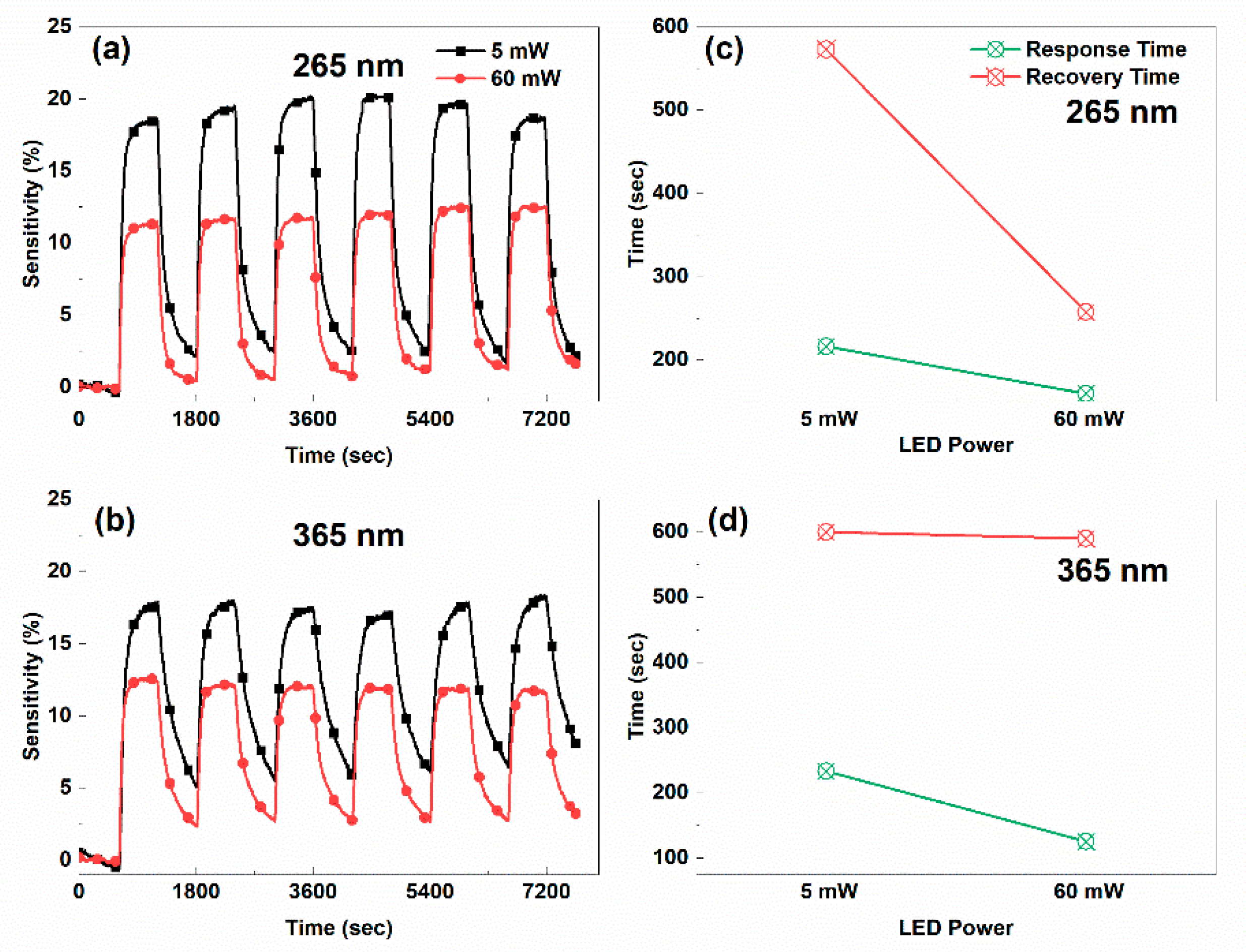
3.1. Photoconductivity
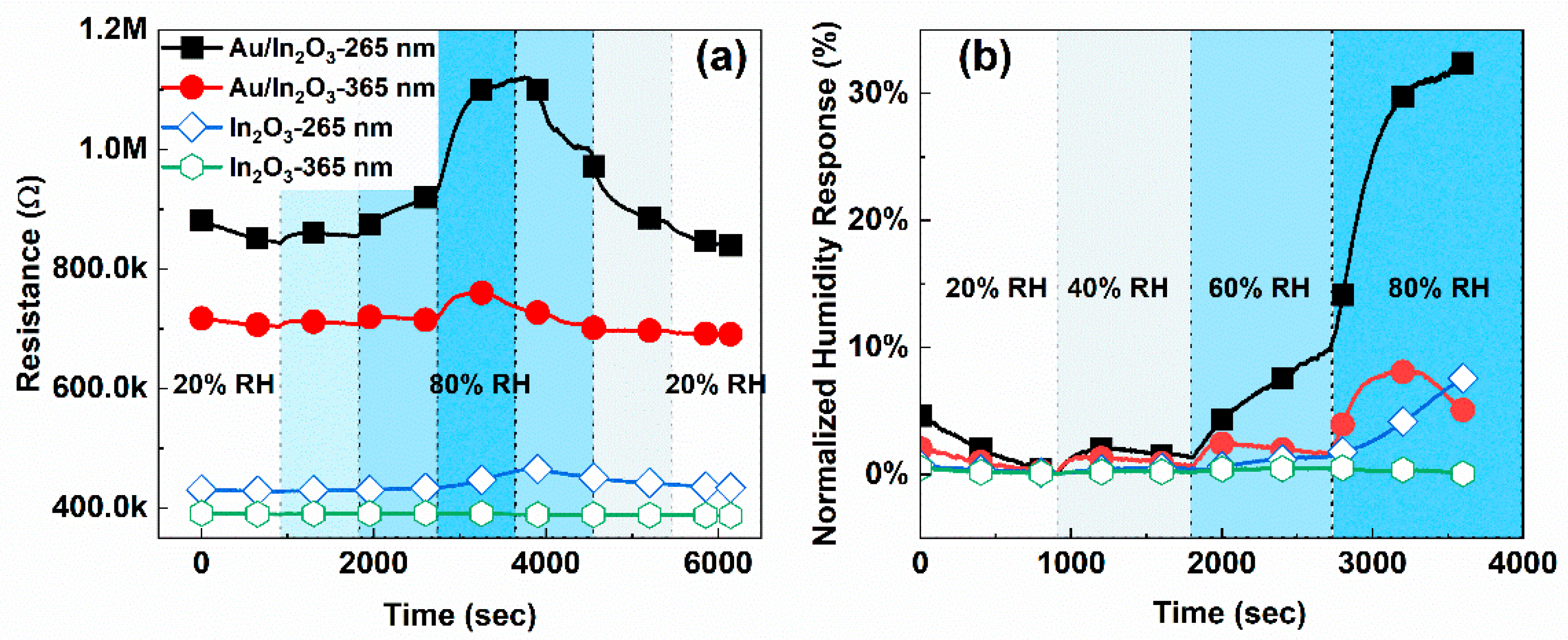
3.2. Humidity Response
3.3. NO2 Response under Various LED Power and Wavelengths
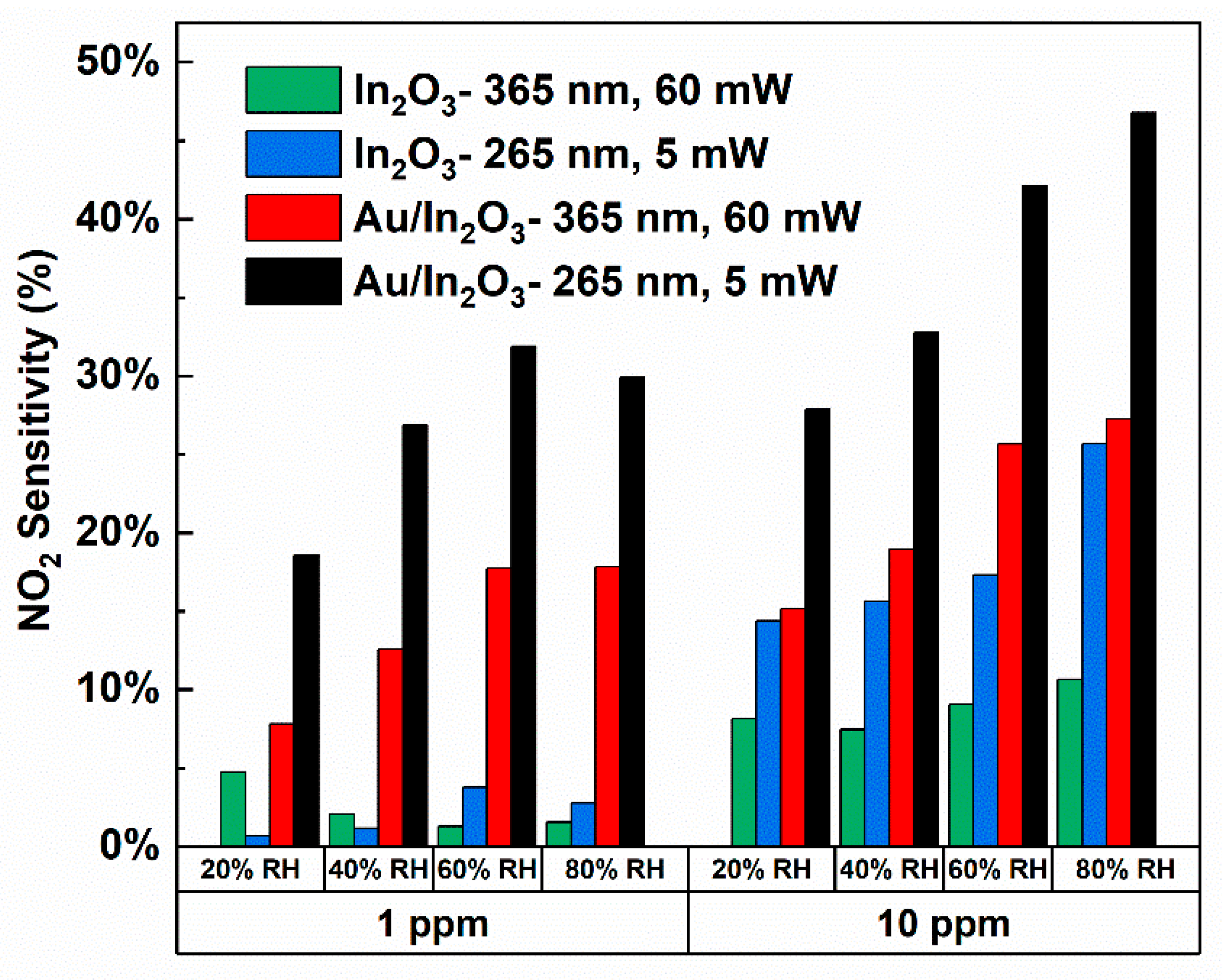
3.4. NO2 Response at Various Relative Humidity Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A Review of Gas Sensors Employed in Electronic Nose Applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penza, M.; Cassano, G.; Rossi, R.; Alvisi, M.; Rizzo, A.; Signore, M.A.; Dikonimos, T.; Serra, E.; Giorgi, R. Enhancement of Sensitivity in Gas Chemiresistors Based on Carbon Nanotube Surface Functionalized with Noble Metal (Au, Pt) Nanoclusters. Appl. Phys. Lett. 2007, 90, 173123. [Google Scholar] [CrossRef]
- Singhal, A.V.; Charaya, H.; Lahiri, I. Noble Metal Decorated Graphene-Based Gas Sensors and Their Fabrication: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 499–526. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mirzaei, A.; Kim, J.-Y.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Optimization of the Surface Coverage of Metal Nanoparticles on Nanowires Gas Sensors to Achieve the Optimal Sensing Performance. Sens. Actuators B Chem. 2020, 302, 127196. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-Activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mater. Sci. 2016, 42, 416–432. [Google Scholar] [CrossRef]
- Xu, F.; HO, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Geng, X.; Liu, X.; Mawella-Vithanage, L.; Hewa-Rahinduwage, C.C.; Zhang, L.; Brock, S.L.; Tan, T.; Luo, L. Photoexcited NO2 Enables Accelerated Response and Recovery Kinetics in Light-Activated NO2 Gas Sensing. ACS Sens. 2021, 6, 4389–4397. [Google Scholar] [CrossRef]
- Šetka, M.; Claros, M.; Chmela, O.; Vallejos, S. Photoactivated Materials and Sensors for NO2 Monitoring. J. Mater. Chem. C 2021, 9, 16804–16827. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liu, Y.; Liu, F.; Liang, X.; Liu, F.; Gao, Y.; Yan, X.; Lu, G. UV-Activated Ultrasensitive and Fast Reversible ppb NO2 Sensing Based on ZnO Nanorod Modified by Constructing Interfacial Electric Field with In2O3 Nanoparticles. Sens. Actuators B Chem. 2020, 305, 127498. [Google Scholar] [CrossRef]
- Weiher, R.L.; Ley, R.P. Optical Properties of Indium Oxide. J. Appl. Phys. 1966, 37, 299–302. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Zhu, Y.; Bao, K.; Lu, Z.; Sun, P.; Sun, Y.; Lu, G. Synthesis and NO2 Gas-Sensing Properties of Coral-like Indium Oxide via a Facile Solvothermal Method. RSC Adv. 2017, 7, 49273–49278. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Zhao, Q.; Wang, D.; Ma, G.; Zhang, M. Facile Synthesis of Nanoparticle Packed In2O3 Nanospheres for Highly Sensitive NO2 Sensing. New J. Chem. 2017, 41, 8530–8535. [Google Scholar] [CrossRef]
- Prajapati, C.S.; Sahay, P.P. Influence of in Doping on the Structural, Optical and Acetone Sensing Properties of ZnO Nanoparticulate Thin Films. Mater. Sci. Semicond. Process. 2013, 16, 200–210. [Google Scholar] [CrossRef]
- Aluri, G.S.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Bertness, K.A.; Rao, M.V. Nitro-Aromatic Explosive Sensing Using GaN Nanowire-Titania Nanocluster Hybrids. IEEE Sens. J. 2013, 13, 1883–1888. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent Progress in Applications of Graphene Oxide for Gas Sensing: A Review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gawali, S.R.; Patil, V.L.; Deonikar, V.G.; Patil, S.S.; Patil, D.R.; Patil, P.S.; Pant, J. Ce Doped NiO Nanoparticles as Selective NO2 Gas Sensor. J. Phys. Chem. Solids 2018, 114, 28–35. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic Effects in Gas Sensing Semiconducting Oxide Nano-Heterostructures: A Review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Reddeppa, M.; Park, B.-G.; Murali, G.; Choi, S.H.; Chinh, N.D.; Kim, D.; Yang, W.; Kim, M.-D. NOx Gas Sensors Based on Layer-Transferred N-MoS2/P-GaN Heterojunction at Room Temperature: Study of UV Light Illuminations and Humidity. Sens. Actuators B Chem. 2020, 308, 127700. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V. Gallium Nitride (GaN) Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2020, 20, 3889. [Google Scholar] [CrossRef] [PubMed]
- Aluri, G.S.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Bertness, K.A.; Sanford, N.A.; Rao, M.V. Highly Selective GaN-Nanowire/TiO2-Nanocluster Hybrid Sensors for Detection of Benzene and Related Environment Pollutants. Nanotechnology 2011, 22, 295503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Rani, A.; Thomson, B.; Debnath, R.; Motayed, A.; Ioannou, D.E.; Li, Q. High-Performance Room-Temperature TiO2-Functionalized GaN Nanowire Gas Sensors. Appl. Phys. Lett. 2019, 115, 121602. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Thomson, B.; Yu, J.; Debnath, R.; Motayed, A.; Rao, M.V. Scalable Metal Oxide Functionalized GaN Nanowire for Precise SO2 Detection. Sens. Actuators B Chem. 2020, 318, 128223. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The Effect of Noble Metal (Au, Pd and Pt) Nanoparticles on the Gas Sensing Performance of SnO2-Based Sensors: A Case Study on the {221} High-Index Faceted SnO2 Octahedra. CrystEngComm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Addabbo, T.; Cappelli, I.; Fort, A.; Mugnaini, M.; Panzardi, E.; Vignoli, V.; Viti, C. The Effect of Au Nanoparticle Addition on Humidity Sensing with Ultra-Small TiO2 Nanoparticles. Chemosensors 2021, 9, 170. [Google Scholar] [CrossRef]
- Miyake, T.; Rolandi, M. Grotthuss Mechanisms: From Proton Transport in Proton Wires to Bioprotonic Devices. J. Phys. Condens. Matter 2015, 28, 023001. [Google Scholar] [CrossRef]
- Munuera, G.; Rives-Arnau, V.; Saucedo, A. Photo-Adsorption and Photo-Desorption of Oxygen on Highly Hydroxylated TiO2 Surfaces. Part 1.—Role of Hydroxyl Groups in Photo-Adsorption. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 736. [Google Scholar] [CrossRef]
- Giberti, A.; Fabbri, B.; Gaiardo, A.; Guidi, V.; Malagù, C. Resonant Photoactivation of Cadmium Sulfide and Its Effect on the Surface Chemical Activity. Appl. Phys. Lett. 2014, 104, 222102. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, H.; Chen, S.; Li, Z.; Li, S.; Wang, M.; Zhu, H.; Chen, X. In Situ Growth of Leakage-Free Direct-Bridging GaN Nanowires: Application to Gas Sensors for Long-Term Stability, Low Power Consumption, and Sub-ppb Detection Limit. Nano Lett. 2019, 19, 3448–3456. [Google Scholar] [CrossRef]
- Hwang, J.; Jung, H.; Shin, H.-S.; Kim, D.-S.; Kim, D.S.; Ju, B.-K.; Chun, M. The Effect of Noble Metals on CO Gas Sensing Properties of In2O3 Nanoparticles. Appl. Sci. 2021, 11, 4903. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2-Sensing Properties of Au-Loaded Porous In2O3 Gas Sensors at Low Operating Temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Choi, S.-W.; Jung, S.-H.; Kim, S.S. Significant Enhancement of the NO2 Sensing Capability in Networked SnO2 Nanowires by Au Nanoparticles Synthesized via γ-Ray Radiolysis. J. Hazard. Mater. 2011, 193, 243–248. [Google Scholar] [CrossRef]
- Mun, Y.; Park, S.; An, S.; Lee, C.; Kim, H.W. NO2 Gas Sensing Properties of Au-Functionalized Porous ZnO Nanosheets Enhanced by UV Irradiation. Ceram. Int. 2013, 39, 8615–8622. [Google Scholar] [CrossRef]
- Shim, Y.-S.; Moon, H.G.; Kim, D.H.; Zhang, L.; Yoon, S.-J.; Yoon, Y.S.; Kang, C.-Y.; Jang, H.W. Au-Decorated WO3 Cross-Linked Nanodomes for Ultrahigh Sensitive and Selective Sensing of NO2 and C2H5OH. RSC Adv. 2013, 3, 10452–10459. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Xie, G.; Yao, M.; Pan, H.; Du, H.; Tai, H.; Du, X.; Su, Y. Enhancing Visible Light-Activated NO2 Sensing Properties of Au NPs Decorated ZnO Nanorods by Localized Surface Plasmon Resonance and Oxygen Vacancies. Mater. Res. Express 2020, 7, 015924. [Google Scholar] [CrossRef]
- Phani, A.R. X-ray Photoelectron Spectrscopy Studies on Pd-Doped SnO2 Liquid Petroleum Gas Sensor. Appl. Phys. Lett. 1998, 71, 2358, Erratum in Appl. Phys. Lett. 1998, 72, 3383–3383. [Google Scholar] [CrossRef]
- Reddeppa, M.; Mitta, S.B.; Park, B.-G.; Kim, S.-G.; Park, S.H.; Kim, M.-D. DNA-CTMA Functionalized GaN Surfaces for NO2 Gas Sensor at Room Temperature under UV Illumination. Org. Electron. 2019, 65, 334–340. [Google Scholar] [CrossRef]
- Reddeppa, M.; KimPhung, N.T.; Murali, G.; Pasupuleti, K.S.; Park, B.-G.; In, I.; Kim, M.-D. Interaction Activated Interfacial Charge Transfer in 2D G-C3N4/Gan Nanorods Heterostructure for Self-Powered UV Photodetector and Room Temperature NO2 Gas Sensor at ppb Level. Sens. Actuators B Chem. 2021, 329, 129175. [Google Scholar] [CrossRef]
- Sun, J.; Sokolovskij, R.; Iervolino, E.; Liu, Z.; Sarro, P.M.; Zhang, G. Suspended AlGaN/GaN HEMT NO2 Gas Sensor Integrated with Micro-Heater. J. Microelectromech. Syst. 2019, 28, 997–1004. [Google Scholar] [CrossRef]
- Shin, J.; Han, S.; Noh, S.; Yu, Y.-T.; Kim, J.S. Room-Temperature Operation of Light-Assisted NO2 Gas Sensor Based on GaN Nanowires and Graphene. Nanotechnology 2021, 32, 505201. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, H.; Li, G.; Zhang, J.; Tian, Y.; Gao, Y.; Guo, Q.; Lu, H.; Gao, J. Surface Functionalization of Porous In2O3 Nanofibers with Zn Nanoparticles for Enhanced Low-Temperature NO2 Sensing Properties. Sens. Actuators B Chem. 2020, 308, 127716. [Google Scholar] [CrossRef]
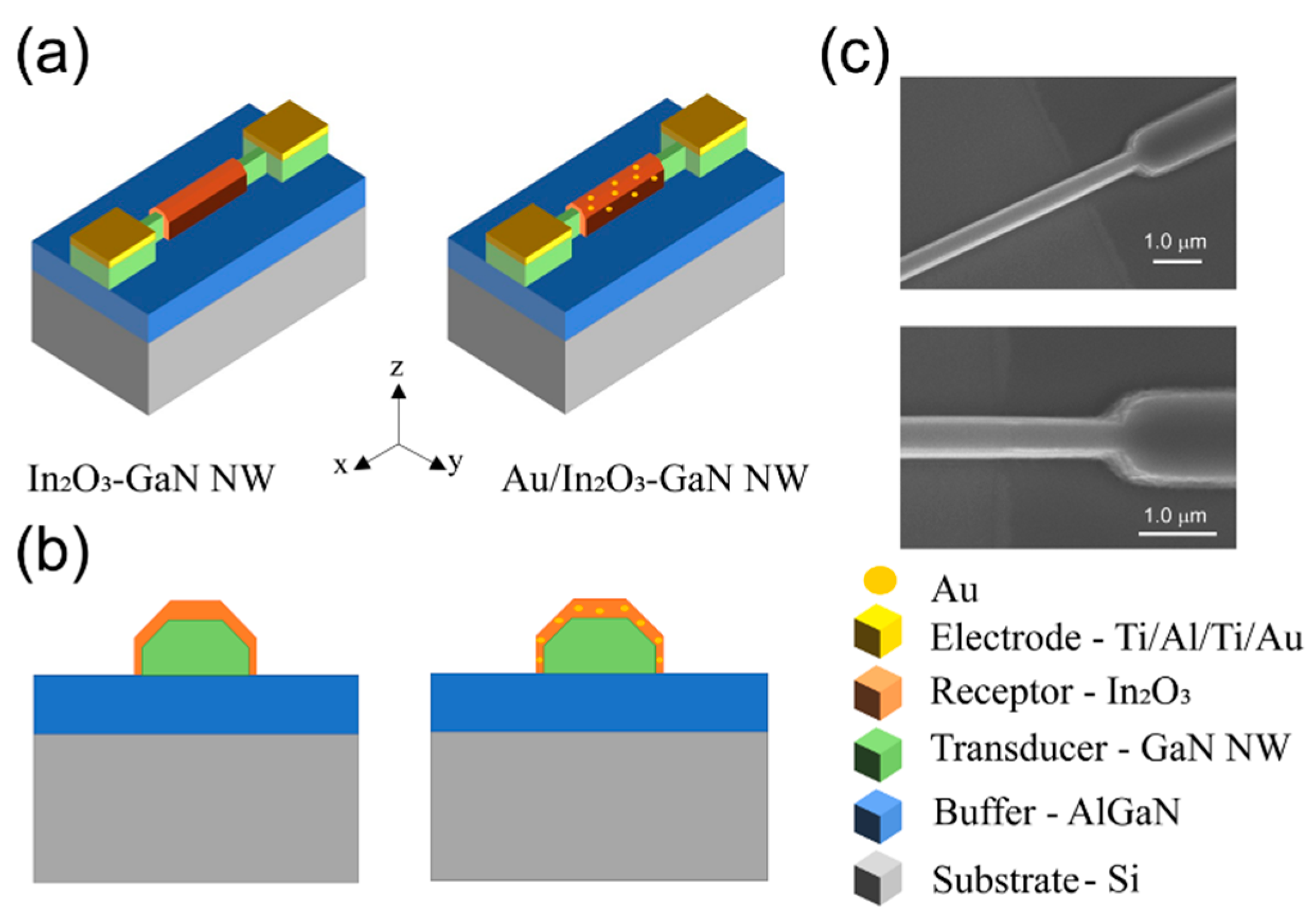
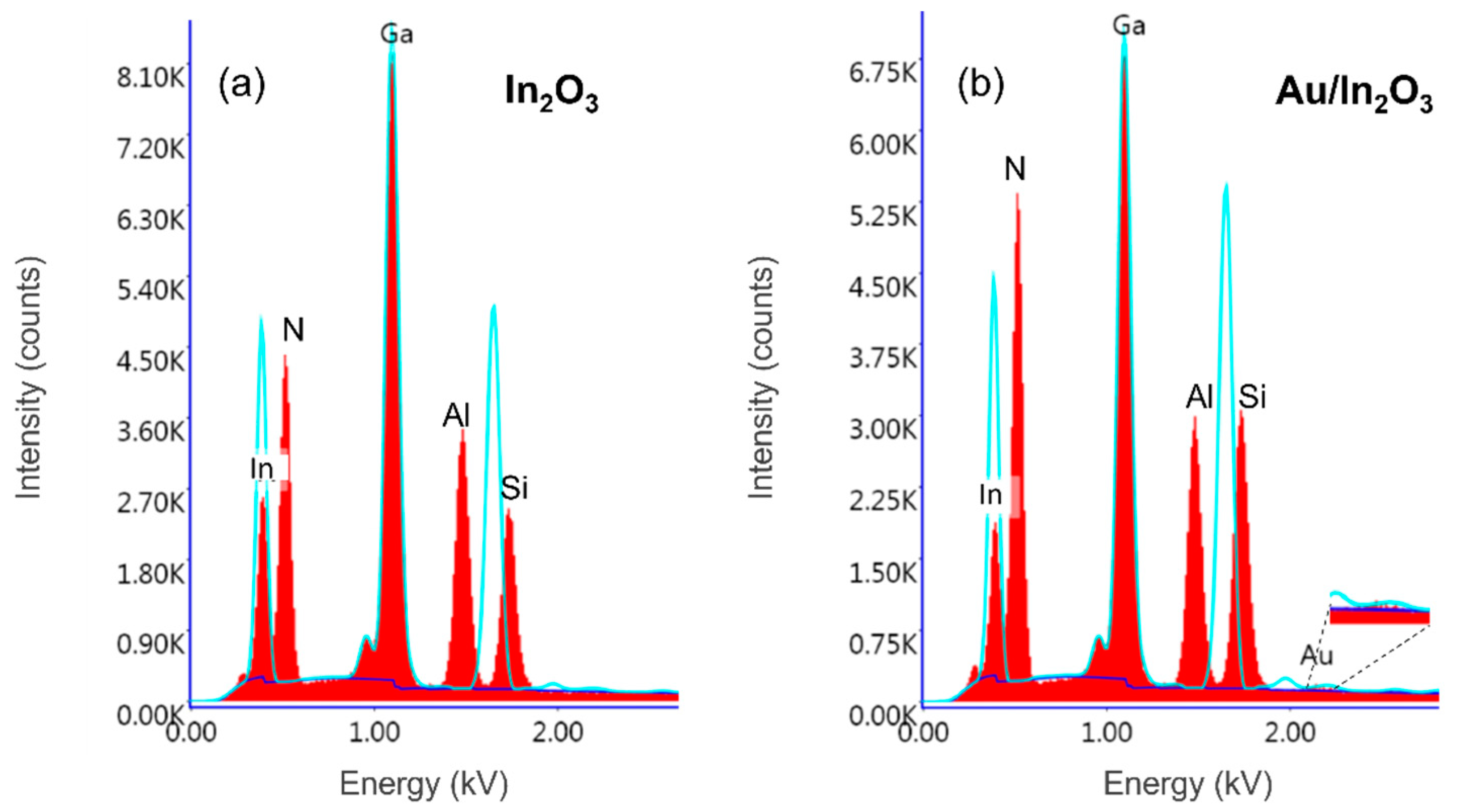


| In2O3 | Au/In2O3 | |||||
|---|---|---|---|---|---|---|
| Element | Weight % | Atomic % | Error % | Weight % | Atomic % | Error % |
| N | 31.84 | 69.93 | 8.27 | 33.73 | 71.73 | 8.28 |
| Ga | 68.16 | 30.07 | 2.47 | 66.15 | 28.26 | 2.52 |
| Au | 0 | 0 | 3.4 | 0.11 | 0.02 | 3.52 |
| Sensing Material | Concentrations (ppm) | λ (nm) | Operating Temp. (°C) | Power Consump. (mW) | Sensitivity (%) | Reference |
|---|---|---|---|---|---|---|
| DNA-CTMA/GaN | 10 | 264, 364 | RT | - | 10 | [39] |
| g-C3N4/GaN NR | 1 | 392 | RT | - | 10 | [40] |
| AlGaN/GaN HMET | 1 | - | 300 | 200 | 1.1 | [41] |
| G/GaN | 10 | 265–350 | RT | - | 8 | [42] |
| In2O3/Zn nanofibers | 1, 10 | - | 50 | - | 2.38, 130 | [43] |
| Au-loaded Porous In2O3 | 1 | - | 100 | - | 900 | [34] |
| Au/In2O3 GaN NW | 1, 10 | 265 | RT | 5 | 27, 42 | Present Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rambeloson, J.; Ioannou, D.E.; Raju, P.; Wang, X.; Motayed, A.; Yun, H.J.; Li, Q. Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature. Chemosensors 2022, 10, 405. https://doi.org/10.3390/chemosensors10100405
Rambeloson J, Ioannou DE, Raju P, Wang X, Motayed A, Yun HJ, Li Q. Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature. Chemosensors. 2022; 10(10):405. https://doi.org/10.3390/chemosensors10100405
Chicago/Turabian StyleRambeloson, Jafetra, Dimitris E. Ioannou, Parameswari Raju, Xiao Wang, Abhishek Motayed, Hyeong Jin Yun, and Qiliang Li. 2022. "Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature" Chemosensors 10, no. 10: 405. https://doi.org/10.3390/chemosensors10100405
APA StyleRambeloson, J., Ioannou, D. E., Raju, P., Wang, X., Motayed, A., Yun, H. J., & Li, Q. (2022). Photoactivated In2O3-GaN Gas Sensors for Monitoring NO2 with High Sensitivity and Ultralow Operating Power at Room Temperature. Chemosensors, 10(10), 405. https://doi.org/10.3390/chemosensors10100405






