New Dinitrophenyl Hydrazones as Colorimetric Probes for Anions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
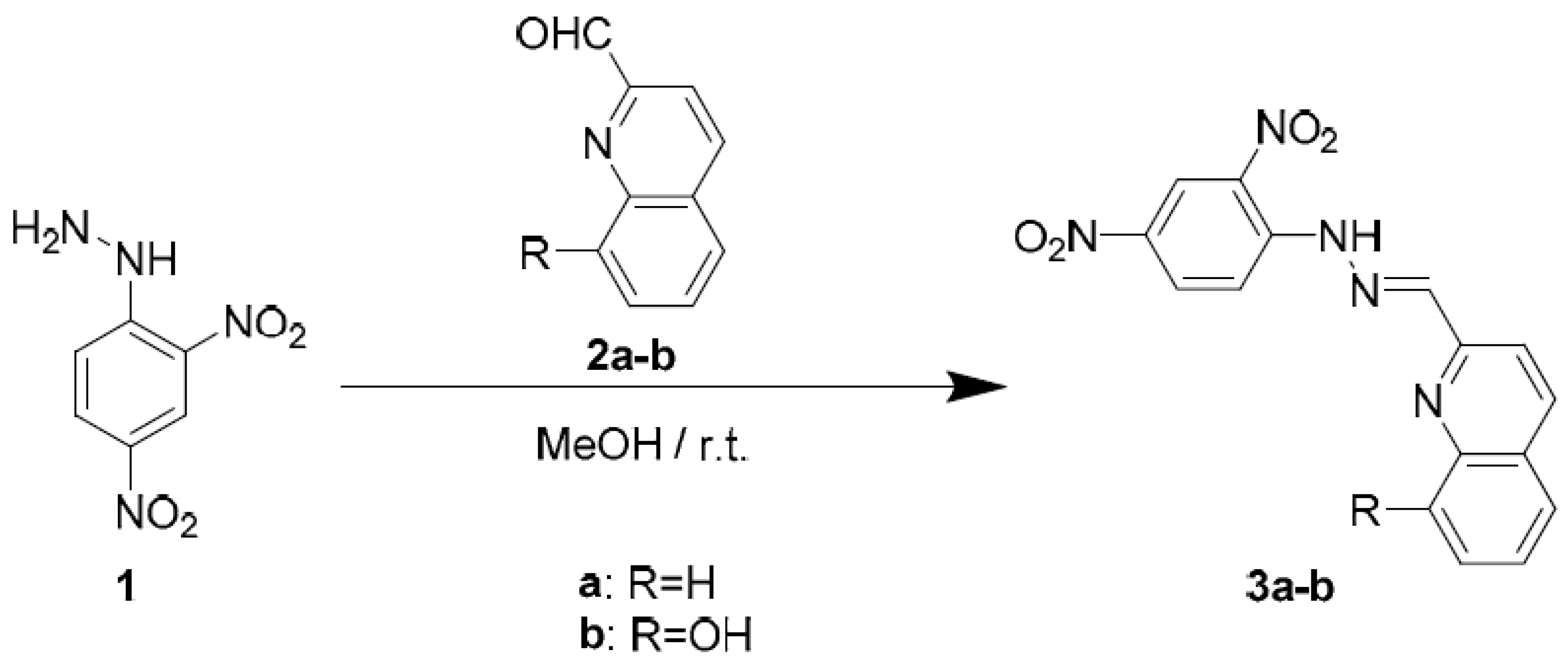
2.2. General Procedure for the Synthesis of Heterocyclic Quinoline-Based Hydrazones
2.2.1. 2-((2-(2,4-Dinitrophenyl)hydrazineylidene)methyl)quinoline 3a
2.2.2. 2-((2-(2,4-Dinitrophenyl)hydrazineylidene)methyl)quinolin-8-ol 3b
2.3. Chemosensing Studies
3. Results and Discussion
3.1. Synthesis of Heterocyclic Quinoline-Based Hydrazones
3.2. Preliminary Chemosensory Tests
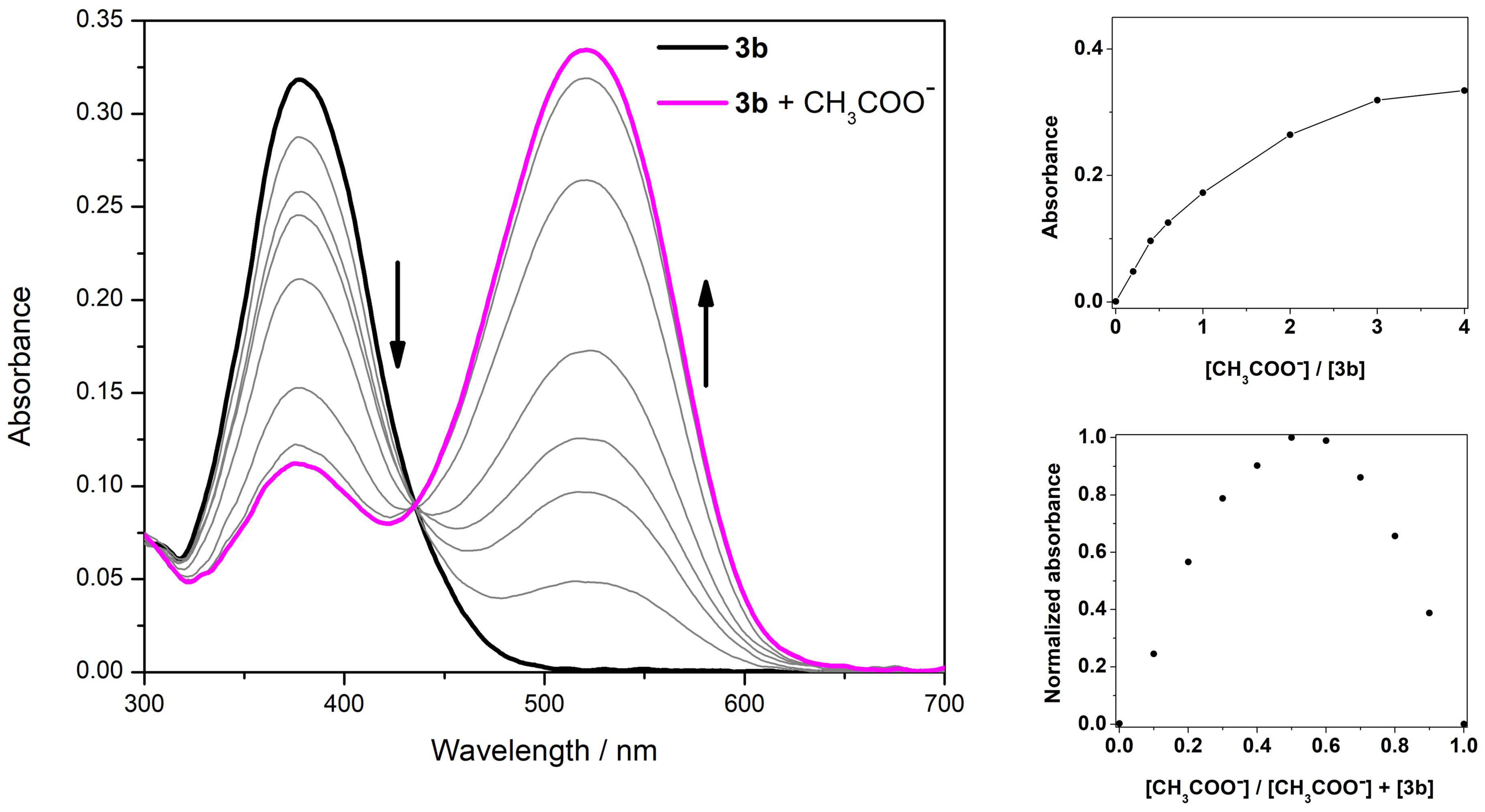
3.3. Spectrophotometric Titrations
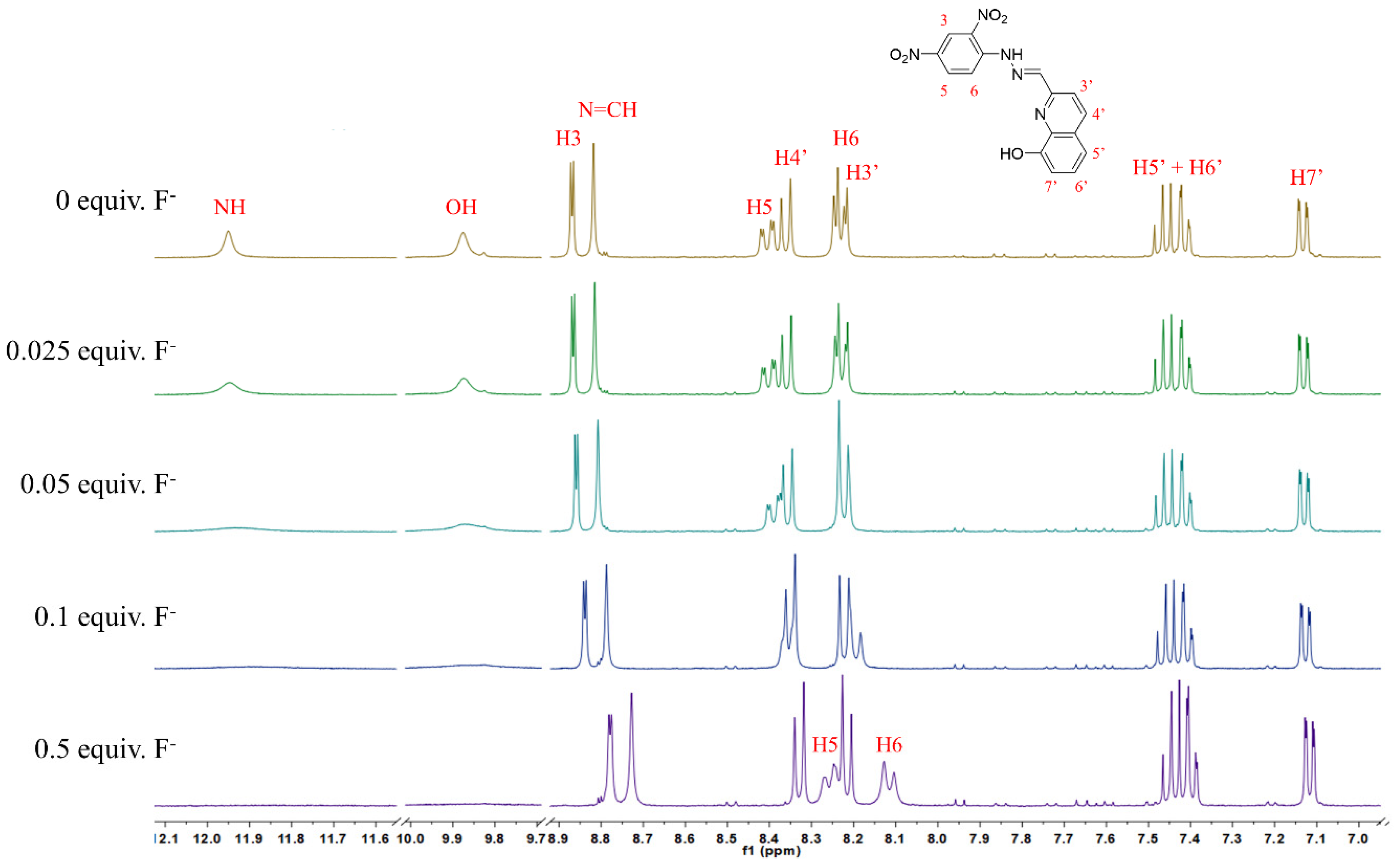
3.4. NMR Titrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blake, A.; Mclean, V. A Calorimetric Consumption Assay for the Measurement by Cultured Ceils of D-Glucose. Anal. Biochem. 1989, 177, 156–160. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.Y.; Chen, C.P.; Lin, C.W. Facile colorimetric detection of human chorionic gonadotropin based on the peptide-induced aggregation of gold nanoparticles. Anal. Methods 2015, 7, 29–33. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Wang, B.; Anslyn, E. Colorimetric sensor design. In Chemosensors: Principles, Strategies, and Applications; Wiley: Hobokan, NJ, USA, 2011; pp. 275–295. [Google Scholar]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.H. A review of the applications of Schiff bases as optical chemical sensors. TrAC—Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Figueira, R.B.; Costa, S.P.G.; Raposo, M.M. Optical fiber sensors for biocide monitoring: Examples, transduction materials, and prospects. ACS Sens. 2020, 5, 3678–3709. [Google Scholar] [CrossRef]
- Junaid, H.M.; Batool, M.; Harun, F.W.; Akhter, M.S.; Shabbir, N. Naked Eye Chemosensing of Anions by Schiff Bases. Crit. Rev. Anal. Chem. 2020, 52, 463–480. [Google Scholar] [CrossRef]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Jali, B.R.; Barick, A.K.; Mohapatra, P.; Sahoo, S.K. A comprehensive review on quinones based fluoride selective colorimetric and fluorescence chemosensors. J. Fluor. Chem. 2021, 244, 109744. [Google Scholar] [CrossRef]
- Udhayakumari, D. Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016. Sens. Actuators B Chem. 2018, 259, 1022–1057. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, X.; Yoon, J. Recent progress in the development of fluorometric and colorimetric chemosensors for detection of cyanide ions. Chem. Soc. Rev. 2014, 43, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2009, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dasgupta, P.K. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Aldrey, A.; Núñez, C.; García, V.; Bastida, R.; Lodeiro, C.; MacÍas, A. Anion sensing properties of new colorimetric chemosensors based on macrocyclic ligands bearing three nitrophenylurea groups. Tetrahedron 2010, 66, 9223–9230. [Google Scholar] [CrossRef]
- Pal, A.; Karmakar, M.; Bhatta, S.R.; Thakur, A. A detailed insight into anion sensing based on intramolecular charge transfer (ICT) mechanism: A comprehensive review of the years 2016 to 2021. Coord. Chem. Rev. 2021, 448, 214167. [Google Scholar] [CrossRef]
- Tay, H.M.; Beer, P. Optical sensing of anions by macrocyclic and interlocked hosts. Org. Biomol. Chem. 2021, 19, 4652–4677. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Jali, B.R.; Baruah, J.B. Recent progress in Schiff bases in detections of fluoride ions. Dyes Pigm. 2021, 194, 109575. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Aljahdali, M.S. Review: Protonation, complex-formation equilibria, and metal–ligand interaction of salicylaldehyde Schiff bases. J. Coord. Chem. 2013, 66, 3423–3468. [Google Scholar] [CrossRef]
- Jabeen, M. A comprehensive review on analytical applications of hydrazone derivatives. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 663–698. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.Y.; Liu, J.; Dong, D. Recent developments in rhodamine salicylidene hydrazone chemosensors. Anal. Methods 2016, 8, 2863–2871. [Google Scholar] [CrossRef]
- Khattab, T.A.; Gaffer, H.E. Synthesis and application of novel tricyanofuran hydrazone dyes as sensors for detection of microbes. Color. Technol. 2016, 132, 460–465. [Google Scholar] [CrossRef]
- Farshbaf, S.; Anzenbacher, P. Fluorimetric sensing of ATP in water by an imidazolium hydrazone based sensor. Chem. Commun. 2019, 55, 1770–1773. [Google Scholar] [CrossRef]
- Wu, W.N.; Wu, H.; Wang, Y.; Mao, X.J.; Liu, B.Z.; Zhao, X.L.; Xu, Z.Q.; Fan, Y.C.; Xu, Z.H. A simple hydrazone as a multianalyte (Cu2+, Al3+, Zn2+) sensor at different pH values and the resultant Al3+ complex as a sensor for F−. RSC Adv. 2018, 8, 5640–5646. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liang, H.; Wen, X.; Li, Z.; Xiong, H.; Tian, Q.; Yan, M.; Tan, Y.; Royal, G. Synchronous colorimetric determination of CN−, F−, and H2PO4− based on structural manipulation of hydrazone sensors. Inorg. Chim. Acta 2022, 532, 120760. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Zhang, C.; Wu, Y.; Liu, Z. Coumarin-hydrazone based high selective fluorescence sensor for copper(II) detection in aqueous solution. Inorg. Chem. Commun. 2013, 34, 8–11. [Google Scholar] [CrossRef]
- Su, X.; Aprahamian, I. Hydrazone-based switches, metallo-assemblies and sensors. Chem. Soc. Rev. 2014, 43, 1963–1981. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of detection in spectroscopy. Spectroscopy 2003, 18, 103. [Google Scholar]
- Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Naphthyl-imidazo-anthraquinones as novel colorimetric and fluorimetric chemosensors for ion sensing. J. Photochem. Photobiol. A Chem. 2013, 259, 33–40. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Selective colorimetric and fluorimetric detection of cyanide in aqueous solution using novel heterocyclic imidazo-anthraquinones. Sens. Actuators B Chem. 2014, 191, 791–799. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.M. An investigation of the deprotonation of hydrazone-based receptors on interaction with anion: Develop a colorimetric system distinguishing cyanide from anions. Tetrahedron 2013, 69, 10267–10271. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Cyanide and fluoride colorimetric sensing by novel imidazo-anthraquinones functionalised with indole and carbazole. Supramol. Chem. 2014, 26, 71–80. [Google Scholar] [CrossRef]

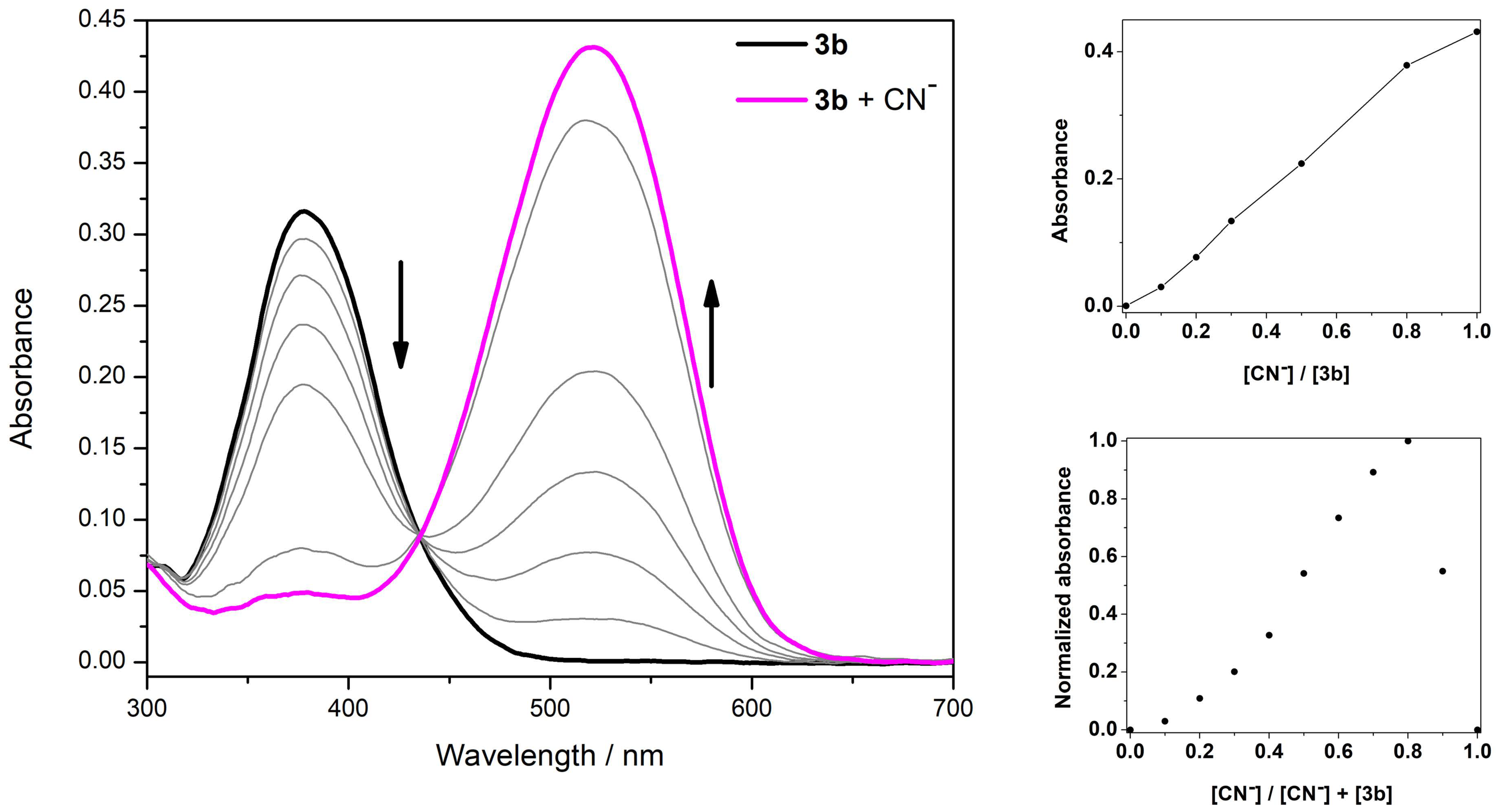
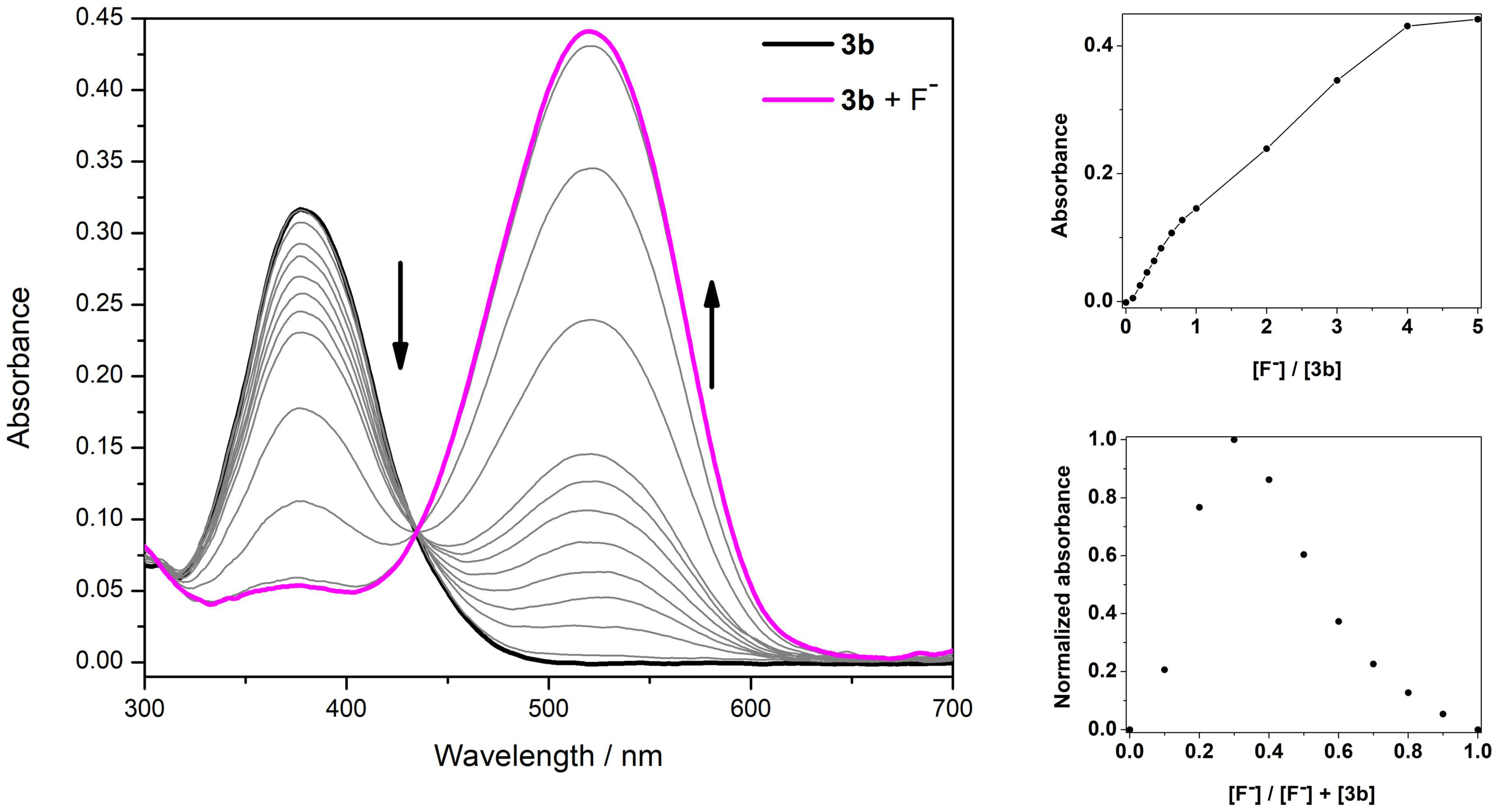
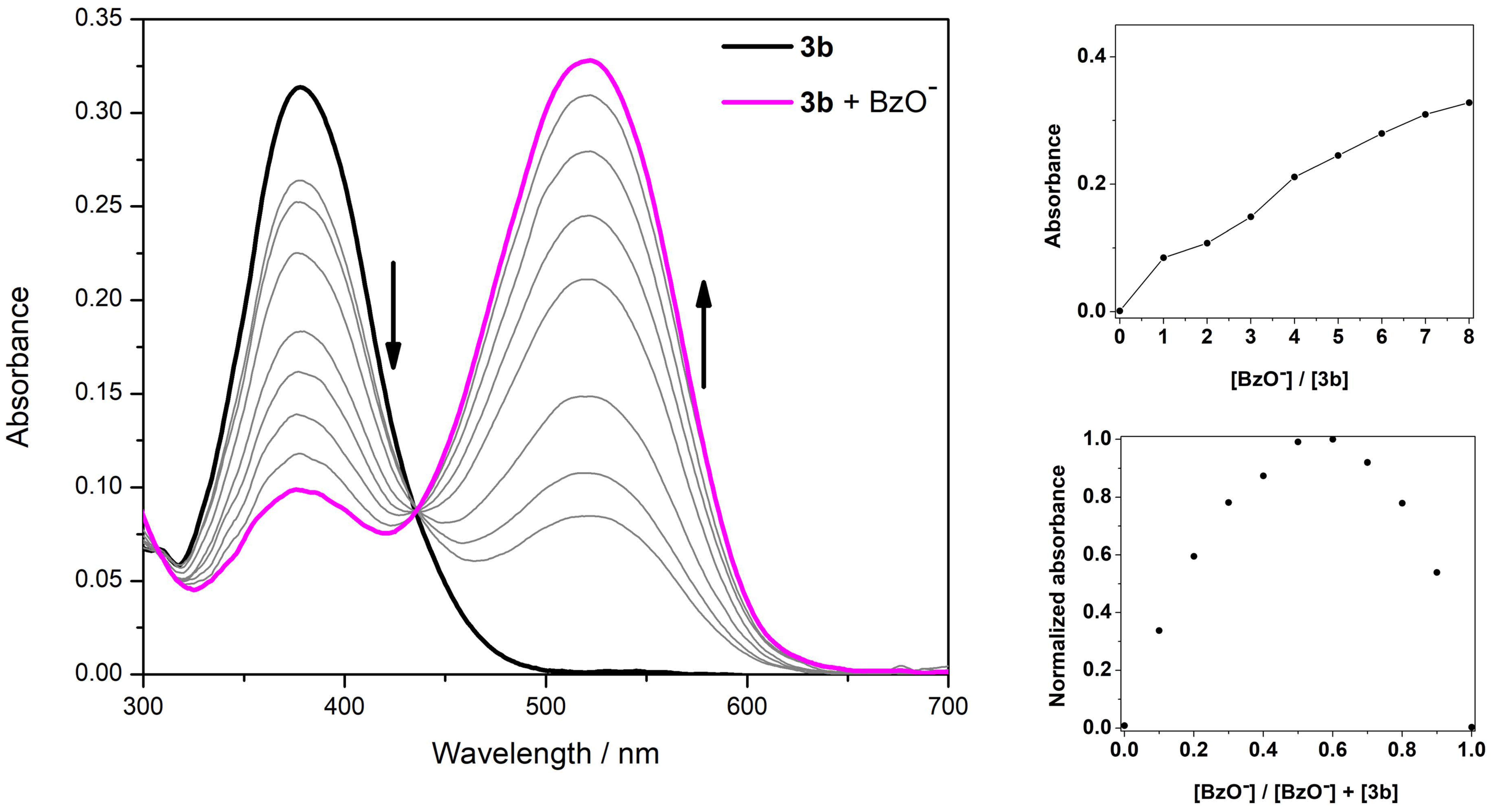


| Compound | CN− | F− | H2PO4− | BzO− | CH3COO− | |
|---|---|---|---|---|---|---|
| LOD (µM) | 3a | 0.58 | 1.35 | 3.03 | 3.13 | 1.20 |
| 3b | 0.35 | 1.21 | 2.27 | 3.22 | 0.90 | |
| LOQ (µM) | 3a | 1.92 | 4.45 | 9.99 | 10.33 | 3.96 |
| 3b | 1.17 | 3.99 | 7.48 | 10.63 | 2.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, R.P.C.L.; Costa, S.P.G.; Figueira, R.B.; Raposo, M.M.M. New Dinitrophenyl Hydrazones as Colorimetric Probes for Anions. Chemosensors 2022, 10, 384. https://doi.org/10.3390/chemosensors10100384
Sousa RPCL, Costa SPG, Figueira RB, Raposo MMM. New Dinitrophenyl Hydrazones as Colorimetric Probes for Anions. Chemosensors. 2022; 10(10):384. https://doi.org/10.3390/chemosensors10100384
Chicago/Turabian StyleSousa, Rui P. C. L., Susana P. G. Costa, Rita B. Figueira, and M. Manuela M. Raposo. 2022. "New Dinitrophenyl Hydrazones as Colorimetric Probes for Anions" Chemosensors 10, no. 10: 384. https://doi.org/10.3390/chemosensors10100384







