Ionophore-Based Potassium Selective Fluorescent Organosilica Nano-Optodes Containing Covalently Attached Solvatochromic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of the Solvatochromic Dye SD
2.3. Preparation of the Potassium Selective Organosilica Nano-Optodes
2.4. Preparation of Potassium Responsive Nylon Film
2.5. Instrumentation and Measurements
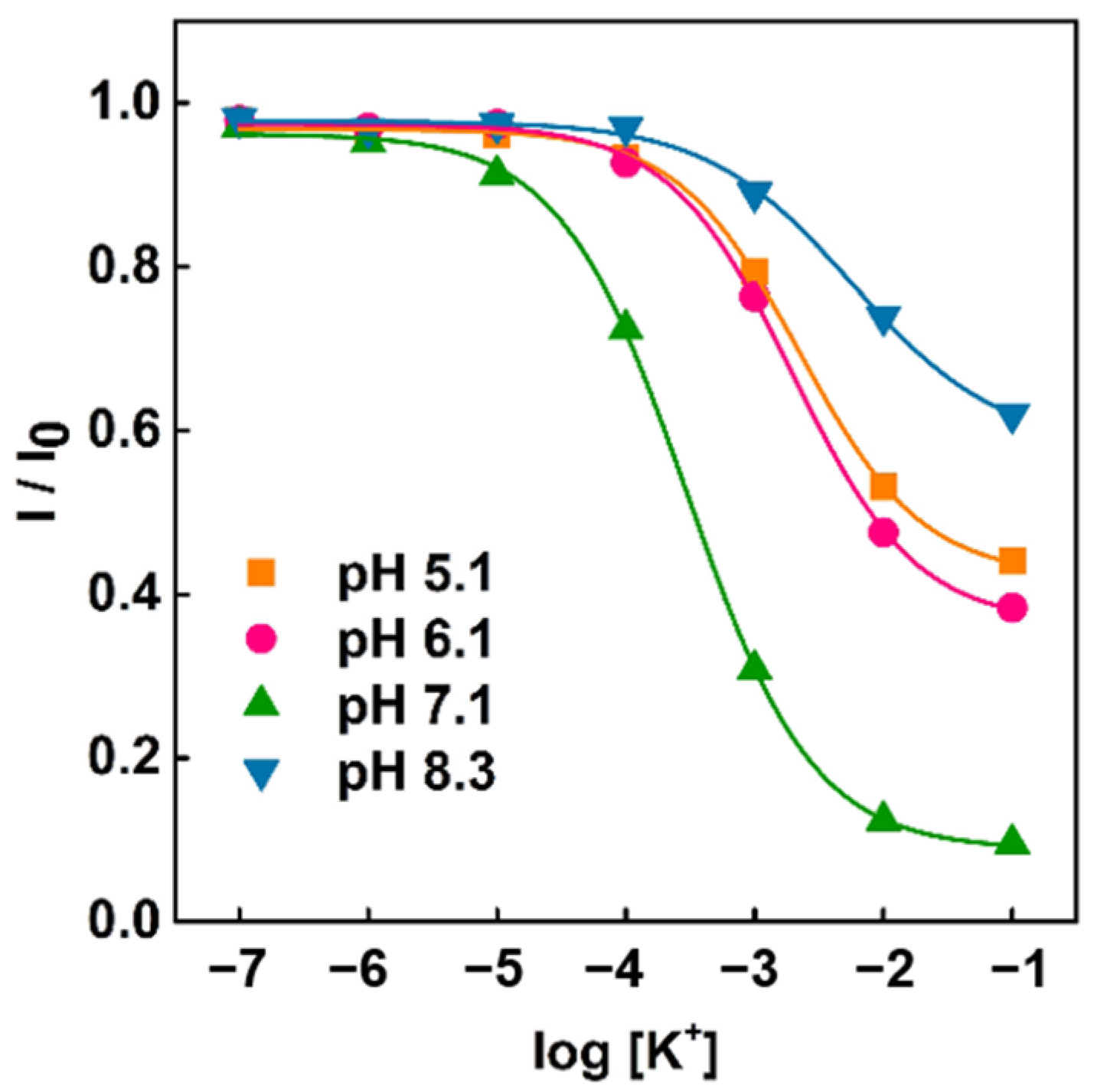
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Ng, R.H.; Sparks, K.M.; Statland, B.E. Colorimetric Determination of Potassium in Plasma and Serum by Reflectance Photometry with a Dry-Chemistry Reagent. Clin. Chem. 1992, 38, 1371–1372. [Google Scholar] [CrossRef]
- Brasuel, M.; Kopelman, R.; Miller, T.J.; Tjalkens, R.; Philbert, M.A. Fluorescent Nanosensors for Intracellular Chemical Analysis: Decyl Methacrylate Liquid Polymer Matrix and Ion-Exchange-Based Potassium PEBBLE Sensors with Real-Time Application to Viable Rat C6 Glioma Cells. Anal. Chem. 2001, 73, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Koronczi, I.; Reichert, J.; Heinzmann, G.; Ache, H.J. Development of a submicron optochemical potassium sensor with enhanced stability due to internal reference. Sens. Actuators B Chem. 1998, 51, 188–195. [Google Scholar] [CrossRef]
- Kong, D.-M.; Ma, Y.-E.; Guo, J.-H.; Yang, W.; Shen, H.-X. Fluorescent Sensor for Monitoring Structural Changes of G-Quadruplexes and Detection of Potassium Ion. Anal. Chem. 2009, 81, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, S.-Y.; Rancic, V.; Aggarwal, A.; Qian, Y.; Miyashita, S.-I.; Ballanyi, K.; Campbell, R.E.; Dong, M. Genetically encoded fluorescent indicators for imaging intracellular potassium ion concentration. Commun. Biol. 2019, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, B.T.T.; Ang, J.Q.; Toh, C.-S. Sensitive detection of potassium ion using Prussian blue nanotube sensor. Electrochem. Commun. 2009, 11, 1861–1864. [Google Scholar] [CrossRef]
- Mistlberger, G.; Crespo, G.A.; Bakker, E. Ionophore-Based Optical Sensors. Annu. Rev. Anal. Chem. 2014, 7, 483–512. [Google Scholar] [CrossRef] [Green Version]
- Kisiel, A.; Maksymiuk, K.; Michalska, A. Capsules as ion-selective optodes—Maximizing sensitivity of ion-selective optodes. Sens. Actuators B Chem. 2018, 273, 1730–1734. [Google Scholar] [CrossRef]
- Rong, G.; Kim, E.H.; Poskanzer, K.E.; Clark, H.A. A method for estimating intracellular ion concentration using optical nanosensors and ratiometric imaging. Sci. Rep. 2017, 7, 10819. [Google Scholar] [CrossRef] [Green Version]
- Ruckh, T.T.; Mehta, A.A.; Dubach, J.M.; Clark, H.A. Polymer-Free Optode Nanosensors for Dynamic, Reversible and Ratiometric Sodium Imaging in the Physiological Range. Sci. Rep. 2013, 3, 3366. [Google Scholar] [CrossRef] [Green Version]
- Kondratyeva, Y.O.; Tolstopjatova, E.G.; Kirsanov, D.O.; Mikhelson, K.N. Chronoamperometric and coulometric analysis with ionophore-based ion-selective electrodes: A modified theory and the potassium ion assay in serum samples. Sens. Actuators B Chem. 2020, 310, 127894. [Google Scholar] [CrossRef]
- Johnson, R.D.; Bachas, L.G. Ionophore-based ion-selective potentiometric and optical sensors. Anal. Bioanal. Chem. 2003, 376, 328–341. [Google Scholar] [CrossRef]
- Kłucińska, K.; Stelmach, E.; Kisiel, A.; Maksymiuk, K.; Michalska, A. Nanoparticles of Fluorescent Conjugated Polymers: Novel Ion-Selective Optodes. Anal. Chem. 2016, 88, 5644–5648. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.S.; Katageri, A.G.; Gohring, G.M.; Cash, K.J. A dual-indicator strategy for controlling the response of ionophore-based optical nanosensors. Sens. Actuators B Chem. 2018, 256, 674–681. [Google Scholar] [CrossRef]
- Stelmach, E.; Kłucińska, K.; Maksymiuk, K.; Michalska, A. Rational design of nanoptodes architecture—Towards multifunctional sensors. Talanta 2019, 196, 226–230. [Google Scholar] [CrossRef]
- Dailey, A.L.; Greer, M.D.; Sodia, T.Z.; Jewell, M.P.; Kalin, T.A.; Cash, K.J. LipiSensors: Exploiting Lipid Nanoemulsions to Fabricate Ionophore-Based Nanosensors. Biosensors 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin-Ince, G.; Dubach, J.M.; Gleason, K.K.; Clark, H.A. Microworm optode sensors limit particle diffusion to enable in vivo measurements. Proc. Natl. Acad. Sci. USA 2011, 108, 2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, C.; Werner, T.; Huber, C.; Wolfbeis, O.S. Emulsion-Based Fluorosensors for Potassium Featuring Improved Stability and Signal Change. Anal. Chem. 1999, 71, 5304–5308. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Lerchi, M.; Rosatzin, T.; Rusterholz, B.; Simon, W. Synthesis and characterization of neutral hydrogen ion-selective chromoionophores for use in bulk optodes. Anal. Chim. Acta 1993, 278, 211–225. [Google Scholar] [CrossRef]
- Lee, C.H.; Folz, J.; Zhang, W.; Jo, J.; Tan, J.W.Y.; Wang, X.; Kopelman, R. Ion-Selective Nanosensor for Photoacoustic and Fluorescence Imaging of Potassium. Anal. Chem. 2017, 89, 7943–7949. [Google Scholar] [CrossRef] [Green Version]
- Kisiel, A.; Baniak, B.; Maksymiuk, K.; Michalska, A. Ion-selective reversing aggregation-caused quenching—Maximizing optodes signal stability. Talanta 2020, 220, 121358. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Du, X.; Ma, X.; Zhai, J.; Xie, X. Ionophore-based pH independent detection of ions utilizing aggregation-induced effects. Analyst 2020, 145, 3846–3850. [Google Scholar] [CrossRef]
- Di, W.; Tan, X.; Calderon, I.A.C.; Reilly, A.E.N.; Niedre, M.; Clark, H.A. Real-time particle-by-particle detection of erythrocyte-camouflaged microsensor with extended circulation time in the bloodstream. Proc. Natl. Acad. Sci. USA 2020, 117, 3509. [Google Scholar] [CrossRef] [PubMed]
- Balaconis, M.K.; Clark, H.A. Biodegradable Optode-Based Nanosensors for in Vivo Monitoring. Anal. Chem. 2012, 84, 5787–5793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruckh, T.T.; Skipwith, C.G.; Chang, W.; Senko, A.W.; Bulovic, V.; Anikeeva, P.O.; Clark, H.A. Ion-Switchable Quantum Dot Förster Resonance Energy Transfer Rates in Ratiometric Potassium Sensors. ACS Nano 2016, 10, 4020–4030. [Google Scholar] [CrossRef] [Green Version]
- Sahari, A.; Ruckh, T.T.; Hutchings, R.; Clark, H.A. Development of an Optical Nanosensor Incorporating a pH-Sensitive Quencher Dye for Potassium Imaging. Anal. Chem. 2015, 87, 10684–10687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, M.P.; Greer, M.D.; Dailey, A.L.; Cash, K.J. Triplet-Triplet Annihilation Upconversion Based Nanosensors for Fluorescence Detection of Potassium. ACS Sens. 2020, 5, 474–480. [Google Scholar] [CrossRef]
- Du, X.; Yang, L.; Hu, W.; Wang, R.; Zhai, J.; Xie, X. A Plasticizer-Free Miniaturized Optical Ion Sensing Platform with Ionophores and Silicon-Based Particles. Anal. Chem. 2018, 90, 5818–5824. [Google Scholar] [CrossRef]
- Xie, X.; Gutiérrez, A.; Trofimov, V.; Szilagyi, I.; Soldati, T.; Bakker, E. Charged Solvatochromic Dyes as Signal Transducers in pH Independent Fluorescent and Colorimetric Ion Selective Nanosensors. Anal. Chem. 2015, 87, 9954–9959. [Google Scholar] [CrossRef]
- Xie, X.; Szilagyi, I.; Zhai, J.; Wang, L.; Bakker, E. Ion-Selective Optical Nanosensors Based on Solvatochromic Dyes of Different Lipophilicity: From Bulk Partitioning to Interfacial Accumulation. ACS Sens. 2016, 1, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Zhai, J.; Bakker, E. Reversible pH-independent optical potassium sensor with lipophilic solvatochromic dye transducer on surface modified microporous nylon. Chem. Commun. 2016, 52, 14254–14257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bakker, E. A tunable detection range of ion-selective nano-optodes by controlling solvatochromic dye transducer lipophilicity. Chem. Commun. 2019, 55, 12539–12542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Du, X.; Xie, X. Ionophore-Based Potassium Selective Fluorescent Organosilica Nano-Optodes Containing Covalently Attached Solvatochromic Dyes. Chemosensors 2022, 10, 23. https://doi.org/10.3390/chemosensors10010023
Zhang Y, Du X, Xie X. Ionophore-Based Potassium Selective Fluorescent Organosilica Nano-Optodes Containing Covalently Attached Solvatochromic Dyes. Chemosensors. 2022; 10(1):23. https://doi.org/10.3390/chemosensors10010023
Chicago/Turabian StyleZhang, Yupu, Xinfeng Du, and Xiaojiang Xie. 2022. "Ionophore-Based Potassium Selective Fluorescent Organosilica Nano-Optodes Containing Covalently Attached Solvatochromic Dyes" Chemosensors 10, no. 1: 23. https://doi.org/10.3390/chemosensors10010023
APA StyleZhang, Y., Du, X., & Xie, X. (2022). Ionophore-Based Potassium Selective Fluorescent Organosilica Nano-Optodes Containing Covalently Attached Solvatochromic Dyes. Chemosensors, 10(1), 23. https://doi.org/10.3390/chemosensors10010023




