Sensors to the Diagnostic Assessment of Anticancer and Antimicrobial Therapies Effectiveness by Drugs a with Pyrazine Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Electrochemical Measurements
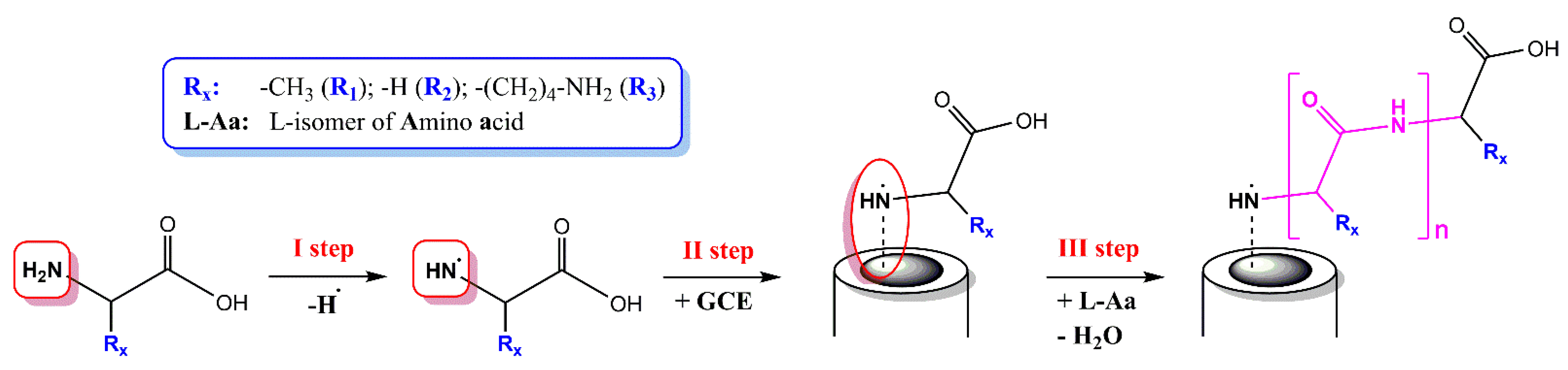
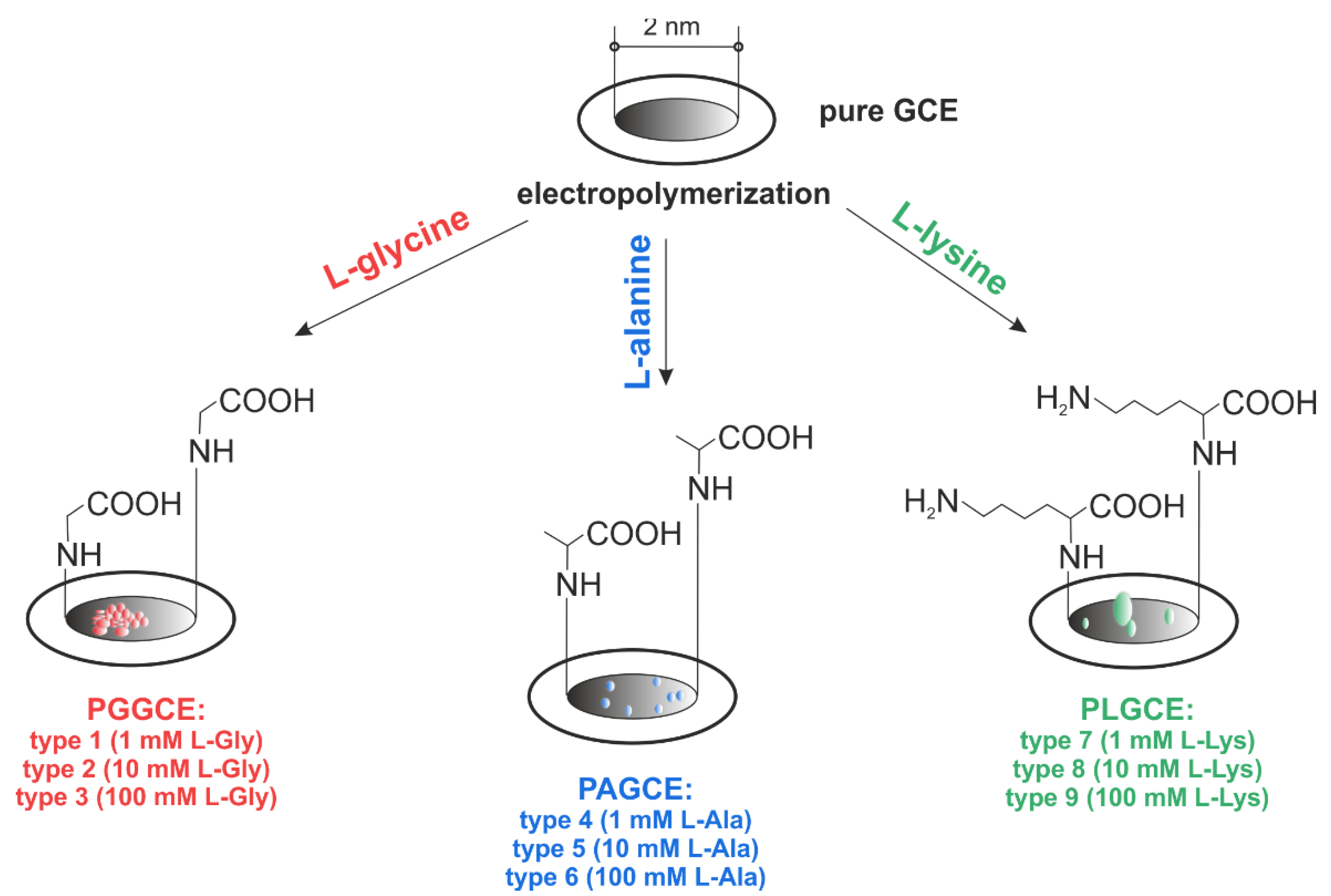
2.3. Electroplolymerization of GCE and Poly-L-Glycine Glassy Carbon Electrodes (PGGCE) Preparing
2.4. Poly-L-Alanine Glassy Carbon Electrodes (PAGCE) Formatting
2.5. Poly-L-Lysine Glassy Carbon Electrodes (PLGCE) Receiving
2.6. Cleaning and Re-Modification of Electrodes
2.7. Microscopic Imaging
3. Results and Discussion
3.1. Determining the Range of Potentials
3.2. Scan Speed Optimization
3.3. Structural Studies of Modified Electrodes
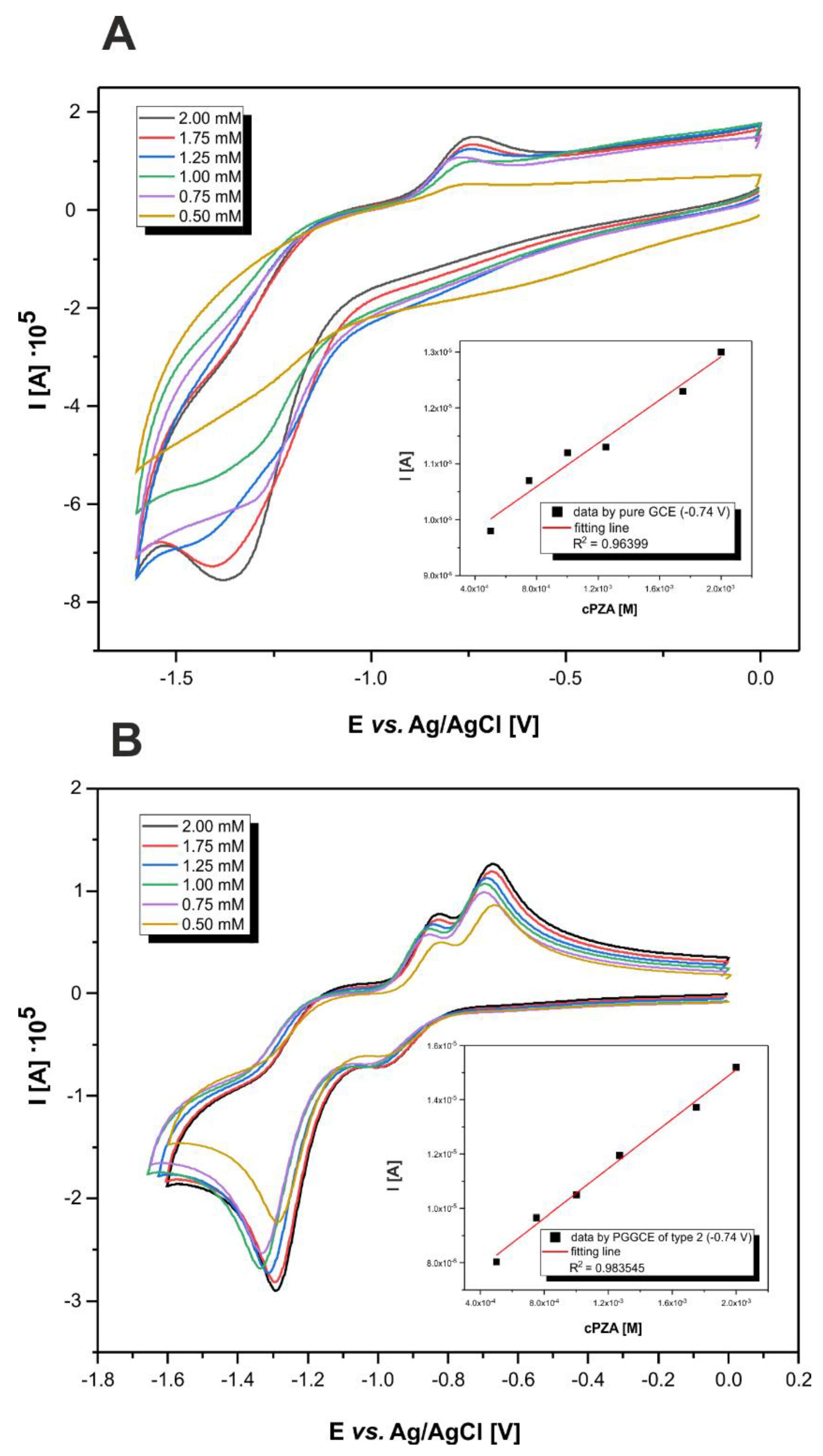
3.4. Analyses with the Use of Modified Electrodes
3.5. Analyses with the Use of Re-Modified Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goh, Z.; Tee, J.K.; Ho, K.H. An Evaluation of the In Vitro Roles and Mechanisms of Silibinin in Reducing Pyrazinamide- and Isoniazid-Induces Hepatocellular Damage. Int. J. Mol. Sci. 2020, 21, 3714. [Google Scholar] [CrossRef]
- Ahmad, Z.; Makaya, N.H.; Grosset, J. History of Drug Discovery: Early Evaluation Studies and Lessons Learnt from Them. Antituber. Chemother. 2011, 40, 2–9. [Google Scholar]
- Zhang, Y.; Mitchison, D. The curious characteristics of pyrazinamide: A review. Int. J. Tuberc. Lung Dis. 2003, 7, 6–21. [Google Scholar]
- Ogryzek, M.; Chylewska, A.; Królicka, A.; Banasiuk, R.; Turecka, K.; Lesiak, D.; Nidzworski, D.; Makowski, M. Coordination chemistry of pyrazine derivatives analogues of PZA: Design, synthesis, characterization and biological activity. RSC Adv. 2016, 6, 64499–64512. [Google Scholar] [CrossRef]
- Yamamoto, S.; Toida, I.; Watanabe, N.; Ura, T. In Vitro Antimycobacterial Activities of Pyrazinamide Analogs. Antimicrob. Agents Chemother. 1995, 9, 2088–2091. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chylewska, A.; Sikorski, A.; Ogryzek, M.; Makowski, M. Attractive S⋯π and π-π interactions in the pyrazine-2-thiocarboxamide structure: Experimental and computational studies in the context of crystal engineering and microbiological properties. J. Mol. Structure 2016, 1105, 96–104. [Google Scholar] [CrossRef]
- Głębocka, A.; Raczyńska, E.D.; Chylewska, A.; Makowski, M. Experimental (FT-IR) and theoretical (DFT) studies on prototropy and H-bond formation for pyrazine-2-amidoxime. J. Phys. Org. Chem. 2016, 29, 326–335. [Google Scholar] [CrossRef]
- Chylewska, A.; Ogryzek, M.; Głębocka, A.; Sikorski, A.; Turecka, K.; Raczyńska, E.D.; Makowski, M. Crystalline pyrazine-2-amidoxime isolated by diffusion method and its structural and behavioral analysis in the context of crystal engineering and microbiological activity. RSC Adv. 2016, 6, 64499–64512. [Google Scholar] [CrossRef]
- Colson, K.; Doss, D.S.; Swift, R.; Tariman, J. Bortezomib, a newly approved proteasome inhibitor for the treatment of multiple myeloma: Nursing implications. Clin. J. Oncol. Nurs. 2004, 8, 473–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piechotta, V.; Jakob, T.; Langer, P.; Monsef, I.; Scheid, C.; Estcourt, L.J.; Ocheni, S.; Theurich, S.; Kuhr, K.; Scheckel, B.; et al. Multiple drug combinations of bortezomib, lenslidomide, and thalidomide for first- line treatment in adults with transplant- ineligible multiple myleoma: A network meta- analysis. Cochrane Database Syst. Rev. 2019, 25, 1–146. [Google Scholar]
- Chauhan, D.; Li, G.; Podar, K.; Hideshima, T.; Shringarpure, R.; Catley, L.; Mitsiades, C.; Munshi, N.; Tai, Y.T.; Suh, N.; et al. The bortezomib/proteasome inhibitor PS-341 and triterpenoid CDDO-Im induce synergistic anti–multiple myeloma (MM) activity and overcome bortezomib resistance. Blood 2004, 103, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Kauffman, M. Development of the Proteasome Inhibitor Velcade™ (Bortezomib). Canc. Investig. 2004, 22, 304–311. [Google Scholar] [CrossRef]
- Schaberg, T.; Rebhan, K.; Lode, H. Risk factors of side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur. Respir J. 1996, 9, 2026–2030. [Google Scholar] [CrossRef]
- Yee, D.; Valiquette, C.; Pelletier, M.; Parisien, I.; Rocher, I.; Menzies, D. Incidence of Serious Side Effects from First-Line Antituberculosis Drugs among Patients Treated for Active Tuberculosis. Am. J. Respir. Crit. Car. Med. 2001, 167, 1472–1477. [Google Scholar] [CrossRef]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Reece, D.E.; Sullivan, D.; Lonial, S.; Mohrbacher, A.F.; Chatta, G.; Shustik, C.; Stewart, A. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Canc. Chem. Pharm. 2010, 67, 57–67. [Google Scholar] [CrossRef][Green Version]
- Mehashe, J. Managing and avoiding bortezomib toxicity. Comm. Oncol. 2007, 4, 480–484. [Google Scholar]
- Pasipanodya, J.G.; Gumbo, T. Clinical and Toxicodynamic Evidence that High-Dose Pyrazinamide Is Not More Hepatotoxic than the Low Doses Currently Used. Antim. Agents Chem. 2010, 54, 2847–2854. [Google Scholar] [CrossRef]
- Sandulescu, R.; Mirel, S.; Oprean, R. The development of spectrophotometric and electroanalytical methods for ascorbic acid and aminophen and their applications in the analyse od effervescent dosage forms. J. Pharm. Biomed. Anal. 2000, 23, 77–78. [Google Scholar] [CrossRef]
- Mani, S.; Cheemalapati, S.; Chen, S.M.; Devadas, B. Anti-tuberculosis Drug Pyrazinamide Determination at Multiwalled Carbon Nanotubes/Graphene Oxide Hybrid Composite Fabricated Electrode. Int. J. Electrochem. Sci. 2015, 10, 7049–7062. [Google Scholar]
- Mulyani, E.; Darmawan, E.; Perwitasari, D.A.; Mulyani, U.A.; Atthobari, J. Validation of pyrazinamide in human plasma using Hplc-Uv for therapeutic drug monitoring. AIP Conf. Proc. 2016, 1746, 20–31. [Google Scholar]
- Conte, J.E.; Lin, E.; Zurlinden, E. High- Performance Liquid Chromatographic Determination of Pyrazinamide in Human Plasma Bronchoalveolar LAvage Fluid, and Alveolar Cells. J. Chrom. Sci. 2000, 38, 33–37. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, M.; Hu, Y.; Zhu, L.; Du, J. An electrochemical sensor based on molecularly imprinted polymer for determination of anticancer drug Mitoxantrone. Sens. Act. B 2018, 255, 544–551. [Google Scholar] [CrossRef]
- Ning, Y.N.; Xiao, B.L.; Niu, N.N.; Moosavi-Movahedi, A.A.; Hong, J. Glucose Oxidase Immobilized on a Functional Polymer Modified Glassy Carbon Electrode and Its Molecule Recognition of Glucose. Polymers 2019, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, M.F.; Santos, D.P.; Zanoni, M.V.B. Determination of isoniazid in human urine using screen-printed carbon electrode modified with poly-L-histidine. Bioelectrochemistry 2010, 77, 133–138. [Google Scholar] [CrossRef]
- Vega, D.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical detection of phenolic estrogenic compounds at carbon nanotube-modified electrodes. Talanta 2007, 71, 1031–1038. [Google Scholar] [CrossRef]
- Venkataprasad, G.; Reddy, T.M.; Shaikshavali, P.; Gopal, P.A. Novel Electrochemical Sensor Based on Multi-walled Carbon Nanotubes/Poly (L-Methionine) for the Investigation of 5-Nitroindazole: A Voltammetric Study. Anal. Chem. Lett. 2018, 8, 457–474. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Maleki, A.A.; Safshekan, S. Simultaneous and sensitive detection of dopamine and uric acid using a poly(L-methionine)/gold nanoparticle-modified glassy carbon electrode. Chin. J. Cat. 2014, 35, 423–429. [Google Scholar] [CrossRef]
- Gomes, E.S.; Leite, F.R.F.; Ferraz, B.R.L.; Mourão, H.A.J.L.; Malagutti, A.R. Voltammetric sensor based on cobalt-poly(methionine)-modified glassy carbon electrode for determination of estriol hormone in pharmaceuticals and urine. J. Pharm. Anal. 2019, 9, 347–357. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Devadas, B.; Chen, S.M. Highly sensitive and selective determination of pyrazinamide at poly-l-methionine/reduced graphene oxide modified electrode by differential pulse voltammetry in human blood plasma and urine samples. J. Coll. Int. Sci. 2014, 418, 132–139. [Google Scholar] [CrossRef]
- Devadas, B.; Cheemalapati, S.; Chen, S.M. Highly sensing graphene oxide/poly-arginine-modified electrode for the simultaneous electrochemical determination of buspirone, isoniazid and pyrazinamide drugs. Ionics 2015, 21, 547–555. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Devadas, B.; Chen, S.M.; Ali, M.A.; Al- Hemaid, F.M.A. Electrochemical determination of selected antihypertensive and antituberculosis drugs at tyrosine modified electrode. Anal. Meth. 2014, 6, 6774–6782. [Google Scholar] [CrossRef]
- Curran, M.P.; McKeage, K. Bortezomib A Review of its Use in Patients with Multiple Myeloma. Drugs 2009, 69, 859–888. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697–702. [Google Scholar] [CrossRef]
- Ferraz, B.R.L.; Leite, F.R.F. Malagutti, Highly sensitive electrocatalytic determination of pyrazinamide using a modified poly(glycine) glassy carbon electrode by square-wave voltammetry. J. Sol. State Electrochem. 2016, 20, 2509–2516. [Google Scholar] [CrossRef]
- Narayana, P.V.; Reddy, T.M.; Gopal, P.; Naidu, G.R. Electrochemical sensing of paracetamol and its simultaneous resolution in the presence of dopamine and folic acid at multi-walled carbon nanotubes/poly(glycine) composite modified electrode: A voltammetric study. Anal. Meth. 2014, 6, 9459–9468. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Song, G.; Li., K.; Zhang, K.; Ye, B. Sensitive voltammetric sensor for bergenin based on poly(l-lysine)/graphene modified glassy carbon electrode. Anal. Meth. 2013, 5, 3895–3902. [Google Scholar] [CrossRef]

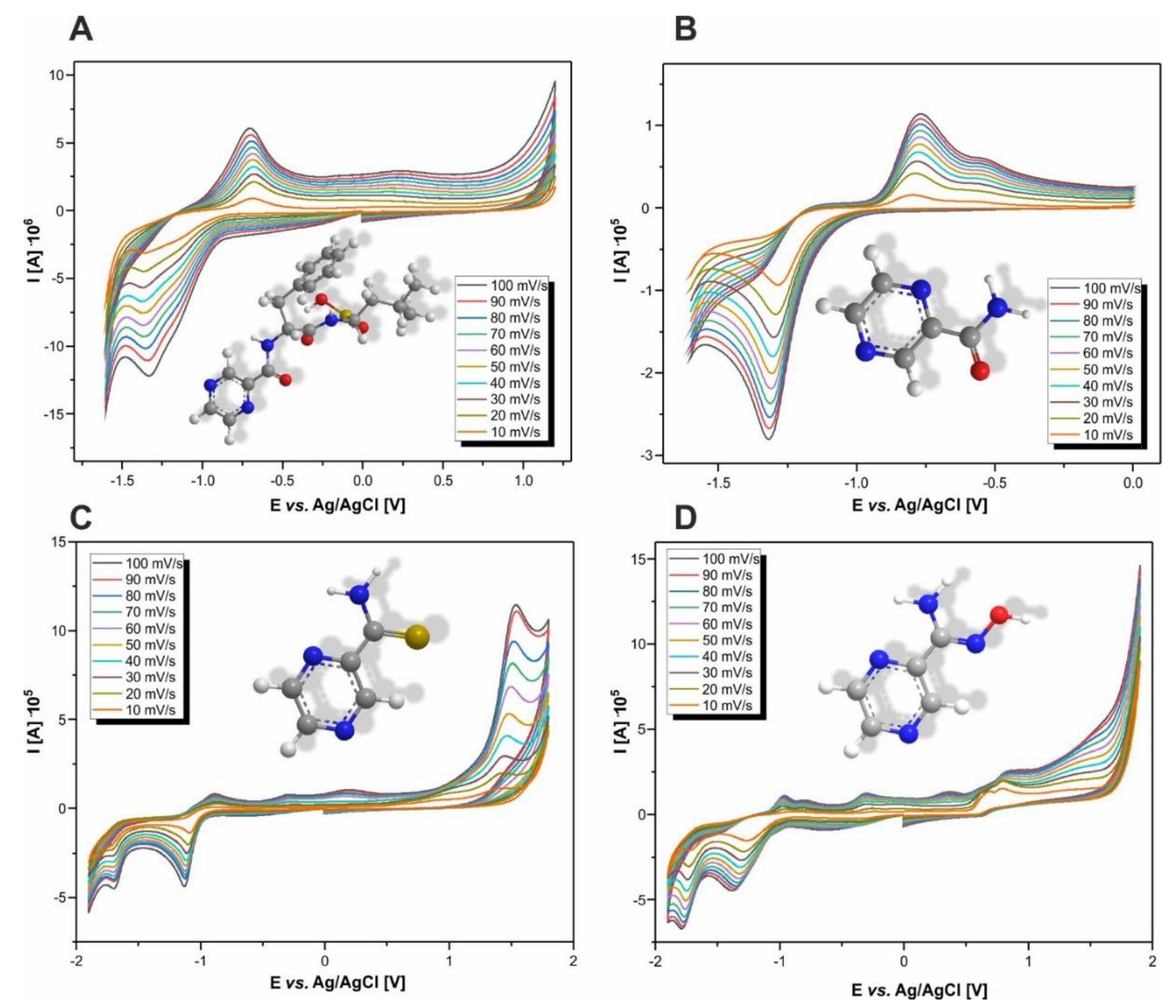




| Compound | Potentials Range (V) | Cathode Peak Potential (V) | Anode Peak Potential (V) |
|---|---|---|---|
| BZM | −1.60 ÷ 1.20 | −1.33 | −0.71 |
| PZA | −1.60 ÷ 0.00 | −1.32 | −0.77 |
| PTCA | −1.90 ÷ 1.80 | −1.13 −1.71 | 1.53 −0.87 |
| PAOX | −1.90 ÷ 1.90 | −1.36 −1.78 | 0.36 0.86 −0.31 −0.97 |
| PGGCE | PAGCE | PLGCE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 | Type 8 | Type 9 | |
| Modified GCE (μm) | 3.15 | 3.87 | 3.75 | 3.23 | 7.90 | 4.35 | 6.37 | 4.32 | 2.65 |
| Clean GCE (μm) | 1.01 | 1.28 | 1.59 | 1.35 | 5.91 | 2.21 | 4.59 | 2.24 | 1.33 |
| Thickness of coating (μm) | 2.14 | 2.59 | 2.16 | 1.88 | 1.99 | 2.14 | 1.78 | 2.08 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domżalska, M.; Dąbrowska, A.M.; Chojnowski, D.; Makowski, M.; Chylewska, A. Sensors to the Diagnostic Assessment of Anticancer and Antimicrobial Therapies Effectiveness by Drugs a with Pyrazine Scaffold. Chemosensors 2022, 10, 24. https://doi.org/10.3390/chemosensors10010024
Domżalska M, Dąbrowska AM, Chojnowski D, Makowski M, Chylewska A. Sensors to the Diagnostic Assessment of Anticancer and Antimicrobial Therapies Effectiveness by Drugs a with Pyrazine Scaffold. Chemosensors. 2022; 10(1):24. https://doi.org/10.3390/chemosensors10010024
Chicago/Turabian StyleDomżalska, Marta, Aleksandra M. Dąbrowska, Dawid Chojnowski, Mariusz Makowski, and Agnieszka Chylewska. 2022. "Sensors to the Diagnostic Assessment of Anticancer and Antimicrobial Therapies Effectiveness by Drugs a with Pyrazine Scaffold" Chemosensors 10, no. 1: 24. https://doi.org/10.3390/chemosensors10010024
APA StyleDomżalska, M., Dąbrowska, A. M., Chojnowski, D., Makowski, M., & Chylewska, A. (2022). Sensors to the Diagnostic Assessment of Anticancer and Antimicrobial Therapies Effectiveness by Drugs a with Pyrazine Scaffold. Chemosensors, 10(1), 24. https://doi.org/10.3390/chemosensors10010024