Socioeconomic Impact of Recurrent Primary Spontaneous Pneumothorax: Should Video-Assisted Thoracoscopic Surgery Be Considered at First Episode of Primary Spontaneous Pneumothorax?
Abstract
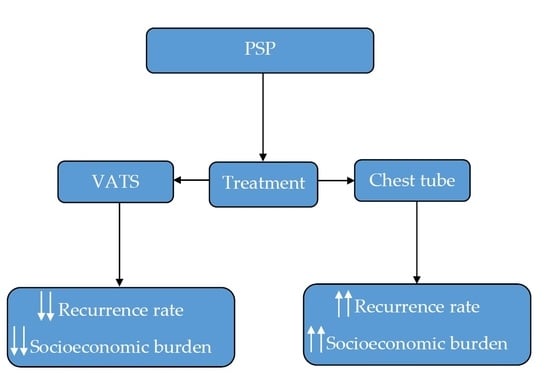
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedure—VATS
2.2. Chest Tube (CT) Treatment
2.3. Statistical Analysis
3. Results
3.1. Length of Hospital Stay (LOS)
3.2. Duration of Chest Tube Drainage
3.3. Treatment of Complications
3.4. Management Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnell, J.; Beer, M.; Eggeling, S.; Gesierich, W.; Gottlieb, J.; Herth, F.J.; Hofmann, H.-S.; Jany, B.; Kreuter, M.; Ley-Zaporozhan, J.; et al. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration 2018, 97, 370–402. [Google Scholar] [CrossRef] [PubMed]
- Sahn, S.A.; Heffner, J.E. Spontaneous pneumothorax. N. Engl. J. Med. 2000, 342, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noppen, M. Management of primary spontaneous pneumothorax. Curr. Opin. Pulm. Med. 2003, 9, 272–275. [Google Scholar] [CrossRef] [PubMed]
- MacDuff, A.; Arnold, A.; Harvey, J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65 (Suppl. 2), ii18–ii31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.H.; Strange, C.; Heffner, J.E.; Light, R.; Kirby, T.J.; Klein, J.; Luketich, J.D.; Panacek, E.A.; Sahn, S.A. Management of spontaneous pneumothorax: An American College of Chest Physicians Delphi consensus statement. Chest 2001, 119, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.-M.; Bintcliffe, O.; Astoul, P.; Canalis, E.; Driesen, P.; Janssen, J.; Krasnik, M.; Maskell, N.; Van Schil, P.; Tonia, T.; et al. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 2015, 46, 321–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noppen, M.; Alexander, P.; Driesen, P.; Slabbynck, H.; Verstraeten, A. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: A multicenter, prospective, randomized pilot study. Am. J. Respir. Crit. Care Med. 2002, 165, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Ayed, A.K.; Chandrasekaran, C.; Sukumar, M. Aspiration versus tube drainage in primary spontaneous pneumothorax: A randomised study. Eur. Respir. J. 2006, 27, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, W.H.; Lindahl-Jacobsen, R.; Katballe, N.; Sindby, J.E.; Titlestad, I.L.; Andersen, P.E.; Licht, P.B. Recurrent Primary Spontaneous Pneumothorax is Common Following Chest Tube and Conservative Treatment. World J. Surg. 2016, 40, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Olesen, W.H.; Katballe, N.; Sindby, J.E.; Titlestad, I.L.; Andersen, P.E.; Lindahl-Jacobsen, R.; Licht, P.B. Surgical treatment versus conventional chest tube drainage in primary spontaneous pneumothorax: A randomized controlled trial. Eur. J. Cardio-Thorac. Surg. 2018, 54, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Watanabe, Y.; Moriyama, S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: Evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005, 127, 2226–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, D.; Klapdor, B.; Ewig, S.; Hecker, E. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: A 10-year experience. Eur. J. Cardio-Thorac. Surg. 2016, 49, 854–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljehani, Y.M.; Almajid, F.M.; Niaz, R.C.; Elghoneimy, Y.F. Management of Primary Spontaneous Pneumothorax: A Single-center Experience. Saudi J. Med. Med. Sci. 2018, 6, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Daemen, J.H.T.; Lozekoot, P.W.J.; Maessen, J.G.; Gronenschild, M.H.M.; Bootsma, G.P.; Hulsewé, K.W.E.; Vissers, Y.J.L.; De Loos, E.R. Chest tube drainage versus video-assisted thoracoscopic surgery for a first episode of primary spontaneous pneumothorax: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2019, 56, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, H.-J.; Kim, S.-W.; Hong, J.-M.; Lee, K.S.; Lee, S.-H. Psychological Problems of Pneumothorax According to Resilience, Stress, and Post-Traumatic Stress. Psychiatry Investig. 2017, 14, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divisi, D.; Di Leonardo, G.; Crisci, R. Video-assisted thoracic surgery versus pleural drainage in the management of the first episode of primary spontaneous pneumothorax. Am. J. Surg. 2015, 210, 68–73. [Google Scholar] [CrossRef]
- Schramel, F.M.; Sutedja, T.G.; Braber, J.C.; van Mourik, J.C.; Postmus, P.E. Cost-effectiveness of video-assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. Eur. Respir. J. 1996, 9, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Torresini, G.; Vaccarili, M.; Divisi, D.; Crisci, R. Is video-assisted thoracic surgery justified at first spontaneous pneumothorax? Eur. J. Cardio-Thorac. Surg. 2001, 20, 42–45. [Google Scholar] [CrossRef]
- Crisci, R.; Coloni, G.F. Video-assisted thoracoscopic surgery versus thoracotomy for recurrent spontaneous pneumothoraxA comparison of results and costs. Eur. J. Cardio-Thorac. Surg. 1996, 10, 556–560. [Google Scholar] [CrossRef] [Green Version]
| Variables | VATS (n = 48) | Chest Tube (n = 48) |
|---|---|---|
| Gender | ||
| Male | 39 (81.3) | 40 (83.3) |
| Female | 9 (18.7) | 8 (16.7) |
| Age (mean) | 24.8 (range 18–39) | 26.2 (range 18–40) |
| BMI (kg/m2) | 20.2 | 21.1 |
| Height (cm) | 180 | 180 |
| Weight (kg) | 65.5 | 70 |
| Variables | VATS (n = 48) | Chest Tube (n = 48) | p-Value |
|---|---|---|---|
| Duration of chest tube (days) | 5 | 6 | 0.06 |
| Mean LOS (days) | 6 | 6 | 1.00 |
| Prolonged air leak > 5 days (n) | 6 (12.5%) | 11 (22.9%) | 0.181 |
| Operation due to prolonged air leak * | 0 (0%) | 10 (20.8%) | 0.001 * |
| Hemothorax (n) | 1 (2.1%) | 0 (0%) | 0.315 |
| Recurrence during follow-up (n) * | 4 (8.3%) | 25 (52.1%) | <0.001 * |
| Variables | VATS (n = 4) | Chest Tube (n = 25) |
|---|---|---|
| Mean LOS at recurrence (days) | 6 | 6 |
| Duration of chest tube (days) | 5 | 5 |
| Cost of hospital day, as per DRG | EUR 148 | EUR 148 |
| Total LOS costs (pp/pg) | EUR 888/EUR 3.552 | EUR 888/EUR 22.200 |
| Surgical material cost (pp/pg) | EUR 465/EUR 1.860 | EUR 465/EUR 11.625 |
| Recurrence treatment costs (pg) | EUR 5.412 | EUR 33.825 |
| Recurrence treatment costs (pp) | EUR 1.353 | EUR 1.353 |
| Variables | VATS (n = 48) | Chest Tube (n = 48) |
|---|---|---|
| Mean LOS (days) | 6 | 6 |
| Cost of hospital day, as per DRG | EUR 148 | EUR 148 |
| Total LOS costs (pp/pg) | EUR 888/EUR 42.624 | EUR 888/EUR 42.624 |
| Surgical material cost (pp/pg) | EUR 465/EUR 22.320 | EUR 77/EUR 3.696 |
| Primary treatment costs (pp) | EUR 1.360 | EUR 1.247 |
| Primary treatment costs (pg) | EUR 65.288 | EUR 59.850 |
| Variables | VATS (Hemothorax, n = 1) | Chest Tube (Prolonged Air Leak, n = 10) |
|---|---|---|
| Mean LOS (days) | 6 | 6 |
| Cost of hospital day, as per DRG | EUR 148 | EUR 148 |
| Total LOS costs (pp/pg) | EUR 888/EUR 888 | EUR 888/EUR 8880 |
| Surgical material cost (pp/pg) | EUR 465/EUR 465 | EUR 465/EUR 4650 |
| Treatment costs (pp) | EUR 1.353 | EUR 1.353 |
| Treatment costs (pg) | EUR 1.353 | EUR 13.530 |
| Variables | VATS (n = 48) | Chest Tube (n = 48) |
|---|---|---|
| Primary treatment costs (pg) | EUR 65.288 | EUR 59.850 |
| Recurrence treatment costs (pg) | EUR 5.412 | EUR 33.825 |
| Total management costs (pg) | EUR 70.700 | EUR 93.675 |
| Total management costs (pp) | EUR 1.473 | EUR 1.952 |
| Total management and complication costs (pg) | EUR 72.053 | EUR 107.205 |
| Total management and complication costs (pp) | EUR 1.501 | EUR 2.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, S.; Alexander, A.; Ashmawy, H.; Dizdar, L.; Safi, S.; Rehders, A.; Fluegen, G.; Knoefel, W.T. Socioeconomic Impact of Recurrent Primary Spontaneous Pneumothorax: Should Video-Assisted Thoracoscopic Surgery Be Considered at First Episode of Primary Spontaneous Pneumothorax? Healthcare 2021, 9, 1236. https://doi.org/10.3390/healthcare9091236
Fung S, Alexander A, Ashmawy H, Dizdar L, Safi S, Rehders A, Fluegen G, Knoefel WT. Socioeconomic Impact of Recurrent Primary Spontaneous Pneumothorax: Should Video-Assisted Thoracoscopic Surgery Be Considered at First Episode of Primary Spontaneous Pneumothorax? Healthcare. 2021; 9(9):1236. https://doi.org/10.3390/healthcare9091236
Chicago/Turabian StyleFung, Stephen, Andrea Alexander, Hany Ashmawy, Levent Dizdar, Sami Safi, Alexander Rehders, Georg Fluegen, and Wolfram Trudo Knoefel. 2021. "Socioeconomic Impact of Recurrent Primary Spontaneous Pneumothorax: Should Video-Assisted Thoracoscopic Surgery Be Considered at First Episode of Primary Spontaneous Pneumothorax?" Healthcare 9, no. 9: 1236. https://doi.org/10.3390/healthcare9091236