Impact of Oxygen Concentration Delivered via Nasal Cannula on Different Lung Conditions: A Bench Study
Abstract
:1. Introduction
2. Materials and Methods
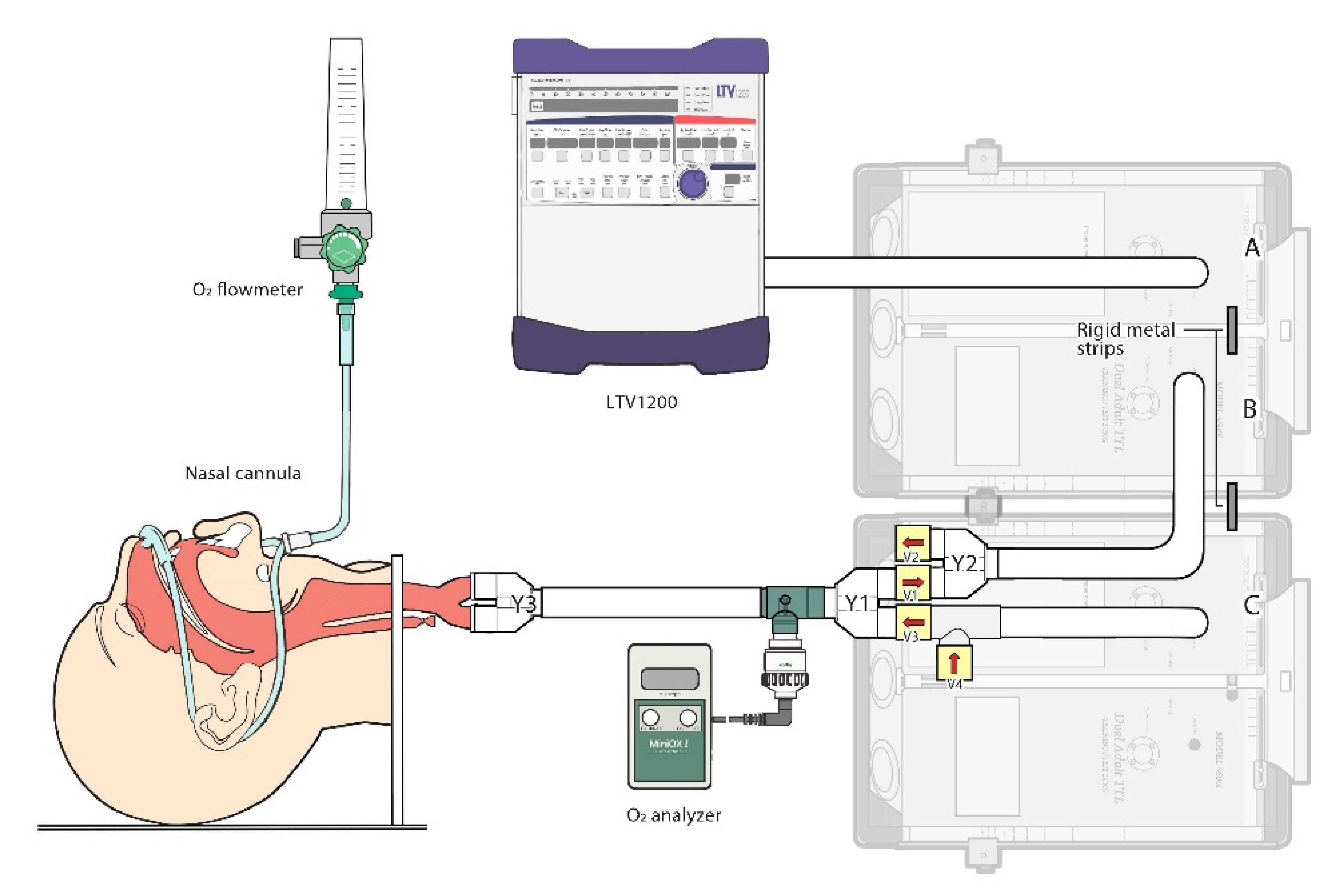
2.1. The Lung Model
2.2. Testing Protocol
2.3. Variables and Measurements
2.4. Statistics
3. Results
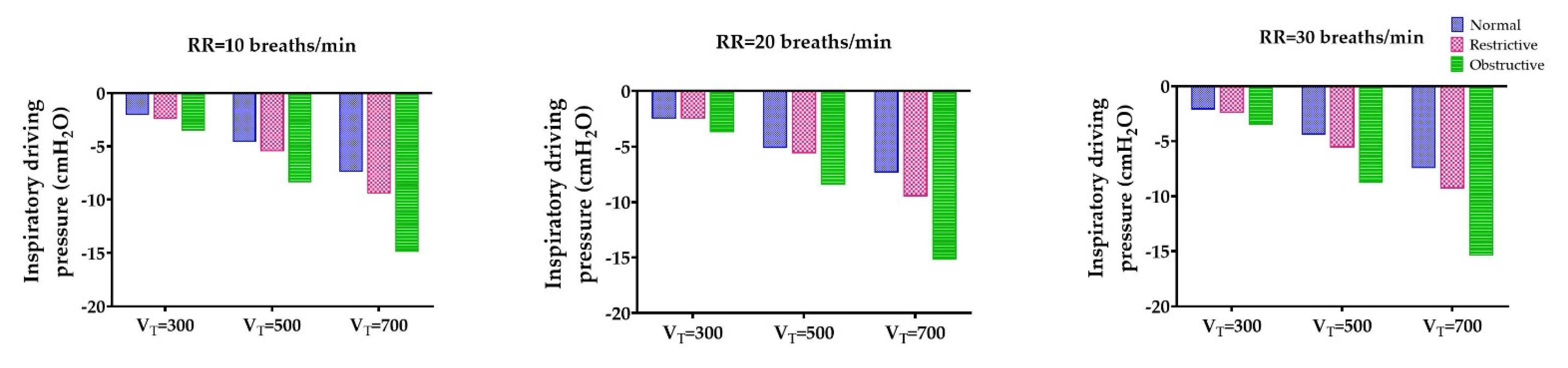
3.1. The Lowest Inspiratory Driving Pressure Obtained through the Obstructive Lung Model
3.2. Decreased Fraction of Inspired Oxygen as Tidal Volumes Increased
3.3. Effect of Fraction of Inspired Oxygen by the Change of Respiratory Rate
3.4. Tidal Volume and Oxygen Flow Rate Were Impact Factors on Oxygen Delivery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, D.R.; Macintyre, N.R.; Galvin, W.F. Respiratory Care: Principles and Practice, 3rd ed.; Jones & Bartlett Learning: Sudbury, MA, USA, 2016. [Google Scholar]
- Kacmarek, R.M.; Stoller, J.K.; Heuer, A.J. Egan’s Fundamentals of Respiratory Care, 11th ed.; Mosby: St. Louis, MO, USA, 2016. [Google Scholar]
- Cairo, J.M. Mosby’s Respiratory Care Equipment, 9th ed.; Mosby: St. Louis, MO, USA, 2013. [Google Scholar]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017, 72 (Suppl. 1), ii1–ii90. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.; Kim, L.H.-Y.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Leigh, J.M. Variation in performance of oxygen therapy devices. Towards the rational employment of ‘The dephlogisticated air described by Priestley’. Ann. R. Coll. Surg. Engl. 1973, 52, 234–253. [Google Scholar] [PubMed]
- Bazuaye, E.A.; Stone, T.N.; Corris, P.A.; Gibson, G.J. Variability of inspired oxygen concentration with nasal cannulas. Thorax 1992, 47, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.J.; Hopkin, J.M. Variability of inspired oxygen concentration with nasal cannulas. Thorax 1992, 47, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, R.; Joshi, P.; Soni, N. An evaluation of oxygen delivery using nasal prongs. Anaesthesia 1992, 47, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.A.B.; Dean, P.; Kinsella, J.; Black, R.; Carter, R.; Hughes, M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated*. Anaesthesia 2008, 63, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Comer, P.B.; Beckham, R.W.; McGraw, C.P. Actual Tracheal Oxygen Concentrations with Commonly Used Oxygen Equipment. Anesthesiology 1976, 44, 71–73. [Google Scholar] [CrossRef]
- McDonald, C.F. Low-flow oxygen: How much is your patient really getting? Respirology 2014, 19, 469–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, N.; Miyashita, T.; Takaki, S.; Goto, T. Effects of Breathing Pattern on Oxygen Delivery Via a Nasal or Pharyngeal Cannula. Respir. Care 2015, 60, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M. High-Flow Nasal Cannula Oxygen Therapy in Adults: Physiological Benefits, Indication, Clinical Benefits, and Adverse Effects. Respir. Care 2016, 61, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Larovere, M.T.; Fanfulla, F.; Navalesi, P.; Delmastro, M.; Mortara, A. Orthopnea and inspiratory effort in chronic heart failure patients. Respir. Med. 2003, 97, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Poletti, V.; Tomassetti, S.; Ravaglia, C. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018, 379, 795–798. [Google Scholar]
- Branson, R.D. Oxygen Therapy in COPD. Respir. Care 2018, 63, 734–748. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, N.; Haynes, A.G.; Stucki, A.; Jüni, P.; Ott, S.R. Long-term oxygen therapy in COPD patients: Population-based cohort study on mortality. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 979–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, V.N. Low-dose oxygen therapy in copd patients: Are there any radiation-like risks? Curr. Opin. Pulm. Med. 2018, 24, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Inwald, D.; Roland, M.; Kuitert, L.; McKenzie, S.A.; Petros, A. Oxygen treatment for acute severe asthma. BMJ 2001, 323, 98–100. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, Y.; Miyagi, S. Oxygen treatment for acute severe asthma. Home oxygenation would be more effective. BMJ 2001, 323, 1069. [Google Scholar] [CrossRef] [PubMed]
- Schacter, E.N.; Littner, M.R.; Luddy, P.; Beck, G.J. Monitoring of oxygen delivery systems in clinical practice. Crit. Care Med. 1980, 8, 405–409. [Google Scholar] [CrossRef]
- Waldau, T.; Larsen, V.H.; Bonde, J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia 1998, 53, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Wettstein, R.B.; Shelledy, D.C.; Peters, J.I. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir. Care 2005, 50, 604–609. [Google Scholar] [PubMed]
- Duprez, F.; Mashayekhi, S.; Cuvelier, G.; Legrand, A.; Reychler, G. A New Formula for Predicting the Fraction of Delivered Oxygen During Low-Flow Oxygen Therapy. Respir. Care 2018, 63, 1528–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Chatburn, R.L. Effect of the Anatomic Reservoir on Low-Flow Oxygen Delivery Via Nasal Cannula: Constant Flow Versus Pulse Flow With Portable Oxygen Concentrator. Respir. Care 2014, 59, 1199–1209. [Google Scholar] [CrossRef] [Green Version]
- Baboi, L.; Subtil, F.; Guérin, C. A bench evaluation of fraction of oxygen in air delivery and tidal volume accuracy in home care ventilators available for hospital use. J. Thorac. Dis. 2016, 8, 3639–3647. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-C.; Orr, J.; Lin, S.-P.; Yu, L.; Tsou, M.-Y.; Westenskow, D.R.; Ting, C.-K. Efficiency of oxygen delivery through different oxygen entrainment devices during sedation under low oxygen flow rate: A bench study. J. Clin. Monit. 2017, 32, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.-M.; Garnero, A.; Saoli, M.; Chatburn, R.L. Parameters for Simulation of Adult Subjects During Mechanical Ventilation. Respir. Care 2018, 63, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.J. High-Flow Oxygen Administration by Nasal Cannula for Adult and Perinatal Patients. Respir. Care 2012, 58, 98–122. [Google Scholar] [CrossRef] [Green Version]
- Wagstaff, T.A.J.; Soni, N. Performance of six types of oxygen delivery devices at varying respiratory rates*. Anaesthesia 2007, 62, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Chikata, Y.; Onodera, M.; Oto, J.; Nishimura, M. FiO2 in an adult model simulating high-flow nasal cannula therapy. Respir. Care 2017, 62, 193–198. [Google Scholar] [CrossRef]
- Caille, V.; Ehrmann, S.; Boissinot, E.; Perrotin, D.; Diot, P.; Dequin, P.-F. Influence of Jet Nebulization and Oxygen Delivery on the Fraction of Inspired Oxygen: An Experimental Model. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Markovitz, G.H.; Colthurst, J.; Storer, T.W.; Cooper, C.B. Effective inspired oxygen concentration measured via transtracheal and oral gas analysis. Respir. Care 2010, 55, 453–459. [Google Scholar] [PubMed]
- O’Reilly-Nugent, A.; Kelly, P.T.; Stanton, J.; Swanney, M.P.; Graham, B.; Beckert, L. Measurement of oxygen concentration delivered via nasal cannulae by tracheal sampling. Respirology 2014, 19, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Models | Variables | Standardized β Coefficients | t Value b | p Value | Collinearity Statistics | ||
|---|---|---|---|---|---|---|---|
| Tolerance | VIF | ||||||
| Normal | Constant | 24.181 | 44.682 | ||||
| O2 flow rate | 0.753 | 13.758 ** | <0.001 | 1.000 | 1.000 | ||
| VT | −0.447 | −8.164 ** | <0.001 | 1.000 | 1.000 | ||
| RR | 0.037 | 0.503 | 0.161 | 1.000 | 1.000 | ||
| Restrictive | Constant | 23.857 | 45.642 | ||||
| O2 flow rate | 0.768 | 13.665 ** | <0.001 | 1.000 | 1.000 | ||
| VT | −0.405 | −7.208 ** | <0.001 | 1.000 | 1.000 | ||
| RR | 0.085 | 1.182 | 0.241 | 1.000 | 1.000 | ||
| Obstructive | Constant | 23.680 | 46.112 | ||||
| O2 flow rate | 0.769 | 13.790 ** | <0.001 | 1.000 | 1.000 | ||
| VT | −0.406 | −7.282 ** | <0.001 | 1.000 | 1.000 | ||
| RR | −1.000 | 0.320 | 1.000 | 1.000 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, H.-Y.; Yang, S.-H.; Chiang, H.-S. Impact of Oxygen Concentration Delivered via Nasal Cannula on Different Lung Conditions: A Bench Study. Healthcare 2021, 9, 1235. https://doi.org/10.3390/healthcare9091235
Tseng H-Y, Yang S-H, Chiang H-S. Impact of Oxygen Concentration Delivered via Nasal Cannula on Different Lung Conditions: A Bench Study. Healthcare. 2021; 9(9):1235. https://doi.org/10.3390/healthcare9091235
Chicago/Turabian StyleTseng, Hui-Yun, Shih-Hsing Yang, and Han-Sun Chiang. 2021. "Impact of Oxygen Concentration Delivered via Nasal Cannula on Different Lung Conditions: A Bench Study" Healthcare 9, no. 9: 1235. https://doi.org/10.3390/healthcare9091235
APA StyleTseng, H.-Y., Yang, S.-H., & Chiang, H.-S. (2021). Impact of Oxygen Concentration Delivered via Nasal Cannula on Different Lung Conditions: A Bench Study. Healthcare, 9(9), 1235. https://doi.org/10.3390/healthcare9091235






