A Comparative Study between 18F-FDG PET/CT and Conventional Imaging in the Evaluation of Progressive Disease and Recurrence in Ovarian Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Positron Emission Tomography/Computed Tomography (PET/CT) Imaging
2.3. Statistical Analysis
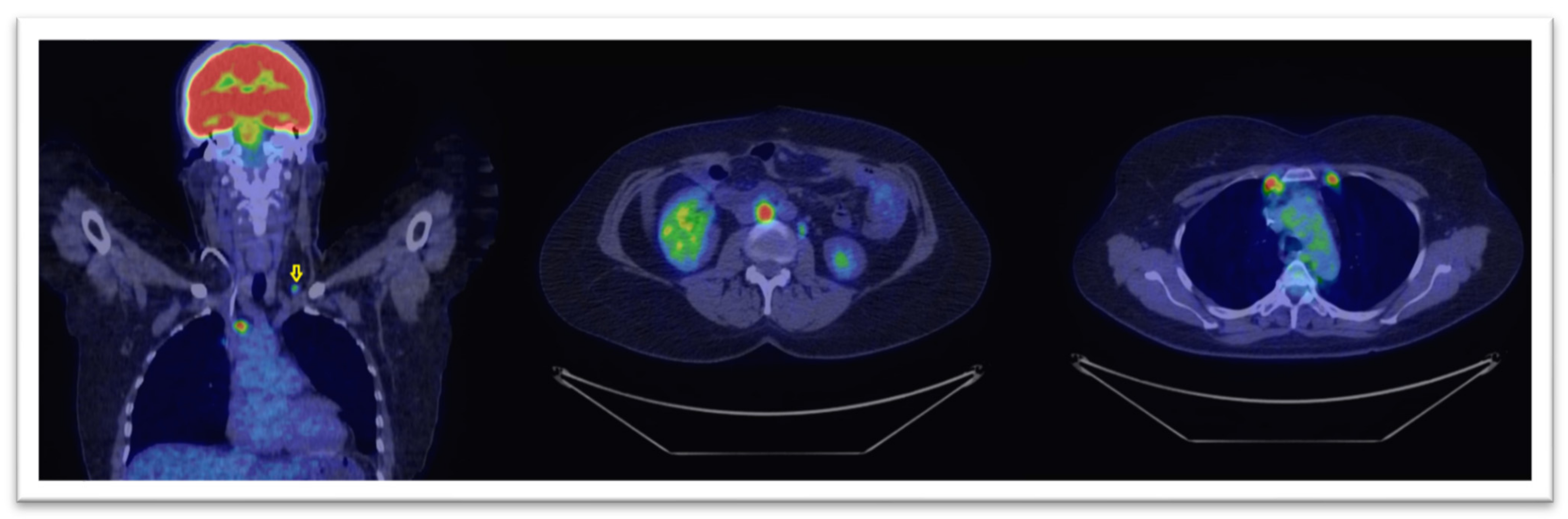
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer, Global Cancer Observatory WHO. Estimated Number of Prevalent Cases (5-Year) in 2018, Worldwide, Females, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=countries&population=900&populations=900&key=asr&sex=2&cancer=39&type=2&statistic=5&prevalence=1&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&in (accessed on 24 July 2020).
- International Agency for Research on Cancer, Global Cancer Observatory WHO. Estimated Number of Deaths in 2018, Worldwide, Females, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=continents&population=900&populations=170&key=asr&sex=2&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&i (accessed on 24 July 2020).
- International Agency for Research on Cancer, Global Cancer Observatory WHO. Estimated Number of New Cases in 2018, Worldwide, Females, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&i (accessed on 24 July 2020).
- International Agency for Research on Cancer, Global Cancer Observatory WHO. Estimated Number of Incident Cases from 2018 to 2040, Ovary, Females, All Ages. Available online: https://gco.iarc.fr/tomorrow/graphic-isotype?type=0&type_sex=0&mode=population&sex=2&populations=900&cancers=25&age_group=value&apc_male=0&apc_female=0&single_unit=1000000&print=0 (accessed on 24 July 2020).
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Ovarian Cancer. Continuous Update Project Expert Report 2018; WCRF: Washington, DC, USA, 2018. [Google Scholar]
- American Cancer Society. Ovarian Cancer Risk Factors. Available online: https://www.cancer.org/cancer/ovarian-cancer/causes-risks-prevention/risk-factors.html (accessed on 23 July 2020).
- Matz, M.; Coleman, M.P.; Sant, M. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1.2021—February 26. 2021. NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 31 May 2020).
- Clarke-Pearson, D.L. Clinical practice. Screening for ovarian cancer. N. Engl. J. Med. 2009, 361, 170–177. [Google Scholar] [CrossRef] [PubMed]
- The American College of Obstetricians and Gynecologists. The Role of the Obstetrician–Gynecologist in the Early Detection of Epithelial Ovarian Cancer. Obstet. Gynecol. 2011, 117, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.T.; Webber, E.M.; Sawaya, G.F. Screening for ovarian cancer updated evidence report and systematic review for the US preventive services task force. JAMA J. Am. Med. Assoc. 2018, 319, 595–606. [Google Scholar] [CrossRef]
- Anton, C.; Carvalho, F.M.; Oliveira, E.I.; Maciel, G.A.R.; Baracat, E.C.; Carvalho, J.P. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics 2012, 67, 437–441. [Google Scholar] [CrossRef]
- Yanaranop, M.; Anakrat, V.; Siricharoenthai, S.; Nakrangsee, S.; Thinkhamrop, B. Is the Risk of Ovarian Malignancy Algorithm Better Than Other Tests for Predicting Ovarian Malignancy in Women with Pelvic Masses? Gynecol. Obstet. Investig. 2017, 82, 47–53. [Google Scholar] [CrossRef]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1079–1091. [Google Scholar] [CrossRef]
- Kemppainen, J.; Hynninen, J.; Virtanen, J.; Seppänen, M. PET/CT for Evaluation of Ovarian Cancer. Semin. Nucl. Med. 2019, 49, 484–492. [Google Scholar] [CrossRef]
- Fruscio, R.; Sina, F.; Dolci, C.; Signorelli, M.; Crivellaro, C.; Dell’Anna, T.; Cuzzocrea, M.; Guerra, L.; Milani, R.; Messa, C. Preoperative 18F-FDG PET/CT in the management of advanced epithelial ovarian cancer. Gynecol. Oncol. 2013, 131, 689–693. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Oguri, H.; Yamada, R.; Maeda, N.; Kohsaki, S.; Fukaya, T. Preoperative evaluation of pelvic masses with combined 18F-fluorodeoxyglucose positron emission tomography and computed tomography. Int. J. Gynecol. Obstet. 2008, 102, 124–127. [Google Scholar] [CrossRef]
- Konishi, H.; Takehara, K.; Kojima, A.; Okame, S.; Yamamoto, Y.; Shiroyama, Y.; Yokoyama, T.; Nogawa, T.; Sugawara, Y. Maximum standardized uptake value of fluorodeoxyglucose positron emission tomography/computed tomography is a prognostic factor in ovarian clear cell adenocarcinoma. Int. J. Gynecol. Cancer. 2014, 24, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Kwon, H.W.; Kang, K.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.-B.; Kim, J.W. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis in patients with epithelial ovarian cancer. Ann. Surg. Oncol. 2012, 19, 1966–1972. [Google Scholar] [CrossRef]
- Vallius, T.; Hynninen, J.; Kemppainen, J.; Alves, V.; Auranen, K.; Matomäki, J.; Oksa, S.; Virtanen, J.; Grenman, S.; Auranen, A.; et al. 18F-FDG-PET/CT based total metabolic tumor volume change during neoadjuvant chemotherapy predicts outcome in advanced epithelial ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Antunovic, L.; Cimitan, M.; Borsatti, E.; Baresic, T.; Sorio, R.; Giorda, G.; Steffan, A.; Balestreri, L.; Tatta, R.; Pepe, G.; et al. Revisiting the Clinical Value of 18F-FDG PET/CT in Detection of Recurrent Epithelial Ovarian Carcinomas. Clin. Nucl. Med. 2012, 37, e184–e188. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Carlier, T.; Colombié, M.; Goulon, D.; Fleury, V.; Rousseau, N.; Berton-Rigaud, D.; Jaffre, I.; Kraeber-Bodéré, F.; Campion, L.; et al. Clinical and survival impact of FDG PET in patients with suspicion of recurrent ovarian cancer: A 6-year follow-up. Front. Med. 2015, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vallius, T.; Peter, A.; Auranen, A.; Carpén, O.; Kemppainen, J.; Matomäki, J.; Oksa, S.; Roering, P.; Seppänen, M.; Grénman, S.; et al. 18F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol. Oncol. 2016, 140, 29–35. [Google Scholar] [CrossRef]
- Caobelli, F.; Young AIMN Working Group; Alongi, P.; Evangelista, L.; Picchio, M.; Saladini, G.; Rensi, M.; Geatti, O.; Castello, A.; Laghai, I.; et al. Predictive value of 18F-FDG PET/CT in restaging patients affected by ovarian carcinoma: A multicentre study. Eur. J. Nucl. Med. Mol. Imaging. 2016, 43, 404–413. [Google Scholar] [CrossRef]
- Piciu, A.; Larg, M.I.; Piciu, D. Correlation between f18-fdg PET/CT imaging and braf V600E genetic mutation for the early assessment of treatment response in papillary thyroid cancers. J. Pers. Med. 2020, 10, 52. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Joo Hyun, O.; Lodge, M.A.; Wahl, R.L. Practical percist: A simplified guide to PET response criteria in solid tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Nam, E.J.; Yun, M.J.; Oh, Y.T.; Kim, J.W.; Kim, S.; Jung, Y.W.; Kim, S.W.; Kim, Y.T. Diagnosis and staging of primary ovarian cancer: Correlation between PET/CT, Doppler US, and CT or MRI. Gynecol. Oncol. 2010, 116, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gu, Z.X.; Tao, X.F.; Liu, S.Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: A meta-analysis. Eur. J. Radiol. 2012, 81, 1002–1006. [Google Scholar] [CrossRef]
- Sebastian, S.; Lee, S.I.; Horowitz, N.S.; Scott, J.A.; Fischman, A.J.; Simeone, J.F.; Fuller, A.F.; Hahn, P.F. PET-CT vs. CT alone in ovarian cancer recurrence. Abdom. Imaging 2008, 33, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Coakley, F.V.; Choi, P.H.; Gougoutas, C.A.; Pothuri, B.; Venkatraman, E.; Chi, D.; Bergman, A.; Hricak, H. Peritoneal metastases: Detection with spiral CT in patients with ovarian cancer. Radiology 2002, 223, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.P.; García, J.M.C.; Rubio, M.D.P.T.; Vicente, A.M.G.; Pardo, F.J.P.; Londoño, G.A.J.; Castrejón, Á.S.; Aguilar, E.A. Value of [18F]FDG-PET/CT and CA125, serum levels and kinetic parameters, in early detection of ovarian cancer recurrence. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Palomar, A.; Nanni, C.; Castellucci, P.; Ambrosini, V.; Montini, G.C.; Allegri, V.; Pettinato, C.; Nahhas, A.; Soriano, Á.; Grassetto, G.; et al. Value of FDG PET/CT in patients with treated ovarian cancer and raised CA125 serum levels. Mol. Imaging Biol. 2012, 14, 123–129. [Google Scholar] [CrossRef]
- Evangelista, L.; Palma, M.D.; Gregianin, M.; Nardin, M.; Roma, A.; Nicoletto, M.O.; Nardelli, G.B.; Zagonel, V. Diagnostic and prognostic evaluation of fluorodeoxyglucose positron emission tomography/computed tomography and its correlation with serum cancer antigen-125 (CA125) in a large cohort of ovarian cancer patients. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 137–144. [Google Scholar] [CrossRef]
- Chu, L.C.; Tsai, H.L.; Wang, H.; Crandall, J.; Javadi, M.S.; Wahl, R.L. Posttreatment FDG PET/CT in predicting survival of patients with ovarian carcinoma. EJNMMI Res. 2016, 6, 4–11. [Google Scholar] [CrossRef][Green Version]
- Soussan, M.; Wartski, M.; Cherel, P.; Fourme, E.; Goupil, A.; Le Stanc, E.; Callet, N.; Alexandre, J.; Pecking, A.-P.; Alberini, J.-L. Impact of FDG PET-CT imaging on the decision making in the biologic suspicion of ovarian carcinoma recurrence. Gynecol. Oncol. 2008, 108, 160–165. [Google Scholar] [CrossRef]
- Chung, H.H.; Kang, W.J.; Kim, J.-W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.-B.; Lee, H.-P. Role of [18F]FDG PET/CT in the assessment of suspected recurrent ovarian cancer: Correlation with clinical or histological findings. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Simcock, B.; Neesham, D.; Quinn, M.; Drummond, E.; Milner, A.; Hicks, R.J. The impact of PET/CT in the management of recurrent ovarian cancer. Gynecol. Oncol. 2006, 103, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Manssor, E.; Abuderman, A.; Osman, S.; Alenezi, S.B.; Almehemeid, S.; Babikir, E.; Alkhorayef, M.; Sulieman, A. Radiation doses in chest, abdomen and pelvis CT procedures. Radiat. Prot. Dosim. 2015, 165, 194–198. [Google Scholar] [CrossRef] [PubMed]
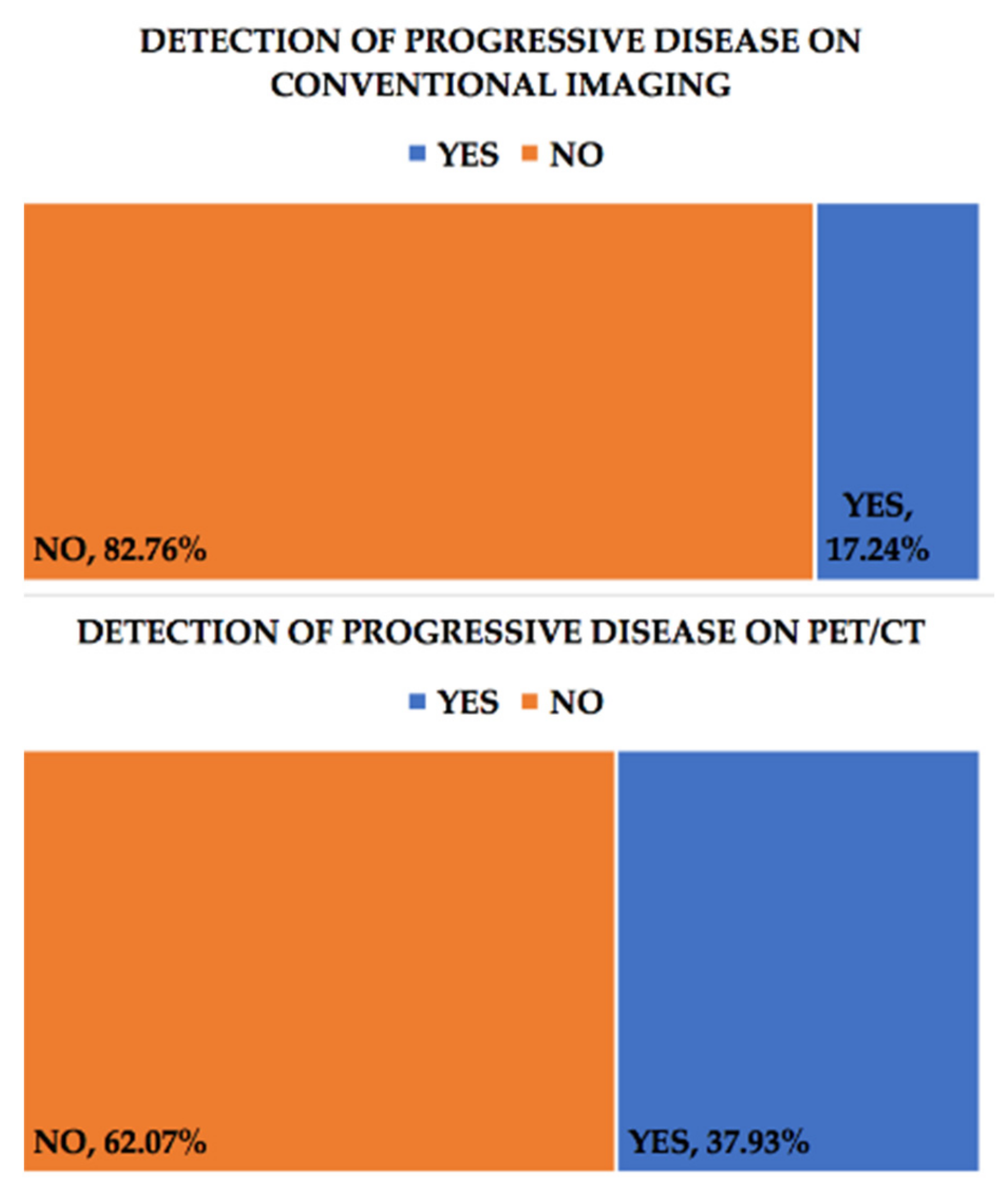
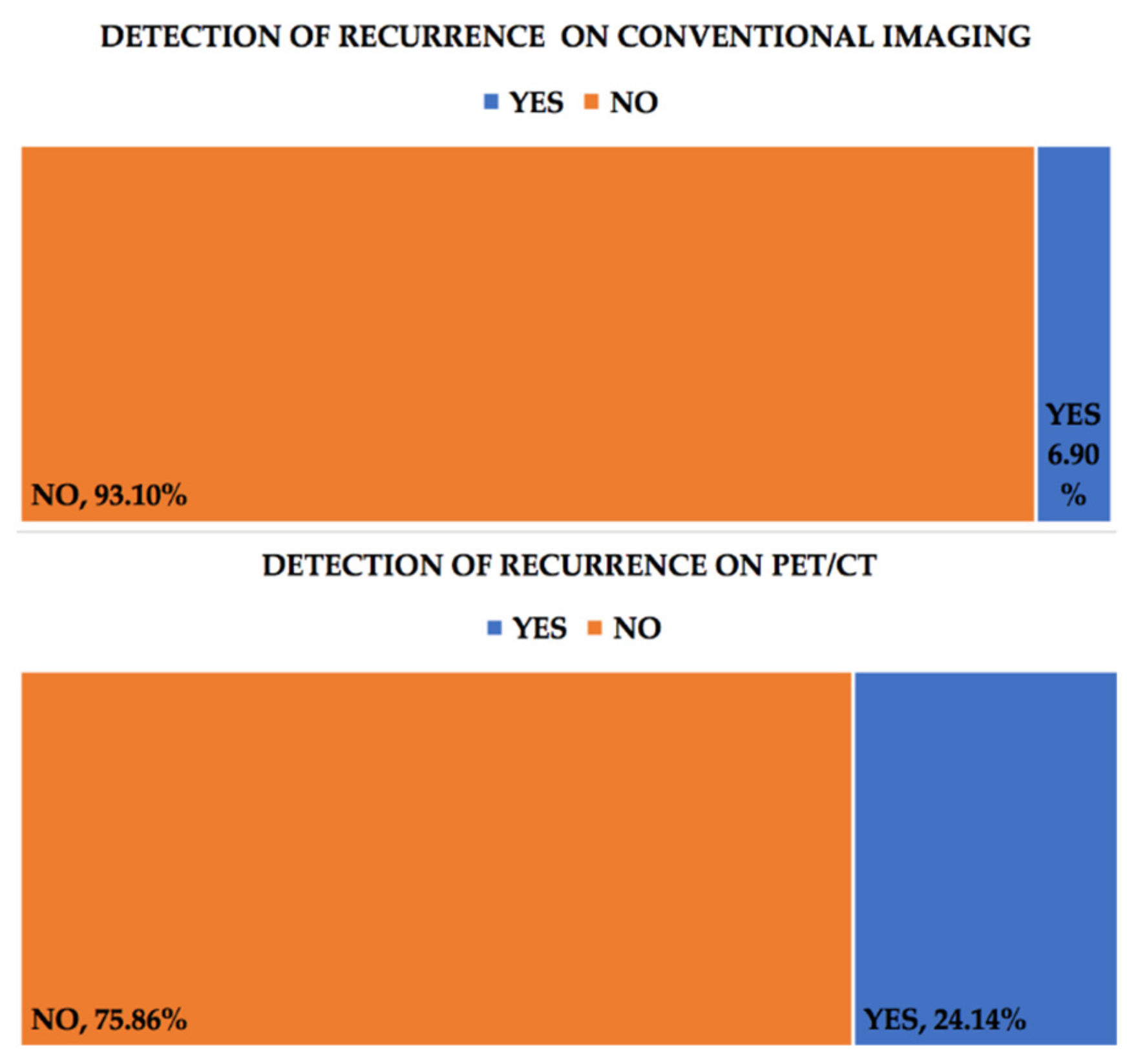
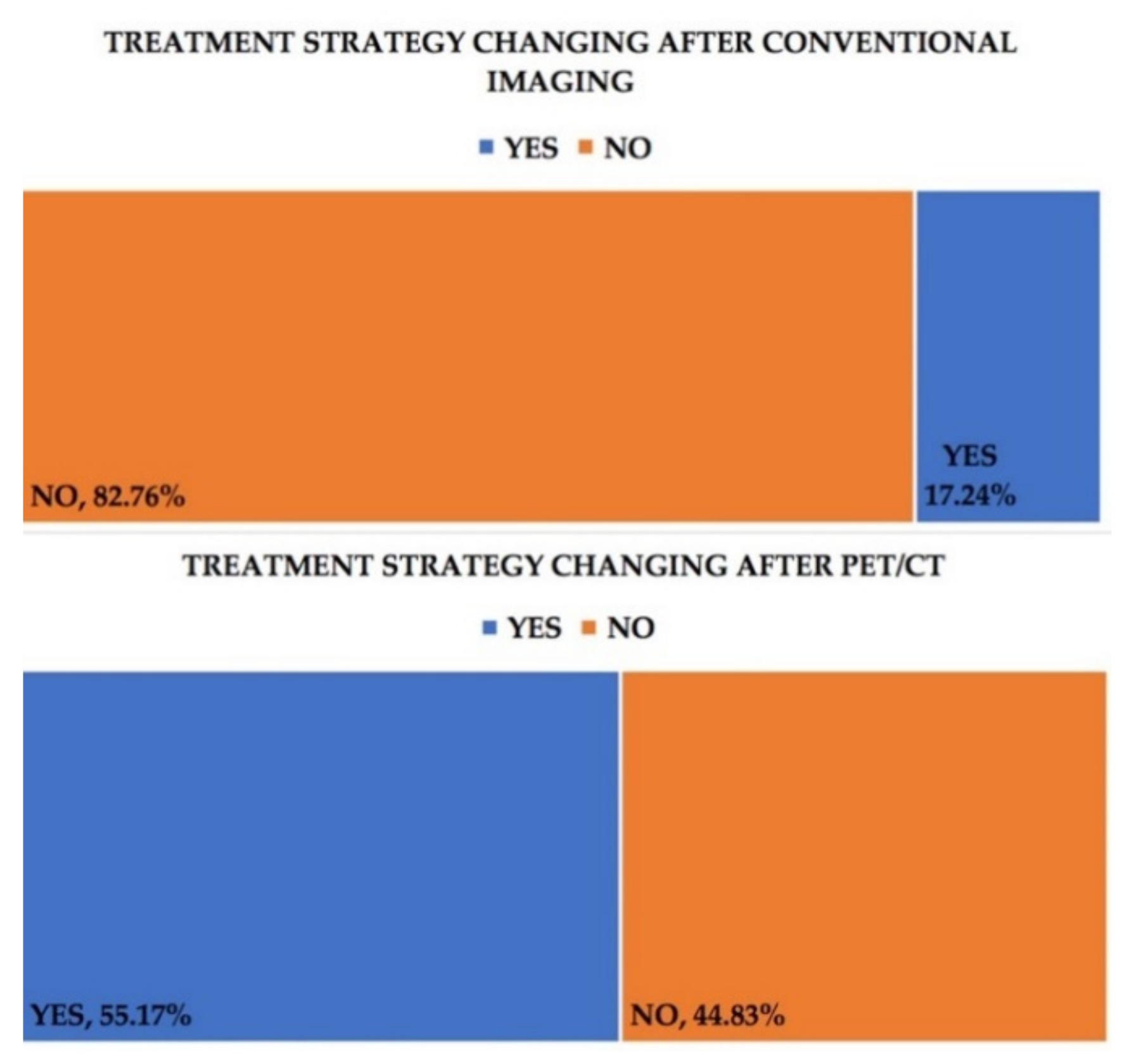
| No. | Age Years | FIGO Staging | CA-125 (U/mL) | Progressive Disease on PET/CT | Progressive Disease on Conventional Imaging | Recurrence on PET/CT | Recurrence on Conventional Imaging |
|---|---|---|---|---|---|---|---|
| 1 | 55 | III | 82.8 | Yes | Yes | No | No |
| 2 | 58 | III | 642 | Yes | No | No | No |
| 3 | 67 | II | <35 | No | No | No | No |
| 4 | 78 | NA | 84.1 | No | No | Yes | No |
| 5 | 55 | III | 24.4 | Yes | Yes | No | No |
| 6 | 56 | III | 79 | No | No | No | No |
| 7 | 52 | III | 280 | Yes | No | No | No |
| 8 | 63 | III | 537 | No | Yes | No | No |
| 9 | 62 | III | <35 | No | No | No | No |
| 10 | 50 | III | 102 | Yes | Yes | No | No |
| 11 | 59 | III | 1125 | Yes | No | No | No |
| 12 | 64 | III | <35 | No | No | Yes | No |
| 13 | 60 | III | 1700 | No | No | No | No |
| 14 | 49 | III | 87.8 | Yes | No | No | No |
| 15 | 55 | III | 19.97 | No | No | No | No |
| 16 | 50 | IV | 126.3 | No | No | Yes | No |
| 17 | 47 | I | 23.1 | No | No | No | No |
| 18 | 72 | III | 463.7 | Yes | Yes | No | No |
| 19 | 64 | III | 37.35 | No | No | No | No |
| 20 | 60 | III | 53.22 | Yes | Yes | No | No |
| 21 | 54 | III | 737 | Yes | No | No | No |
| 22 | 51 | III | 1245 | No | No | Yes | Yes |
| 23 | 70 | III | <35 | No | No | No | No |
| 24 | 60 | NA | <35 | No | No | No | No |
| 25 | 51 | III | 85 | No | No | Yes | No |
| 26 | 65 | III | <35 (10.4) | No | No | Yes | No |
| 27 | 45 | IV | <35 (14.51) | No | No | No | No |
| 28 | 63 | III | 90.9 | No | No | Yes | No |
| 29 | 49 | III | <35 (5.2) | Yes | No | No | No |
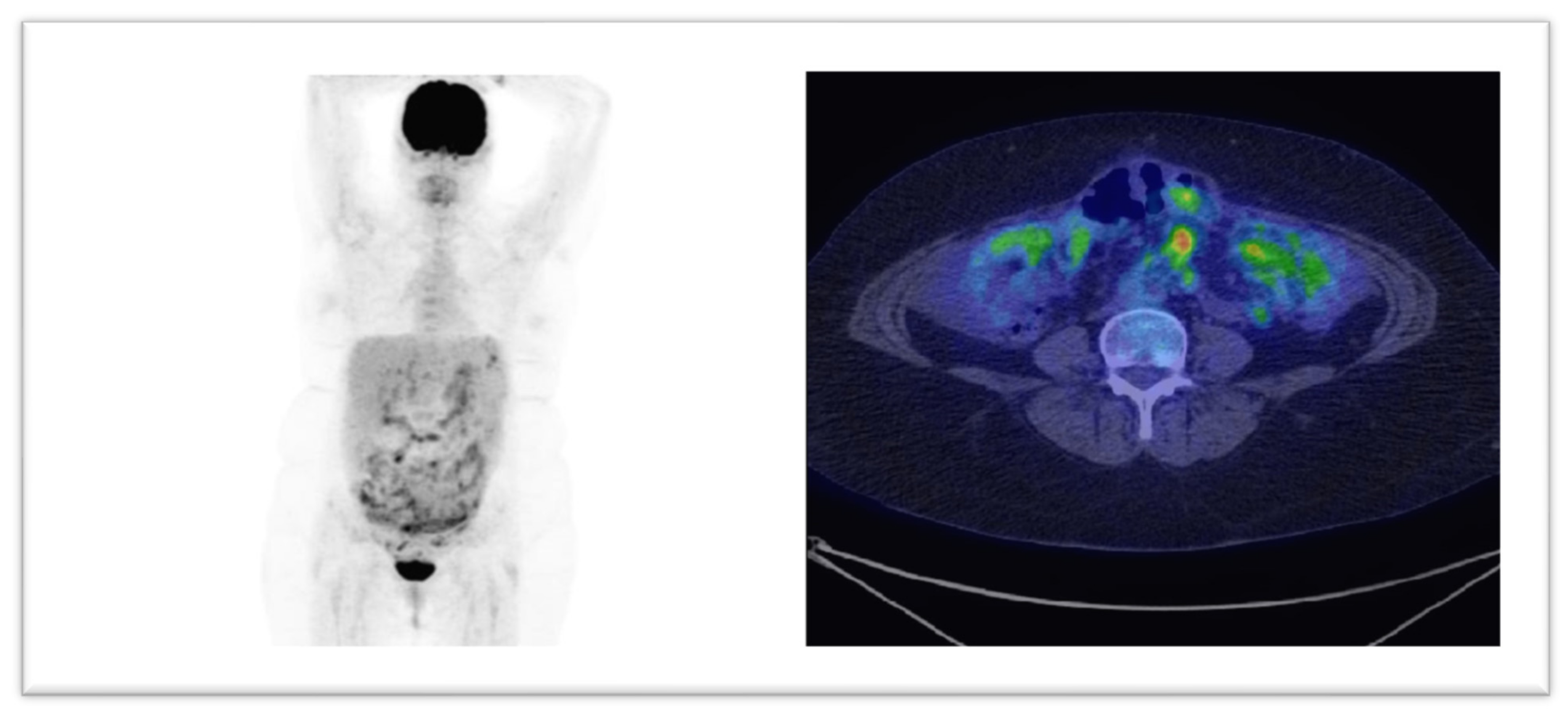
| Site Where Disease Progression Was Observed through F-18 FDG PET/CT Imaging | Number of Cases/Percent Out of Total Number of Patients with Progressive Disease |
|---|---|
| Peritoneal | 8 (72.7%) |
| Hepatic | 4 (27.27%) |
| Splenic | 2 (18.18%) |
| Subdiaphragmatic lymph nodes | 5 (45.45%) |
| Supradiaphragmatic lymph nodes | 3 (27.27%) |
| No. | Treatment before 18F-FDG PET-CT Scan | Treatment after 18F-FDG PET-CT Scan |
|---|---|---|
| 1 | Gemcitabine + Cisplatin | Cyclophosphamide + Cisplatin |
| 2 | Paclitaxel | Topotecan |
| 3 | Gemcitabine + Cisplatin | Cyclophosphamide + Cisplatin |
| 4 | Follow-up | Carboplatin + Paclitaxel |
| 5 | Olaparib | Trabectedin |
| 6 | Carboplatin + Paclitaxel | Carboplatin + Paclitaxel + Bevacizumab |
| 7 | Follow-up | Carboplatin + Paclitaxel |
| 8 | Follow-up | Carboplatin + Paclitaxel |
| 9 | Doxorubicin + Bevacizumab | Paclitaxel + Bevacizumab |
| 10 | Doxorubicin + Carboplatin | Topotecan |
| 11 | Carboplatin + Paclitaxel | Paclitaxel + Bevacizumab |
| 12 | Carboplatin + Paclitaxel | Carboplatin + Paclitaxel + Bevacizumab |
| 13 | Olaparib | Carboplatin + Paclitaxel |
| 14 | Gemcitabine | Surgery |
| 15 | Carboplatin + Paclitaxel | Gemcitabine + Carboplatin |
| 16 | Carboplatin + Paclitaxel | Doxorubicin + Bevacizumab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, G.; Achimaș-Cadariu, P.; Piciu, A.; Căinap, S.S.; Căinap, C.; Piciu, D. A Comparative Study between 18F-FDG PET/CT and Conventional Imaging in the Evaluation of Progressive Disease and Recurrence in Ovarian Carcinoma. Healthcare 2021, 9, 666. https://doi.org/10.3390/healthcare9060666
Rusu G, Achimaș-Cadariu P, Piciu A, Căinap SS, Căinap C, Piciu D. A Comparative Study between 18F-FDG PET/CT and Conventional Imaging in the Evaluation of Progressive Disease and Recurrence in Ovarian Carcinoma. Healthcare. 2021; 9(6):666. https://doi.org/10.3390/healthcare9060666
Chicago/Turabian StyleRusu, George, Patriciu Achimaș-Cadariu, Andra Piciu, Simona Sorana Căinap, Călin Căinap, and Doina Piciu. 2021. "A Comparative Study between 18F-FDG PET/CT and Conventional Imaging in the Evaluation of Progressive Disease and Recurrence in Ovarian Carcinoma" Healthcare 9, no. 6: 666. https://doi.org/10.3390/healthcare9060666
APA StyleRusu, G., Achimaș-Cadariu, P., Piciu, A., Căinap, S. S., Căinap, C., & Piciu, D. (2021). A Comparative Study between 18F-FDG PET/CT and Conventional Imaging in the Evaluation of Progressive Disease and Recurrence in Ovarian Carcinoma. Healthcare, 9(6), 666. https://doi.org/10.3390/healthcare9060666







