Abstract
(1) Background: Stretching is known to improve range of motion (ROM), and evidence has suggested that strength training (ST) is effective too. However, it is unclear whether its efficacy is comparable to stretching. The goal was to systematically review and meta-analyze randomized controlled trials (RCTs) assessing the effects of ST and stretching on ROM (INPLASY 10.37766/inplasy2020.9.0098). (2) Methods: Cochrane Library, EBSCO, PubMed, Scielo, Scopus, and Web of Science were consulted in October 2020 and updated in March 2021, followed by search within reference lists and expert suggestions (no constraints on language or year). Eligibility criteria: (P) Humans of any condition; (I) ST interventions; (C) stretching (O) ROM; (S) supervised RCTs. (3) Results: Eleven articles (n = 452 participants) were included. Pooled data showed no differences between ST and stretching on ROM (ES = −0.22; 95% CI = −0.55 to 0.12; p = 0.206). Sub-group analyses based on risk of bias, active vs. passive ROM, and movement-per-joint analyses showed no between-protocol differences in ROM gains. (4) Conclusions: ST and stretching were not different in their effects on ROM, but the studies were highly heterogeneous in terms of design, protocols and populations, and so further research is warranted. However, the qualitative effects of all the studies were quite homogeneous.
1. Introduction
Joint range of motion (ROM) is the angle by which a joint moves from its resting position to the extremities of its motion in any given direction [1]. Improving ROM is a core goal for the general population [2], as well as in clinical contexts [3], such as in treating acute respiratory failure [4], plexiform neurofibromas [5], recovering from breast cancer-related surgery [6], and total hip replacement [7]. Several common clinical conditions negatively affect ROM, such as ankylosing spondylitis [8], cerebral palsy [9], Duchenne muscular dystrophy [10], osteoarthritis [11] rheumatoid arthritis [12]. Unsurprisingly, ROM gains are also relevant in different sports [13], such as basketball, baseball and rowing [14,15,16]. ROM is improved through increased stretch tolerance, augmented fascicle length and changes in pennation angle [17], as well as reduced tonic reflex activity [18]. Stretching is usually prescribed for increasing ROM in sports [19,20], clinical settings, such as chronic low back pain [21], rheumatoid arthritis [22], and exercise performance in general [23]. Stretching techniques, include static (active or passive), dynamic, or proprioceptive neuromuscular facilitation (PNF), all of which can improve ROM [2,24,25,26,27].
It should be noted that muscle weakness is associated with diminished ROM [28,29,30]. Strength training (ST) can be achieved through a number of methods, as long as resistance is applied to promote strength gains, and includes methods as diverse as using free weights or plyometrics [31]. Although ST primarily addresses muscle weakness, it has been shown to increase ROM [32]. For example, hip flexion and extension ROM of adolescent male hurdles was improved using plyometrics [33], while judo fighters improved ROM (shoulder flexion, extension, abduction and adduction; trunk flexion and extension; and hip flexion and extension) through resistance training [34]. The ROM gains, using resistance training, have also been described in relation to healthy elderly people for hip flexion and cervical extension [35], and isometric neck strength training, with an elastic band, in women with chronic nonspecific neck pain improved neck flexion, extension, rotation and lateral flexion [36]. ST that is focused on concentric and eccentric contractions has been shown to increase fascicle length [37,38,39]. Improvements in agonist-antagonist co-activation [40], reciprocal inhibition [41], and potentiated stretch-shortening cycles due to greater active muscle stiffness [42] may also explain why ST is a suitable method for improving ROM.
Nevertheless, studies comparing the effects of ST and stretching in ROM have presented conflicting evidence [43,44], and many have small sample sizes [45,46]. Developing a systematic review and meta-analysis may help summarize this conflicting evidence and increase statistical power, thus, providing clearer guidance for interventions [47]. Therefore, the aim of this systematic review and meta-analysis was to compare the effects of supervised and randomized ST versus stretching protocols on ROM in participants of any health and training status.
2. Materials and Methods
2.1. Protocol and Registration
The methods and protocol registration were preregistered prior to conducting the review: INPLASY, no.202090098, DOI:10.37766/inplasy2020.9.0098.
2.2. Eligibility Criteria
Articles were eligible for inclusion if published in peer-reviewed journals, with no restrictions in language or publication date. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were adopted [48]. Participants, interventions, comparators, outcomes, and study design (P.I.C.O.S.) were established as follows: (i) Participants with no restriction regarding health, sex, age, or training status; (ii) ST interventions supervised by a certified professional. ST was defined as any method focused on developing strength, ranging from resistance training to plyometrics [31]; no limitations were placed with regard to intensity, volume, type of contractions and frequency, as it could excessively narrow the searches; (iii) comparators were supervised groups performing any form of stretching, including static stretching, passive stretching, dynamic stretching, and PNF [2], regardless of their intensity, duration or additional features; (iv) outcomes were ROM assessed in any joint, preferably through goniometry, but standardized tests such as the sit-and-reach were also acceptable; (v) randomized controlled trials (RCTs). RCTs reduce bias and better balance participant features between the groups [47], and are important for the advancement of sports science [49]. There were no limitations regarding intervention length.
The study excluded reviews, letters to editors, trial registrations, proposals for protocols, editorials, book chapters, and conference abstracts. Exclusion criteria, based on P.I.C.O.S., included: (i) Research with non-human animals; (ii) non-ST protocols or ST interventions combined with other methods (e.g., endurance); unsupervised interventions; (iii) stretching or ST + stretching interventions combined with other training methods (e.g., endurance); protocols without stretching; unsupervised interventions; (iv) studies not reporting ROM; (v) non-randomized interventions.
2.3. Information Sources and Search
Six databases were used to search and retrieve the articles in early October 2020: Cochrane Library, EBSCO, PubMed (including MEDLINE), Scielo, Scopus, and Web of Science (Core Collection). Boolean operators were applied to search the article title, abstract and/or keywords: (“strength training” OR “resistance training” OR “weight training” OR “plyometric*” OR “calisthenics”) AND (“flexibility” OR “stretching”) AND “range of motion” AND “random*”. The specificities of each search engine included: (i) Cochrane Library, items were limited to trials, including articles but excluding protocols, reviews, editorials and similar publications; (ii) EBSCO, the search was limited to articles in scientific, peer-reviewed journals (iii) PubMed, the search was limited to title or abstract; publications were limited to RCTs and clinical trials, excluding books and documents, meta-analyses, reviews and systematic reviews; (iv) in Scielo, Scopus and Web of Science, the publication type was limited to article; and (v) Web of Science, “topic” is the term used to refer to title, abstract and keywords.
An additional search was conducted within the reference lists of the included records. The list of articles and inclusion criteria were then sent to four experts to suggest additional references. The search strategy and consulted databases were not provided in this process to avoid biasing the experts’ searches. More detailed information is available as supplementary material.
Updated searches: on 8 March 2021, we conducted new searches in the databases. However, each database has specific approaches to filtering the searches by date. In Cochrane, we searched for articles entering the database in the previous 6 months. In EBSCO, we searched for all fields starting from October 2020 onwards. In PubMed, the entry date was set to 1 October 2020, onwards. In Scielo, Scopus and Web of Science, publication date was limited to 2020 and 2021.
2.4. Search Strategy
Here, we provide the specific example of search conducted in PubMed:
(((“strength training” [Title/Abstract] OR “resistance training” [Title/Abstract] OR “weight training” [Title/Abstract] OR “plyometric*” [Title/Abstract] OR “calisthenics” [Title/Abstract]) AND (“flexibility” [Title/Abstract] OR “stretching” [Title/Abstract])) AND (“range of motion” [Title/Abstract])) AND (“random*” [Title/Abstract]).
After this search, the filters RCT and Clinical Trial were applied.
2.5. Study Selection
J.A. and F.M.C. each conducted the initial search and selection stages independently, and then compared result to ensure accuracy. J.F. and T.R. independently reviewed the process to detect potential errors. When necessary, re-analysis was conducted until a consensus was achieved.
2.6. Data Collection Process
J.A., F.M.C., A.A.M. and J.F. extracted the data, while J.M., T.R., R.Z. and A.M. independently revised the process. Data for the meta-analysis were extracted by JA and independently verified by A.A.M. and R.R.C. Data were available for sharing.
2.7. Data Items
Data items: (i) Population: subjects, health status, sex/gender, age, training status, selection of subjects; (ii) intervention and comparators: Study length in weeks, weekly frequency of the sessions, weekly training volume in minutes, session duration in minutes, number of exercises per session, number of sets and repetitions per exercise, load (e.g., % 1 Repetition Maximum), full versus partial ROM, supervision ratio; in the comparators, modality of stretching applied was also considered; adherence rates were considered a posteriori; (iii) ROM testing: joints and actions, body positions (e.g., standing, supine), mode of testing (i.e., active, passive, both), pre-testing warm-up, timing (e.g., pre- and post-intervention, intermediate assessments), results considered for a given test (e.g., average of three measures), data reliability, number of testers and instructions provided during testing; (iv) Outcomes: changes in ROM for intervention and comparator groups; (vi) funding and conflicts of interest.
2.8. Risk of Bias in Individual Studies
The risk of bias (RoB) in individual studies was assessed using the Cochrane risk-of-bias tool for randomized trials (RoB 2) [50]. J.A. and A.M. independently completed RoB analysis, which was reviewed by F.M.C. Where inconsistencies emerged, the original articles were re-analyzed until a consensus was achieved.
2.9. Summary Measures
Meta-analysis was conducted when ≥3 studies were available [51]. Pre- and post-intervention means and standard deviations (SDs) for dependent variables were used after being converted to Hedges’s g effect size (ES) [51]. When means and SDs were not available, they were calculated from 95% confidence intervals (CIs) or standard error of mean (SEM), using Cochrane’s RevMan Calculator for Microsoft Excel [52]. When ROM data from different groups (e.g., men and women) or different joints (e.g., knee and ankle) was pooled, weighted formulas were applied [47].
2.10. Synthesis of Results
The inverse variance random-effects model for meta-analyses [53,54] was used to allocate a proportionate weight to trials based on the size of their individual standard errors [55], and accounting for heterogeneity across studies [56]. The ESs were presented alongside 95% CIs and interpreted using the following thresholds [57]: <0.2, trivial; 0.2–0.6, small; >0.6–1.2, moderate; >1.2–2.0, large; >2.0–4.0, very large; >4.0, extremely large. Heterogeneity was assessed using the I2 statistic, with values of <25%, 25–75%, and >75% considered to represent low, moderate, and high levels of heterogeneity, respectively [58]. Data used for meta-analysis is available in a supplementary Excel file.
2.11. Risk of Bias Across Studies
Publication bias was explored using the extended Egger’s test [59], with p < 0.05 implying bias. To adjust for publication bias, a sensitivity analysis was conducted using the trim and fill method [60], with L0 as the default estimator for the number of missing studies [61].
2.12. Moderator Analyses
Using a random-effects model and independent computed single factor analysis, potential sources of heterogeneity likely to influence the effects of training interventions were selected, including (i) ROM type (i.e., passive versus active), (ii) studies RoB in randomization, and (iii) studies RoB in measurement of the outcome [62]. These analyses were decided post-protocol registration.
All analyses were carried out using the Comprehensive Meta-Analysis program (version 2; Biostat, Englewood, NJ, USA). Statistical significance was set at p ≤ 0.05. Data for the meta-analysis were extracted by JA and independently verified by A.A.M. and R.R.C.
2.13. Quality and Confidence in Findings
Although not planned in the registered protocol, we decided to abide by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [63], which addresses five dimensions that can downgrade studies when assessing the quality of evidence in RCTs. RoB, inconsistency (through heterogeneity measures), and publication bias were addressed above and were considered a priori. Directness was guaranteed by design, as no surrogates were used for any of the pre-defined P.I.C.O. dimensions. Imprecision was assessed on the basis of 95% CIs.
3. Results
3.1. Study Selection
An initial search returned 194 results (52 in Cochrane Library, 11 in EBSCO, 11 in PubMed, 9 in Scielo, 88 in Scopus, and 23 in Web of Science). After removal of duplicates, 121 records remained. Screening the titles and abstracts for eligibility criteria resulted in the exclusion of 106 articles: 26 were not original research articles (e.g., trial registrations, reviews), 24 were out of scope, 48 did not have the required intervention or comparators, five did not assess ROM, two were non-randomized and one was unsupervised. Fifteen articles were eligible for full-text analysis. One article did not have the required intervention [64], and two did not have the needed comparators [65,66]. In one article, the ST and stretching groups performed a 20–30 min warm-up following an unspecified protocol [67]. In another, the intervention and comparator were unsupervised [68], and in one the stretching group was unsupervised [69]. Finally, in one article, 75% of the training sessions were unsupervised [70]. Therefore, eight articles were included at this stage [33,43,44,45,46,71,72,73].
A manual search within the reference lists of the included articles revealed five additional potentially fitting articles. Two lacked the intervention group required [74,75], and two were non-randomized [76,77]. One article met the inclusion criteria [78]. Four experts revised the inclusion criteria and the list of articles and suggested eight articles based on their titles and abstracts. Six were excluded: interventions were multicomponent [79,80]; comparators performed no exercise [81,82]; out of scope [83]; and unsupervised stretching group [84]. Two articles were included [85,86], increasing the list to eleven articles [33,43,44,45,46,71,72,73,78,85,86], with 452 participants eligible for meta-analysis (Figure 1). Updated searches: in the renewed searches, 28 records emerged, of which two passed the screening. However, these two records had already been included in our final sample. Therefore, no new article was included.
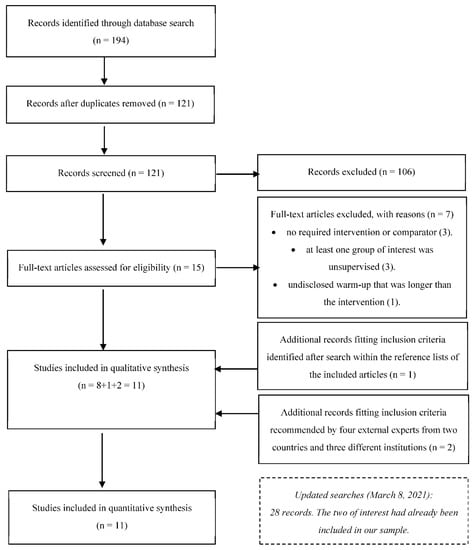
Figure 1.
Flowchart describing the study selection process.
3.2. Study Characteristics and Results
The data items can be found in Table 1. The study of Wyon, Smith and Koutedakis [73] required consultation of a previous paper [87] to provide essential information. Samples ranged from 27 [46] to 124 subjects [43], including: Trained participants, i.e., engaging in systematic exercise programs [33,45,72,73], healthy sedentary participants [44,71,78], sedentary and trained participants [86], workers with chronic neck pain [46], participants with fibromyalgia [85], and elderly participants with difficulties in at least one of four tasks: transferring, bathing, toileting, and walking [43]. Seven articles included only women [45,73,78,85] or predominantly women [43,44,46]; three investigated only men [33,86] or predominantly men [71]; and one article had a balanced mixture of men and women [72].

Table 1.
Characteristics of included randomized trials.
Interventions lasted between five [71] and 16 weeks [78]. Minimum weekly training frequency was two sessions [46,85] and maximum was five [73]. Six articles provided insufficient information concerning session duration [44,45,72,73,78,86]. Ten articles vaguely defined training load for the ST and stretching groups [33,43,46,71,72,85,86], or for stretching groups [44,45,78]. Six articles did not report on using partial or full ROM during ST exercises [33,43,45,46,72,78]. Different stretching modalities were implemented: static active [44,46,71,78,85,86], dynamic [43,45], dynamic with a 10-s hold [33], static active in one group and static passive in another [73], and a combination of dynamic, static active, and PNF [72].
Hip joint ROM was assessed in seven articles [33,43,45,71,72,73,78], knee ROM in five [43,44,45,71,86], shoulder ROM in four [43,45,71,85], elbow and trunk ROM in two [43,45], and cervical spine [46] and the ankle joint ROM in one article [43]. In one article, active ROM (AROM) was tested for the trunk, while passive ROM (PROM) was tested for the other joints [43]. In one article, PROM was tested for goniometric assessments and AROM for hip flexion [45]. In another, AROM was assessed for the shoulder and PROM for the hip and knee [71]. Three articles only assessed PROM [44,72,86], and four AROM [33,46,78,85], while one assessed both for the same joint [73].
In seven articles [33,46,71,72,78,85], ST and stretching groups significantly improved ROM, and the differences between the groups were non-significant. In one article, the ST group had significant improvements in 8 of 10 ROM measures, while dynamic stretching did not lead to improvement in any of the groups [43]. In another article, the three groups significantly improved PROM, without between-group differences; the ST and the static active stretching groups also significantly improved AROM [73]. In two articles, none of the groups improved ROM [44,45].
3.3. Risk of Bias in Individual Studies
Table 2 presents assessments of RoB. Bias arising from the randomization process was low in four articles [43,45,73,85], moderate in one [46] and high in six [33,44,71,72,78,86]. Bias due to deviations from intended interventions, missing outcome data, and selection of the reported results was low. Bias in measurement of the outcome was low in six articles [44,45,46,78,85,86], but high in five [33,43,71,72,73].

Table 2.
Assessments of risk of bias (Cochrane’s RoB 2).
3.4. Synthesis of Results
Comparisons were performed between ST and stretching groups, involving eleven articles and 452 participants. Global effects on ROM were achieved pooling data from the different joints. One article did not have the data required [44], but the authors supplied it upon request. For another article [45], we also requested data relative to the goniometric evaluations, but obtained no response. Therefore, only data from the sit-and-reach test were used. For one article [46], means and SDs were obtained from 95% CIs, while in another [85], SDs were extracted from SEMs using Cochrane’s RevMan Calculator.
From the five articles, including both genders, four provided pooled data, with no distinction between genders [43,44,46,71]. One article presented data separated by gender, without significant differences between men and women in response to interventions [72]. Weighted formulas were applied sequentially for combining means and SDs of groups within the same study [47]. Two studies presented the results separated by left and right lower limbs, with both showing similar responses to the interventions [33,73]; outcomes were combined using the same weighted formulas for the means and SDs. Five articles only presented one decimal place [33,43,46,72,78], and so all values were rounded for uniformity.
Effects of ST versus stretching on ROM: no significant difference was noted between ST and stretching (ES = −0.22; 95% CI = −0.55 to 0.12; p = 0.206; I2 = 65.4%; Egger’s test p = 0.563; Figure 2). The relative weight of each study in the analysis ranged from 6.4% to 12.7% (the size of the plotted squares in Figure 2 reflects the statistical weight of each study).
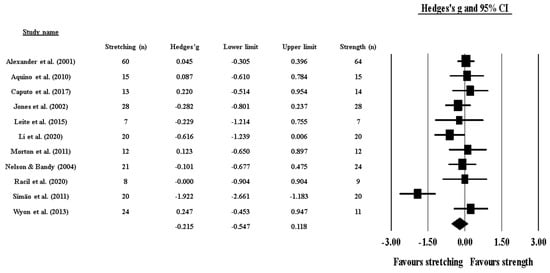
Figure 2.
Forest plot of changes in ROM after participating in stretching-based compared to Scheme 95. confidence intervals (CI). The size of the plotted squares reflects the statistical weight of the study.
3.5. Additional Analyses
Effects of ST versus stretching on ROM, moderated by study RoB in randomization: No significant sub-group differences in ROM changes (p = 0.256) was found when programs with high RoB (6 studies; ES = −0.41; 95% CI = −1.02 to 0.20; within-group I2 = 77.5%) were compared to programs with low RoB (4 studies; ES = −0.03; 95% CI = −0.29 to 0.23; within-group I2 = 0.0%) (Figure 3).
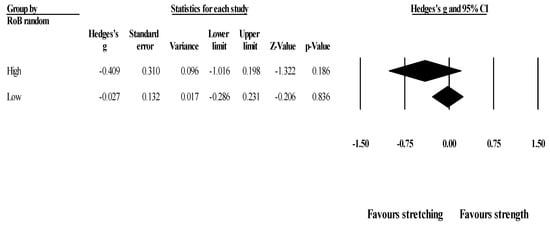
Figure 3.
Forest plot of changes in ROM after participating in stretching-based compared to Scheme 95. confidence intervals (CI).
Effects of ST versus stretching on ROM, moderated by study RoB in measurement of the outcome: No significant sub-group difference in ROM changes (p = 0.320) was found when programs with high RoB (5 studies; ES = −0.04; 95% CI = −0.31 to 0.24; within-group I2 = 8.0%) were compared to programs with low RoB (6 studies; ES = −0.37; 95% CI = −0.95 to 0.22; within-group I2 = 77.3%) (Figure 4).
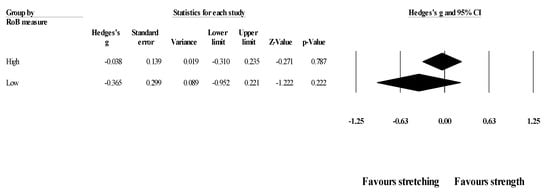
Figure 4.
Forest plot of changes in ROM after participating in stretching-based compared to Scheme 95. confidence intervals (CI).
Effects of ST versus stretching on ROM, moderated by ROM type (active vs. passive): No significant sub-group difference in ROM changes (p = 0.642) was found after training programs that assessed active (8 groups; ES = −0.15; 95% CI = −0.65 to 0.36; within-group I2 = 78.7%) compared to passive ROM (6 groups; ES = −0.01; 95% CI = −0.27 to 0.24; within-group I2 = 15.3%) (Figure 5).
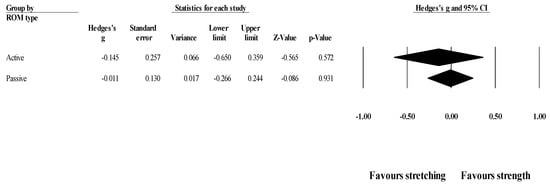
Figure 5.
Forest plot of changes in ROM after participating in stretching-based compared to Scheme 95. confidence intervals (CI).
Effects of ST versus stretching on hip flexion ROM: Seven studies provided data for hip flexion ROM (pooled n = 294). There was no significant difference between ST and stretching interventions (ES = −0.24; 95% CI = −0.82 to 0.34; p = 0.414; I2 = 80.5%; Egger’s test p = 0.626; Figure 6). The relative weight of each study in the analysis ranged from 12.0% to 17.4% (the size of the plotted squares in Figure 6 reflects the statistical weight of each study).
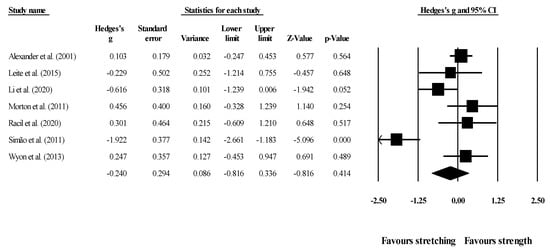
Figure 6.
Forest plot of changes in hip flexion ROM after participating in stretching-based compared to strength-based training interventions. Values shown are effect sizes (Hedges’s g) with 95% confidence intervals (CI). The size of the plotted squares reflects the statistical weight of the study.
Effects of ST versus stretching on hip flexion ROM, moderated by study RoB in randomization: No significant sub-group difference in hip flexion ROM changes (p = 0.311) was found when programs with high RoB in randomization (4 studies; ES = −0.46; 95% CI = −1.51 to 0.58; within-group I2 = 86.9%) were compared to programs with low RoB in randomization (3 studies; ES = 0.10; 95% CI = −0.20 to 0.40; within-group I2 = 0.0%) (Figure 7).
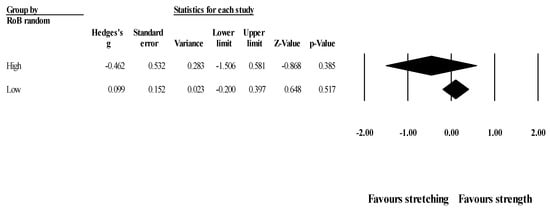
Figure 7.
Forest plot of changes in hip flexion ROM after participating in stretching-based compared to strength-based training interventions with high versus low RoB in randomization. Values shown are effect sizes (Hedges’s g) with 95% confidence intervals (CI).
Effects of ST versus stretching on hip flexion ROM, moderated by ROM type (active vs. passive): No significant sub-group difference in hip flexion ROM changes (p = 0.466) was found after the programs assessed active (4 groups; ES = −0.38; 95% CI = −1.53 to 0.76; within-group I2 = 87.1%) compared to passive ROM (4 groups; ES = 0.08; 95% CI = −0.37 to 0.52; within-group I2 = 56.5%) (Figure 8).
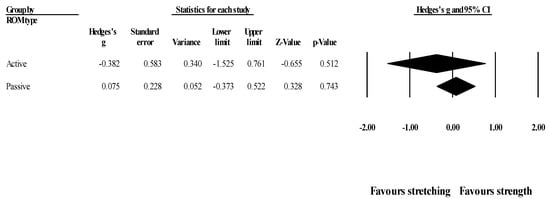
Figure 8.
Forest plot of changes in hip flexion ROM after participating in stretching-based compared to strength-based training interventions assessing active or passive ROM. Values shown are effect sizes (Hedges’s g) with 95% confidence intervals (CI).
Effects of ST versus stretching on knee extension ROM: Four studies provided data for knee extension ROM (pooled n = 223). There was no significant difference between ST and stretching interventions (ES = 0.25; 95% CI = −0.02 to 0.51; p = 0.066; I2 = 0.0%; Egger’s test p = 0.021; Figure 9). After the application of the trim and fill method, the adjusted values changed to ES = 0.33 (95% CI = 0.10 to 0.57), favoring ST. The relative weight of each study in the analysis ranged from 11.3% to 54.2% (the size of the plotted squares in Figure 9 reflects the statistical weight of each study).
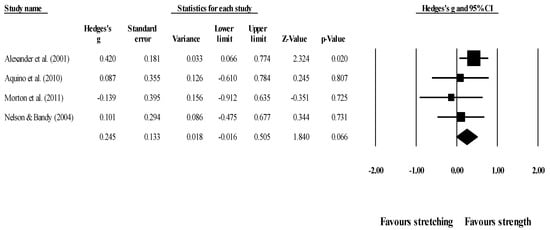
Figure 9.
Forest plot of changes in knee extension ROM after participating in stretching-based compared to strength-based training interventions (all assessed passive ROM). Values shown are effect sizes (Hedges’s g) with 95% confidence intervals (CI). The size of the plotted squares reflects the statistical weight of the study.
One article behaved as an outlier in all comparisons, favoring stretching [78], but after sensitivity analysis the results remained unchanged (p > 0.05), with all ST versus stretching comparisons remaining non-significant.
3.6. Confidence in Cumulative Evidence
Table 3 presents GRADE assessments. ROM is a continuous variable, and so a high degree of heterogeneity was expected [88]. Imprecision was moderate, likely reflecting the fact that ROM is a continuous variable. Overall, both ST and stretching consistently promoted ROM gains, but no recommendation could be made favoring one protocol.

Table 3.
GRADE assessments for the certainty of evidence.
4. Discussion
4.1. Summary of Evidence
The aim of this systematic review and meta-analysis was to compare the effects of supervised and randomized ST compared to stretching protocols on ROM, in participants of any health and training status. Qualitative synthesis showed that ST and stretching interventions were not statistically different in improving ROM. However, the studies were highly heterogeneous with regard to the nature of the interventions and moderator variables, such as gender, health, or training status. This had been reported in the original manuscripts as well. A meta-analysis, including 11 articles and 452 participants, showed that ST and stretching interventions were not statistically different in active and passive ROM changes, regardless of RoB in the randomization process, or in measurement of the outcome. RoB was low for deviations from intended interventions, missing outcome data, and selection of the reported results. No publication bias was detected.
High heterogeneity is expected in continuous variables [88], such as ROM. However, more research should be conducted to afford sub-group analysis according to characteristics of the analyzed population, as well as protocol features. For example, insufficient reporting of training volume and intensity meant it was impossible to establish effective dose-response relationships, although a minimum of five weeks of intervention [71], and two weekly sessions were sufficient to improve ROM [46,85]. Studies were not always clear with regard to the intensity used in ST and stretching protocols. Assessment of stretching intensity is complex, but a practical solution may be to apply scales of perceived exertion [73], or the Stretching Intensity Scale [89]. ST intensity may also moderate effects on ROM [90], and ST with full versus partial ROM may have distinct neuromuscular effects [81] and changes in fascicle length [37]. Again, the information was insufficient to discuss these factors, which could potentially explain part of the heterogeneity of results. This precludes advancing stronger conclusions and requires further research to be implemented.
Most studies showed ROM gains in ST and stretching interventions, but in two studies, neither group showed improvements [44,45]. Although adherence rates were unreported by Aquino, Fonseca, Goncalves, Silva, Ocarino and Mancini [44], they were above 91.7% in Leite, De Souza Teixeira, Saavedra, Leite, Rhea and Simão [45], thus providing an unlikely explanation for these results. In the study by Aquino, Fonseca, Goncalves, Silva, Ocarino and Mancini [44], the participants increased their stretch tolerance, and the ST group changed the peak torque angle, despite no ROM gains. The authors acknowledged that there was high variability in measurement conditions (e.g., room temperature), which could have interfered with calculations. Leite, De Souza Teixeira, Saavedra, Leite, Rhea and Simão [45] suggested that the use of dynamic instead of static stretching could explain the lack of ROM gains in the stretching and stretching + ST groups. However, other studies using dynamic stretching have shown ROM gains [33,43]. Furthermore, Leite, De Souza Teixeira, Saavedra, Leite, Rhea and Simão [45] provided no interpretation for the lack of ROM gains in the ST group.
Globally, however, both ST and stretching were effective in improving ROM. We asked what the reason for ST to improve ROM in a manner that is not statistically distinguishable from stretching? A first thought might be to speculate that perhaps the original studies used sub-threshold stretching intensities and/or durations. However, the hypothesis that ST has intrinsic merit for improving ROM should also be considered. ST with an eccentric focus demands the muscles to produce force on elongated positions, and a meta-analysis showed limited-to-moderate evidence that eccentric ST is associated with increases in fascicle length [91]. Likewise, a recent study showed that 12 sessions of eccentric ST increased fascicle length of the biceps femoris long head [38]. However, ST with an emphasis in concentric training has been shown to increase fascicle length when full ROM was required [37]. In a study with nine older adults, ST increased fascicle length in both the eccentric and concentric groups, albeit more prominently in the former [92]. Conversely, changes in pennation angle were superior in the concentric group (35% increase versus 5% increase). Plyometric training can also increase plantar flexor tendon extensibility [42].
One article showed significant reductions in pain associated with increases in strength [46]. Therefore, decreased pain sensitivity may be another mechanism by which ST promotes ROM gains. An improved agonist-antagonist coactivation is another possible mechanism promoting ROM gains, through better adjusted force ratios [40,73]. Also, some articles included in the meta-analysis assessed other outcomes in addition to ROM, and these indicated that ST programs may have additional advantages when compared to stretching, such as greater improvements in neck flexors endurance [46], ten repetition maximum Bench Press and Leg Press [45,78], and countermovement jump and 60-m sprint with hurdles [33] which may favor the choice of ST over stretching interventions.
4.2. Limitations
After protocol registration, we chose to improve upon the design, namely adding two dimensions (directness and imprecision) that would provide a complete GRADE assessment. Furthermore, subgroup analyses were not planned a priori. There is a risk of multiple subgroup analyses generating a false statistical difference, merely to the number of analyses conducted [47]. However, all analyses showed an absence of significant differences and therefore provide a more complete understanding that the effects of ST or stretching on ROM are consistent across conditions. Looking backwards, perhaps removing the filters used in the initial searches could have provided a greater number of records. Notwithstanding, it would also likely provide a huge number of non-relevant records, including opinion papers and reviews. Moreover, consultation with four independent experts may hopefully have resolved this shortcoming.
Due to the heterogeneity of populations analysed, sub-group analysis according to sex or age group were not possible, and so it would be important to explore if these features interact with the protocols in meaningful ways. Moreover, there was a predominance of studies with women, meaning more research with men is advised. There was also a predominance of assessments of hip joint ROM, followed by knee and shoulder, with the remaining joints receiving little to no attention. In addition, dose-response relationships could not be addressed, mainly due to poor reporting. However, the qualitative findings of all the studies were very homogeneous, with statistical significance tests failing to show differences between ST and stretching protocols.
5. Conclusions
Overall, ST and stretching were not statistically different in ROM improvements, both in short-term interventions [71], and in longer-term protocols [78], suggesting that a combination of neural and mechanical factors is at play. However, the heterogeneity of study designs and populations precludes any definite conclusions and invites researchers to delve deeper into this phenomenon. Notwithstanding this observation, the qualitative effects were quite similar across studies. Therefore, if ROM gains are a desirable outcome, both ST and stretching reveal promising effects, but future research should better explore this avenue. In addition, the studies included in this review showed that ST had a few advantages in relation to stretching, as was explored in the discussion. Furthermore, session duration may negatively impact adherence to an exercise program [93]. If future research confirms that ST generates ROM gains similar to those obtained with stretching, clinicians may prescribe smaller, more time-effective programs when deemed convenient and appropriate, thus eventually increasing patient adherence rates. Alternatively, perhaps studies using stretching exercises should better assess their intensity and try to establish minimum thresholds for their efficacy in improving ROM.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/healthcare9040427/s1.
Author Contributions
We followed ICJME guidelines. Therefore, all authors have provided substantial contributions for the conceptualization and design of the study, acquisition, analysis and interpretation of data, as well as drafting and revising the manuscript critically. J.A., R.R.-C. and F.M.C. conceptualized the work and were actively involved in all stages of the manuscript. A.M., A.A.M., J.F., J.M., T.R. and R.Z. were more deeply involved in the methods and results. H.S. was more deeply invested in the rationale and discussion. All authors have read and agreed to the published version of the manuscript. Furthermore, all authors agree to be accountable for all aspects of the work. Contributors that did not meet these parameters are not listed as authors but are named in the acknowledgements.
Funding
No funding to declare.
Institutional Review Board Statement
Not applicable, since it was a review.
Informed Consent Statement
Not applicable, since it was a review.
Data Availability Statement
Our data were made available with the submission.
Acknowledgments
Richard Inman: language editing and proof reading. Pedro Morouço: pre-submission scientific review of the manuscript. Daniel Moreira-Gonçalves and Fábio Nakamura, plus two experts that chose to remain anonymous: Review of inclusion criteria and included articles, and proposal of additional articles to be included in the systematic review and meta-analysis. Filipe Manuel Clemente: This work is supported by Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior through national funds and when applicable co-funded EU funds under the project UIDB/50008/2020.
Conflicts of Interest
J.M. owns a company focused on Personal Trainer’s education but made no attempt to bias the team in protocol design and search process, and had no role in study selection or in extracting data for meta-analyses. The multiple cross-checks described in the methods provided objectivity to data extraction and analysis. Additionally, J.M. had no financial involvement in this manuscript. The other authors have no conflict of interest to declare.
References
- Kent, M. The Oxford Dictionary of Sports Science & Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Bayles, M.P., Swank, A.M., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- De Zoete, R.M.; Armfield, N.R.; McAuley, J.H.; Chen, K.; Sterling, M. Comparative effectiveness of physical exercise interventions for chronic non-specific neck pain: A systematic review with network meta-analysis of 40 randomised controlled trials. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Morris, P.E.; Berry, M.J.; Files, D.C.; Thompson, J.C.; Hauser, J.; Flores, L.; Dhar, S.; Chmelo, E.; Lovato, J.; Case, L.D.; et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA 2016, 315, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in children with inoperable plexiform neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.; Haddad, C.A.S.; Giron, P.S.; Figueira, P.V.G.; Estevão, A.; Elias, S.; Nazário, A.C.P.; Facina, G. Exercise protocol with limited shoulder range of motion for 15 or 30 days after conservative surgery for breast cancer with oncoplastic technique: A randomized clinical trial. Am. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Mou, P.; Zeng, W.N.; Chen, Y.; Zhou, Z. Synchronous or sequential cementless bilateral total hip arthroplasty for osseous ankylosed hips with ankylosing spondylitis. BMC Musculoskelet. Disord. 2021, 22, 302. [Google Scholar] [CrossRef]
- Bekteshi, S.; Vanmechelen, I.; Konings, M.; Ortibus, E.; Feys, H.; Monbaliu, E. Clinical presentation of spasticity and passive range of motion deviations in dyskinetic cerebral palsy in relation to dystonia, choreoathetosis, and functional classification systems. Dev. Neurorehabil. 2021, 24, 205–213. [Google Scholar] [CrossRef]
- Lloyd Morris, E.H.; Estilow, T.; Glanzman, A.M.; Cusack, S.V.; Yum, S.W. Improving temporomandibular range of motion in people with duchenne muscular dystrophy and spinal muscular atrophy. Am. J. Occup. Ther. 2020, 74. [Google Scholar] [CrossRef]
- Benner, R.W.; Shelbourne, K.D.; Bauman, S.N.; Norris, A.; Gray, T. Knee osteoarthritis: Alternative range of motion treatment. Orthop. Clin. N. Am. 2019, 50, 425–432. [Google Scholar] [CrossRef]
- Kojima, T.; Ishikawa, H.; Tanaka, S.; Haga, N.; Nishida, K.; Yukioka, M.; Hashimoto, J.; Miyahara, H.; Niki, Y.; Kimura, T.; et al. Characteristics of functional impairment in patients with long-standing rheumatoid arthritis based on range of motion of joints: Baseline data from a multicenter prospective observational cohort study to evaluate the effectiveness of joint surgery in the treat-to-target era. Mod. Rheumatol. 2018, 28, 474–481. [Google Scholar] [CrossRef]
- Pozzi, F.; Plummer, H.A.; Shanley, E.; Thigpen, C.A.; Bauer, C.; Wilson, M.L.; Michener, L.A. Preseason shoulder range of motion screening and in-season risk of shoulder and elbow injuries in overhead athletes: Systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, V.; del Coso, J.; Raya-González, J.; Nakamura, F.Y.; Castillo, D. Effects of basketball match-play on ankle dorsiflexion range of motion and vertical jump performance in semi-professional players. J. Sports Med. Phys. Fit. 2020, 60, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Wasserberger, K.; Oliver, G.D. Influence of a pre-throwing protocol on range of motion and strength in baseball athletes. Int. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Koldenhoven, R.M.; Jiwan, N.C.; Zhan, J.; Liu, T. Trunk and shoulder kinematics of rowing displayed by Olympic athletes. Sports Biomech. 2020. [Google Scholar] [CrossRef] [PubMed]
- Blazevich, A.; Cannavan, D.; Waugh, C.M.; Miller, S.C.; Thorlund, J.B.; Aagaard, P.; Kay, A.D. Range of motion, neuromechanical, and architectural adaptations to plantar flexor stretch training in humans. J. Appl. Physiol. 2014, 117, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Guissard, N.; Duchateau, J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve 2004, 29, 248–255. [Google Scholar] [CrossRef]
- Lima, C.D.; Brown, L.E.; Li, Y.; Herat, N.; Behm, D. Periodized versus non-periodized stretch training on gymnasts flexibility and performance. Int. J. Sports Med. 2019, 40, 779–788. [Google Scholar] [CrossRef]
- Behm, D.G.; Kay, A.D.; Trajano, G.S.; Blazevich, A.J. Mechanisms underlying performance impairments following prolonged static stretching without a comprehensive warm-up. Eur. J. Appl. Physiol. 2020. [Google Scholar] [CrossRef]
- Deyo, R.A.; Walsh, N.E.; Martin, D.C.; Schoenfeld, L.S.; Ramamurthy, S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N. Engl. J. Med. 1990, 322, 1627–1634. [Google Scholar] [CrossRef]
- Lamb, S.E.; Williamson, E.M.; Heine, P.J.; Adams, J.; Dosanjh, S.; Dritsaki, M.; Glover, M.J.; Lord, J.; McConkey, C.; Nichols, V.; et al. Exercises to improve function of the rheumatoid hand (SARAH): A randomised controlled trial. Lancet 2015, 385, 421–429. [Google Scholar] [CrossRef]
- Kokkonen, J.; Nelson, A.G.; Eldredge, C.; Winchester, J.B. Chronic static stretching improves exercise performance. Med. Sci. Sports Exerc. 2007, 39, 1825–1831. [Google Scholar] [CrossRef]
- Santos, C.; Beltrão, N.B.; Pirauá, A.L.T.; Durigan, J.L.Q.; Behm, D.; de Araújo, R.C. Static stretching intensity does not influence acute range of motion, passive torque, and muscle architecture. J. Sport Rehabil. 2020, 29, 1–6. [Google Scholar] [CrossRef]
- Iwata, M.; Yamamoto, A.; Matsuo, S.; Hatano, G.; Miyazaki, M.; Fukaya, T.; Fujiwara, M.; Asai, Y.; Suzuki, S. Dynamic stretching has sustained effects on range of motion and passive stiffness of the hamstring muscles. J. Sports Sci. Med. 2019, 18, 13–20. [Google Scholar]
- Lempke, L.; Wilkinson, R.; Murray, C.; Stanek, J. The effectiveness of PNF versus static Stretching on increasing hip-flexion range of motion. J. Sport Rehabil. 2018, 27, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.S.; Mazzone, M.A.; Taylor, K. The effect of 4 different durations of static hamstring stretching on passive knee-extension range of motion. J. Sport Rehabil. 2005, 14, 95–107. [Google Scholar] [CrossRef]
- Frasson, V.B.; Vaz, M.A.; Morales, A.B.; Torresan, A.; Telöken, M.A.; Gusmão, P.D.F.; Crestani, M.V.; Baroni, B.M. Hip muscle weakness and reduced joint range of motion in patients with femoroacetabular impingement syndrome: A case-control study. Braz. J. Phys. Ther. 2020, 24, 39–45. [Google Scholar] [CrossRef]
- Pettersson, H.; Boström, C.; Bringby, F.; Walle-Hansen, R.; Jacobsson, L.T.H.; Svenungsson, E.; Nordin, A.; Alexanderson, H. Muscle endurance, strength, and active range of motion in patients with different subphenotypes in systemic sclerosis: A cross-sectional cohort study. Scand. J. Rheumatol. 2019, 48, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Nakagawa, T.; Nakamura, K.; Engebretsen, L. Prevention and management of knee osteoarthritis and knee cartilage injury in sports. Br. J. Sports Med. 2011, 45, 304–309. [Google Scholar] [CrossRef]
- Bompa, T.O.; Buzzichelli, C.A. Periodization: Theory and Methodology of Training, 6th ed.; Human Kinetics: Champaign, IL, USA, 2018. [Google Scholar]
- Moscão, J.; Vilaça-Alves, J.; Afonso, J. A review of the effects of static stretching in human mobility and strength training as a more powerful alternative: Towards a different paradigm. Motricidade 2020, 16, 18–27. [Google Scholar] [CrossRef]
- Racil, G.; Jlid, M.C.; Bouzid, M.S.; Sioud, R.; Khalifa, R.; Amri, M.; Gaied, S.; Coquart, J. Effects of flexibility combined with plyometric exercises vs. isolated plyometric or flexibility mode in adolescent male hurdlers. J. Sports Med. Phys. Fit. 2020, 60, 45–52. [Google Scholar] [CrossRef]
- Saraiva, A.R.; Reis, V.M.; Costa, P.B.; Bentes, C.M.; Costae Silva, G.V.; Novaes, J.S. Chronic effects of different resistance training exercise orders on flexibility in elite judo athletes. J. Hum. Kinet. 2014, 40, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, N.H.; Ribeiro, A.S.; Nascimento, M.A.; Gobbo, L.A.; Schoenfeld, B.J.; Achour, A.; Gobbi, S.; Oliveira, A.R.; Cyrino, E.S. Effects of different resistance training frequencies on flexibility in older women. Clin. Interv. Aging 2015, 10, 531–538. [Google Scholar] [CrossRef]
- Ylinen, J.; Takala, E.P.; Nykänen, M.; Häkkinen, A.; Mälkiä, E.; Pohjolainen, T.; Karppi, S.L.; Kautiainen, H.; Airaksinen, O. Active neck muscle training in the treatment of chronic neck pain in women: A randomized controlled trial. JAMA 2003, 289, 2509–2516. [Google Scholar] [CrossRef]
- Valamatos, M.J.; Tavares, F.; Santos, R.M.; Veloso, A.P.; Mil-Homens, P. Influence of full range of motion vs. equalized partial range of motion training on muscle architecture and mechanical properties. Eur. J. Appl. Physiol. 2018, 118, 1969–1983. [Google Scholar] [CrossRef]
- Marušič, J.; Vatovec, R.; Marković, G.; Šarabon, N. Effects of eccentric training at long-muscle length on architectural and functional characteristics of the hamstrings. Scand. J. Med. Sci. Sports 2020. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.N.; Duhig, S.J.; Timmins, R.G.; Williams, M.D.; Opar, D.A.; Al Najjar, A.; Kerr, G.K.; Shield, A.J. Impact of the Nordic hamstring and hip extension exercises on hamstring architecture and morphology: Implications for injury prevention. Br. J. Sports Med. 2017, 51, 469–477. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.D.; Morse, C.I.; Thom, J.M.; de Haan, A.; Narici, M.V. Changes in antagonist muscles’ coactivation in response to strength training in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1022–1027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva-Batista, C.; Mattos, E.C.; Corcos, D.M.; Wilson, J.M.; Heckman, C.J.; Kanegusuku, H.; Piemonte, M.E.; Túlio de Mello, M.; Forjaz, C.; Roschel, H.; et al. Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson’s disease. J. Appl. Physiol. 2017, 122, 1–10. [Google Scholar] [CrossRef]
- Kubo, K.; Ishigaki, T.; Ikebukuro, T. Effects of plyometric and isometric training on muscle and tendon stiffness in vivo. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.B.; Galecki, A.T.; Grenier, M.L.; Nyquist, L.V.; Hofmeyer, M.R.; Grunawalt, J.C.; Medell, J.L.; Fry-Welch, D. Task-specific resistance training to improve the ability of activities of daily living-impaired older adults to rise from a bed and from a chair. J. Am. Geriatr. Soc. 2001, 49, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.F.; Fonseca, S.T.; Goncalves, G.G.; Silva, P.L.; Ocarino, J.M.; Mancini, M.C. Stretching versus strength training in lengthened position in subjects with tight hamstring muscles: A randomized controlled trial. Man. Ther. 2010, 15, 26–31. [Google Scholar] [CrossRef]
- Leite, T.; de Souza Teixeira, A.; Saavedra, F.; Leite, R.D.; Rhea, M.R.; Simão, R. Influence of strength and flexibility training, combined or isolated, on strength and flexibility gains. J. Strength Cond. Res. 2015, 29, 1083–1088. [Google Scholar] [CrossRef]
- Caputo, G.M.; di Bari, M.; Naranjo Orellana, J. Group-based exercise at workplace: Short-term effects of neck and shoulder resistance training in video display unit workers with work-related chronic neck pain-a pilot randomized trial. Clin. Rheumatol. 2017, 36, 2325–2333. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; MacAuley, D. The quality of research in sports journals. Br. J. Sports Med. 2002, 36, 124. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Skrede, T.; Steene-Johannessen, J.; Anderssen, S.A.; Resaland, G.K.; Ekelund, U. The prospective association between objectively measured sedentary time, moderate-to-vigorous physical activity and cardiometabolic risk factors in youth: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 55–74. [Google Scholar] [CrossRef]
- Drahota, A.; Beller, E. RevMan Calculator for Microsoft Excel; Cochrane: London, UK, 2020. [Google Scholar]
- Chiu, Y.H.; Chang, K.V.; Chen, I.J.; Wu, W.T.; Özçakar, L. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 6663–6672. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lee, T.M.; Han, D.S.; Chang, K.V. The prevalence of sarcopenia and its impact on clinical outcomes in lumbar degenerative spine disease—A systematic review and meta-analysis. J. Clin. Med. 2021, 10. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions: The Cochrane Collaboration; Higgins, J.P., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2008; pp. 243–296. [Google Scholar]
- Kontopantelis, E.; Springate, D.A.; Reeves, D. A re-analysis of the Cochrane Library data: The dangers of unobserved heterogeneity in meta-analyses. PLoS ONE 2013, 8, e69930. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, L. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahab, M.G.A.; Salem, E.E.S.; El-Hadidy, E.I.; El-Barbary, H.M. Effect of plyometric training on shoulder strength and active movements in children with Erb’s palsy. Int. J. Pharmtech. Res. 2016, 9, 25–33. [Google Scholar]
- Fernandez-Fernandez, J.; Ellenbecker, T.; Sanz-Rivas, D.; Ulbricht, A.; Ferrauti, A. Effects of a 6-week junior tennis conditioning program on service velocity. J. Sports Sci. Med. 2013, 12, 232–239. [Google Scholar]
- De Resende-Neto, A.G.; do Nascimento, M.A.; de Sa, C.A.; Ribeiro, A.S.; Desantana, J.M.; da Silva-Grigoletto, M.E. Comparison between functional and traditional training exercises on joint mobility, determinants of walking and muscle strength in older women. J. Sports Med. Phys. Fit. 2019, 59, 1659–1668. [Google Scholar] [CrossRef]
- Hajihosseini, E.; Norasteh, A.; Shamsi, A.; Daneshmandi, H.; Shahheidari, S. Effects of strengthening, stretching and comprehensive exercise program on the strength and range of motion of the shoulder girdle muscles in upper crossed syndrome. Med. Sport 2016, 69, 24–40. [Google Scholar]
- Fukuchi, R.K.; Stefanyshyn, D.J.; Stirling, L.; Ferber, R. Effects of strengthening and stretching exercise programmes on kinematics and kinetics of running in older adults: A randomised controlled trial. J. Sports Sci. 2016, 34, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, A.; Kautiainen, H.; Hannonen, P.; Ylinen, J. Strength training and stretching versus stretching only in the treatment of patients with chronic neck pain: A randomized one-year follow-up study. Clin. Rehabil. 2008, 22, 592–600. [Google Scholar] [CrossRef]
- Kalkman, B.M.; Holmes, G.; Bar-On, L.; Maganaris, C.N.; Barton, G.J.; Bass, A.; Wright, D.M.; Walton, R.; O’Brien, T.D. Resistance training combined with stretching increases tendon stiffness and is more effective than stretching alone in children with cerebral palsy: A randomized controlled trial. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.K.; Whitehead, J.R.; Brinkert, R.H.; Caine, D.J. Resistance training vs. static stretching: Effects on flexibility and strength. J. Strength Cond. Res. 2011, 25, 3391–3398. [Google Scholar] [CrossRef]
- Li, S.; Garrett, W.E.; Best, T.M.; Li, H.; Wan, X.; Liu, H.; Yu, B. Effects of flexibility and strength interventions on optimal lengths of hamstring muscle-tendon units. J. Sci. Med. Sport 2020, 23, 200–205. [Google Scholar] [CrossRef]
- Wyon, M.A.; Smith, A.; Koutedakis, Y. A comparison of strength and stretch interventions on active and passive ranges of movement in dancers: A randomized controlled trial. J. Strength Cond. Res. 2013, 27, 3053–3059. [Google Scholar] [CrossRef]
- Girouard, C.K.; Hurley, B.F. Does strength training inhibit gains in range of motion from flexibility training in older adults? Med. Sci. Sports Exerc. 1995, 27, 1444–1449. [Google Scholar] [CrossRef]
- Raab, D.M.; Agre, J.C.; McAdam, M.; Smith, E.L. Light resistance and stretching exercise in elderly women: Effect upon flexibility. Arch. Phys. Med. Rehabil. 1988, 69, 268–272. [Google Scholar] [PubMed]
- Klinge, K.; Magnusson, S.P.; Simonsen, E.B.; Aagaard, P.; Klausen, K.; Kjaer, M. The effect of strength and flexibility training on skeletal muscle electromyographic activity, stiffness, and viscoelastic stress relaxation response. Am. J. Sports Med. 1997, 25, 710–716. [Google Scholar] [CrossRef]
- Nóbrega, A.C.; Paula, K.C.; Carvalho, A.C. Interaction between resistance training and flexibility training in healthy young adults. J. Strength Cond. Res. 2005, 19, 842–846. [Google Scholar] [CrossRef]
- Simão, R.; Lemos, A.; Salles, B.; Leite, T.; Oliveira, É.; Rhea, M.; Reis, V.M. The influence of strength, flexibility, and simultaneous training on flexibility and strength gains. J. Strength Cond. Res. 2011, 25, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, C.-R.; Liang, W.-A.; Huang, C.-Y. Motor control integrated into muscle strengthening exercises has more effects on scapular muscle activities and joint range of motion before initiation of radiotherapy in oral cancer survivors with neck dissection: A randomized controlled trial. PLoS ONE 2020, 15, e0237133. [Google Scholar] [CrossRef]
- Venkataraman, K.; Tai, B.C.; Khoo, E.Y.H.; Tavintharan, S.; Chandran, K.; Hwang, S.W.; Phua, M.S.L.A.; Wee, H.L.; Koh, G.C.H.; Tai, E.S. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: A randomised controlled trial. Diabetologia 2019, 62, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, J.G.; Cava, A.M.; Courel-Ibáñez, J.; González-Badillo, J.J.; Morán-Navarro, R. Full squat produces greater neuromuscular and functional adaptations and lower pain than partial squats after prolonged resistance training. Eur. J. Sport Sci. 2020, 20, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Çergel, Y.; Topuz, O.; Alkan, H.; Sarsan, A.; Sabir Akkoyunlu, N. The effects of short-term back extensor strength training in postmenopausal osteoporotic women with vertebral fractures: Comparison of supervised and home exercise program. Arch. Osteoporos. 2019, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Albino, I.L.R.; Freitas, C.D.L.R.; Teixeira, A.R.; Gonçalves, A.K.; Santos, A.M.P.V.D.; Bós, Â.J.G. Influência do treinamento de força muscular e de flexibilidade articular sobre o equilíbrio corporal em idosas. Rev. Bras. Geriatr. Gerontol. 2012, 15, 17–25. [Google Scholar] [CrossRef]
- LeCheminant, J.D.; Hinman, T.; Pratt, K.B.; Earl, N.; Bailey, B.W.; Thackeray, R.; Tucker, L.A. Effect of resistance training on body composition, self-efficacy, depression, and activity in postpartum women. Scand. J. Med. Sci. Sports 2014, 24, 414–421. [Google Scholar] [CrossRef]
- Jones, K.D.; Burckhardt, C.S.; Clark, S.R.; Bennett, R.M.; Potempa, K.M. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J. Rheumatol. 2002, 29, 1041–1048. [Google Scholar]
- Nelson, R.T.; Bandy, W.D. Eccentric training and static stretching improve hamstring flexibility of high school males. J. Athl. Train. 2004, 39, 254–258. [Google Scholar]
- Wyon, M.A.; Felton, L.; Galloway, S. A comparison of two stretching modalities on lower-limb range of motion measurements in recreational dancers. J. Strength Cond. Res. 2009, 23, 2144–2148. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.R.; Vaz, J.R.; Gomes, L.; Silvestre, R.; Hilário, E.; Cordeiro, N.; Carnide, F.; Pezarat-Correia, P.; Mil-homens, P. A new tool to assess the perception of stretching intensity. J. Strength Cond. Res. 2015, 29, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Kambas, A.; Katrabasas, I.; Leontsini, D.; Chatzinikolaou, A.; Jamurtas, A.Z.; Douroudos, I.; Aggelousis, N.; Taxildaris, K. Resistance training and detraining effects on flexibility performance in the elderly are intensity-dependent. J. Strength Cond. Res. 2006, 20, 634–642. [Google Scholar] [CrossRef]
- Gérard, R.; Gojon, L.; Decleve, P.; van Cant, J. The effects of eccentric training on biceps femoris architecture and strength: A systematic review with meta-analysis. J. Athl. Train. 2020, 55, 501–514. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Longo, S.; Narici, M.V. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp. Physiol. 2009, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mirapeix, F.; Escolar-Reina, P.; Gascón-Cánovas, J.J.; Montilla-Herrador, J.; Jimeno-Serrano, F.J.; Collins, S.M. Predictive factors of adherence to frequency and duration components in home exercise programs for neck and low back pain: An observational study. BMC Musculoskelet. Disord. 2009, 10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).