External Validation of the Acute Kidney Injury Risk Prediction Score for Critically Ill Surgical Patients Who Underwent Major Non-Cardiothoracic Surgery
Abstract
1. Introduction
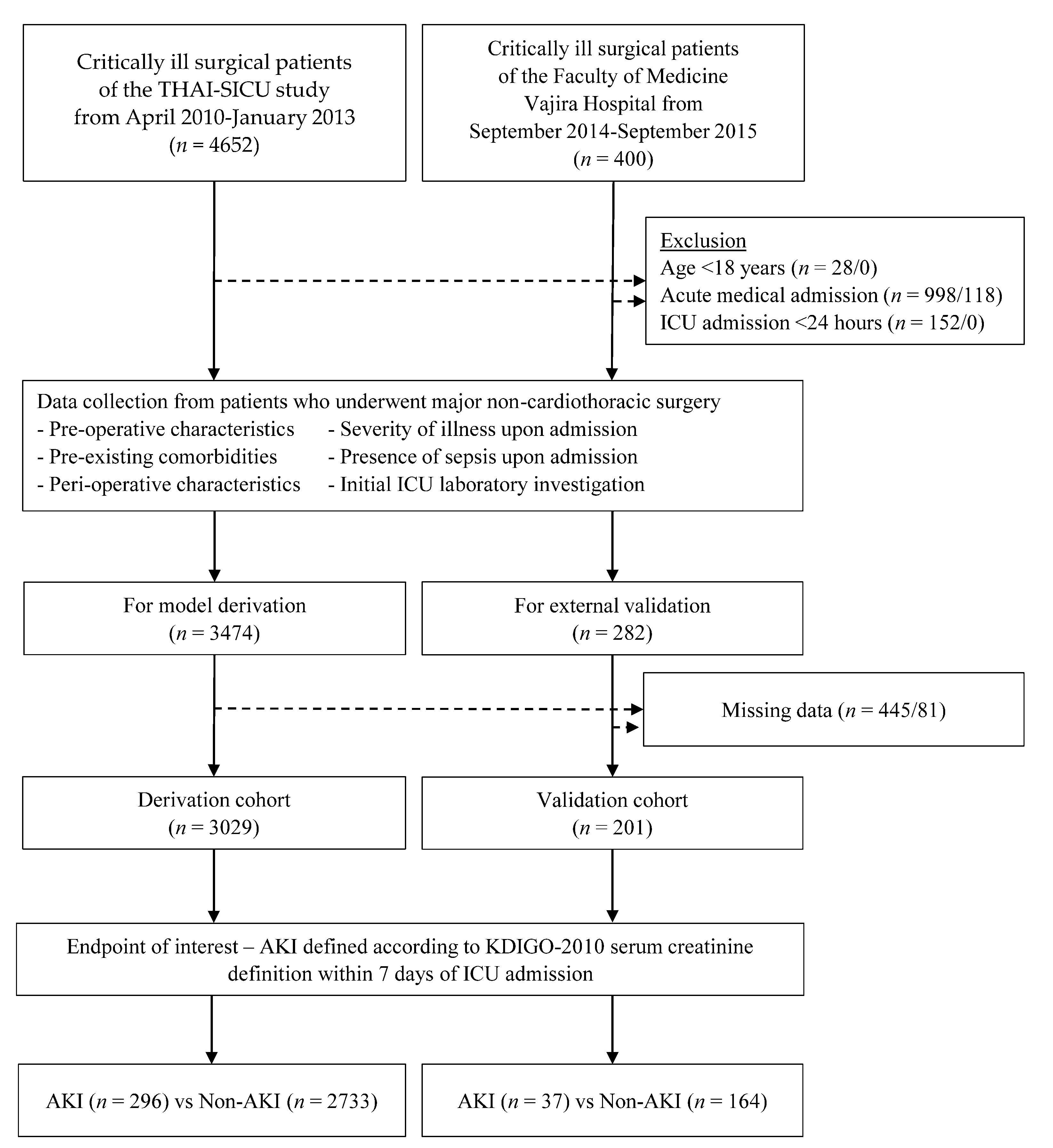
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W.; et al. Acute Kidney Injury: An Increasing Global Concern. Lancet Lond. Engl. 2013, 382, 170–179. [Google Scholar] [CrossRef]
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising Awareness of Acute Kidney Injury: A Global Perspective of a Silent Killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.-H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2015, 10, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Srisawat, N.; Kulvichit, W.; Mahamitra, N.; Hurst, C.; Praditpornsilpa, K.; Lumlertgul, N.; Chuasuwan, A.; Trongtrakul, K.; Tasnarong, A.; Champunot, R.; et al. The Epidemiology and Characteristics of Acute Kidney Injury in the Southeast Asia Intensive Care Unit: A Prospective Multicentre Study. Nephrol. Dial. Transplant. 2020, 35, 1729–1738. [Google Scholar] [CrossRef]
- Trongtrakul, K.; Sawawiboon, C.; Wang, A.Y.; Chitsomkasem, A.; Limphunudom, P.; Kurathong, S.; Prommool, S.; Trakarnvanich, T.; Srisawat, N. Acute Kidney Injury in Critically Ill Surgical Patients: Epidemiology, Risk Factors and Outcomes. Nephrology 2019, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef]
- Go, A.S.; Hsu, C.-Y.; Yang, J.; Tan, T.C.; Zheng, S.; Ordonez, J.D.; Liu, K.D. Acute Kidney Injury and Risk of Heart Failure and Atherosclerotic Events. Clin. J. Am. Soc. Nephrol. 2018, 13, 833–841. [Google Scholar] [CrossRef]
- Pisitsak, C.; Chittawatanarat, K.; Wacharasint, P.; Chaiwat, O.; Komonhirun, R.; Morakul, S. Prevalence, outcomes and risk factors of acute kidney injury in surgical intensive care unit: A multi-center Thai university-based surgical intensive care units study (THAI-SICU Study). J. Med. Assoc. Thail. 2016, 99, S193–S200. [Google Scholar]
- Gameiro, J.; Fonseca, J.A.; Neves, M.; Jorge, S.; Lopes, J.A. Acute Kidney Injury in Major Abdominal Surgery: Incidence, Risk Factors, Pathogenesis and Outcomes. Ann. Intensive Care 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Sear, J.W. Kidney Dysfunction in the Postoperative Period. Br. J. Anaesth. 2005, 95, 20–32. [Google Scholar] [CrossRef]
- Kim, J.M.; Jo, Y.Y.; Na, S.W.; Kim, S.I.; Choi, Y.S.; Kim, N.O.; Park, J.E.; Koh, S.O. The Predictors for Continuous Renal Replacement Therapy in Liver Transplant Recipients. Transplant. Proc. 2014, 46, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Wijeysundera, D.N.; Tait, G.A.; Beattie, W.S. Association of Intraoperative Hypotension with Acute Kidney Injury after Elective Noncardiac Surgery. Anesthesiology 2015, 123, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 Initiative for Acute Kidney Injury (Zero Preventable Deaths by 2025): A Human Rights Case for Nephrology. Lancet Lond. Engl. 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.-P.; Paganini, E.P. A Clinical Score to Predict Acute Renal Failure after Cardiac Surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef]
- Palomba, H.; de Castro, I.; Neto, A.L.C.; Lage, S.; Yu, L. Acute Kidney Injury Prediction Following Elective Cardiac Surgery: AKICS Score. Kidney Int. 2007, 72, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Dekker, F.W.; Vadiveloo, T.; Marwick, C.; Deshmukh, H.; Donnan, P.T.; Diepen, M.V. Risk of Postoperative Acute Kidney Injury in Patients Undergoing Orthopaedic Surgery—Development and Validation of a Risk Score and Effect of Acute Kidney Injury on Survival: Observational Cohort Study. BMJ 2015, 351. [Google Scholar] [CrossRef]
- Slankamenac, K.; Breitenstein, S.; Held, U.; Beck-Schimmer, B.; Puhan, M.A.; Clavien, P.-A. Development and Validation of a Prediction Score for Postoperative Acute Renal Failure Following Liver Resection. Ann. Surg. 2009, 250, 720–728. [Google Scholar] [CrossRef]
- Rueggeberg, A.; Boehm, S.; Napieralski, F.; Mueller, A.R.; Neuhaus, P.; Falke, K.J.; Gerlach, H. Development of a Risk Stratification Model for Predicting Acute Renal Failure in Orthotopic Liver Transplantation Recipients. Anaesthesia 2008, 63, 1174–1180. [Google Scholar] [CrossRef]
- Wilson, T.; Quan, S.; Cheema, K.; Zarnke, K.; Quinn, R.; de Koning, L.; Dixon, E.; Pannu, N.; James, M.T. Risk Prediction Models for Acute Kidney Injury Following Major Noncardiac Surgery: Systematic Review. Nephrol. Dial. Transplant. 2016, 31, 231–240. [Google Scholar] [CrossRef]
- Malhotra, R.; Kashani, K.B.; Macedo, E.; Kim, J.; Bouchard, J.; Wynn, S.; Li, G.; Ohno-Machado, L.; Mehta, R. A Risk Prediction Score for Acute Kidney Injury in the Intensive Care Unit. Nephrol. Dial. Transplant. 2017, 32, 814–822. [Google Scholar] [CrossRef]
- Bell, S.; Prowle, J. Postoperative AKI—Prevention Is Better than Cure? J. Am. Soc. Nephrol. 2019, 30, 4–6. [Google Scholar] [CrossRef]
- Trongtrakul, K.; Patumanond, J.; Kongsayreepong, S.; Morakul, S.; Pipanmekaporn, T.; Akaraborworn, O.; Poopipatpab, S. Acute Kidney Injury Risk Prediction Score for Critically-Ill Surgical Patients. BMC Anesthesiol. 2020, 20, 140. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk Prediction Models: II. External Validation, Model Updating, and Impact Assessment. Heart Br. Card. Soc. 2012, 98, 691–698. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Chittawatanarat, K.; Chaiwat, O.; Morakul, S.; Pipanmekaporn, T.; Thawitsri, T.; Wacharasint, P.; Fuengfoo, P.; Chatmongkolchart, S.; Akaraborworn, O.; Pathonsamit, C.; et al. A Multi-Center Thai University-Based Surgical Intensive Care Units Study (THAI-SICU Study): Methodology and ICU Characteristics. J. Med. Assoc. Thail. 2014, 97, 45. [Google Scholar]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Vergouwe, Y. Towards Better Clinical Prediction Models: Seven Steps for Development and an ABCD for Validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Springer International Publishing: New York, NY, USA, 2009. [Google Scholar]
- Vickers, A.J.; van Calster, B.; Steyerberg, E.W. A Simple, Step-by-Step Guide to Interpreting Decision Curve Analysis. Diagn. Progn. Res. 2019, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Saville, B.R.; Lewis, R.J. Decision Curve Analysis. JAMA 2015, 313, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Grab, J.D.; O’Brien, S.M.; Bridges, C.R.; Gammie, J.S.; Haan, C.K.; Ferguson, T.B.; Peterson, E.D. Bedside Tool for Predicting the Risk of Postoperative Dialysis in Patients Undergoing Cardiac Surgery. Circulation 2006, 114, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Karkouti, K.; Dupuis, J.-Y.; Rao, V.; Chan, C.T.; Granton, J.T.; Beattie, W.S. Derivation and Validation of a Simplified Predictive Index for Renal Replacement Therapy after Cardiac Surgery. JAMA 2007, 297, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, H.; Park, S.; Lee, S.; Kim, K.; Yoon, H.J.; Park, J.; Choi, Y.; Lee, S.; Kim, J.H.; et al. Simple Postoperative AKI Risk (SPARK) Classification before Noncardiac Surgery: A Prediction Index Development Study with External Validation. J. Am. Soc. Nephrol. 2019, 30, 170–181. [Google Scholar] [CrossRef]
- Debray, T.P.A.; Vergouwe, Y.; Koffijberg, H.; Nieboer, D.; Steyerberg, E.W.; Moons, K.G.M. A New Framework to Enhance the Interpretation of External Validation Studies of Clinical Prediction Models. J. Clin. Epidemiol. 2015, 68, 279–289. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Derivation (n = 3029) | Validation (n = 201) | Standardized Difference |
|---|---|---|---|
| Pre-operative | |||
| Age (year, mean ± SD) | 61.8 ± 16.7 | 62.5 ± 17.6 | −0.042 |
| Female, n (%) | 1312 (43.4) | 106 (52.7) | 0.092 |
| Body weight (kg, mean ± SD) | 60.3 ± 15.9 | 61.6 ± 16.9 | −0.081 |
| Body mass index (kg/m2, mean ± SD) | 23.3 ± 5.6 | 24.0 ± 5.9 | −0.125 |
| Pre-existing comorbidities | |||
| Diabetes mellitus, n (%) | 645 (21.3) | 51 (25.4) | 0.057 |
| Hypertension, n (%) | 1512 (49.9) | 108 (53.7) | 0.037 |
| Cardiovascular diseases, n (%) | 634 (20.9) | 28 (13.9) | 0.110 |
| Respiratory diseases, n (%) | 246 (8.1) | N/A | N/A |
| Chronic kidney disease, n (%) | 267 (8.8) | 38 (18.9) | 0.242 |
| Malignancy, n (%) | 437 (14.4) | 77 (38.3) | 0.356 |
| Others, n (%) | 235 (7.8) | 35 (17.4) | 0.255 |
| Peri-operative | |||
| ASA classification, n (%) | 0.259 | ||
| I | 193 (6.4) | 12 (6.0) | |
| II–III | 2470 (81.5) | 179 (89.1) | |
| IV–V | 366 (12.1) | 10 (5.0) | |
| Emergency operation, n (%) | 956 (31.6) | 61 (30.4) | 0.014 |
| Operative sites, n (%) | |||
| Neuro, head and neck surgery | 344 (11.4) | 22 (11.0) | 0.010 |
| Gastrointestinal surgery | 1857 (61.3) | 116 (42.3) | 0.186 |
| Orthopedics surgery | 435 (14.4) | 55 (27.4) | 0.215 |
| Others | 494 (16.3) | 39 (19.4) | 0.053 |
| Operative duration (min, mean ± SD) | 280 ± 175 | 281 ± 147 | −0.006 |
| Peri-operative blood loss (mL, mean ± SD) | 1026 ± 1787 | 728 ± 1077 | 0.170 |
| Peri-operative fluid balance (mL, mean ± SD) | 2112 ± 1852 | 1604 ± 1455 | 0.231 |
| Peri-operative urine output (mL, mean ± SD) | 505 ± 574 | 584 ± 537 | −0.138 |
| Post-operative (at ICU admission) | |||
| APACHE - II score (mean ± SD) | 10.6 ± 6.2 | 13.5 ± 5.8 | −0.470 |
| SOFA score (mean ± SD) | 2.8 ± 3.0 | 3.9 ± 2.3 | −0.371 |
| SOFA non-renal score (mean ± SD) | 2.2 ± 2.7 | 3.6 ± 2.2 | −0.524 |
| Sepsis | 283 (9.3) | 28 (13.9%) | 0.117 |
| Laboratory investigations | |||
| Hemoglobin (gm/dL, mean ± SD) | 10.7 ± 2.0 | 11.4 ± 1.9 | −0.351 |
| Albumin (gm/dL, mean ± SD) | 2.78 ± 0.81 | 3.04 ± 0.75 | −0.322 |
| Blood glucose (mg/dL, mean ± SD) | 166.8 ± 55.9 | N/A | N/A |
| PaO2/FiO2 ratio (mean ± SD) | 339 ± 129 | N/A | N/A |
| Abnormal chest film, n (%) | 436 (14.9) | N/A | N/A |
| Abnormal ECG, n (%) | 678 (23.6) | N/A | N/A |
| Serum creatinine at ICU (mg/dL, mean ± SD) | 1.19 ± 1.36 | 0.94 ± 0.59 | 0.189 |
| Reference serum creatinine, n (%) | 0.340 | ||
| Renal dysfunction value | 570 (18.8) | 42 (20.9) | |
| MDRD recalculated value | 943 (31.1) | 34 (16.8) | |
| Lowest value during admission | 1516 (50.1) | 125 (62.2) | |
| Clinical endpoints | |||
| AKI in 7 days of ICU admission, n (%) | 296 (9.8) | 37 (18.4) | 0.198 |
| ICU mortality, n (%) | 159 (5.3) | 10 (5.0) | 0.014 |
| Day-28 mortality, n (%) | 251 (8.3) | 12 (6.0) | 0.080 |
| ICU length of stay (day, mean ± SD) | 3.2 ± 4.6 | 4.8 ± 5.8 | −0.341 |
| Hospital length of stay (day, mean ± SD) | 20.9 ± 18.3 | 23.4 ± 19.3 | −0.136 |
| Average AKI prediction score | |||
| AKI (mean ± SD) | 8.5 ± 3.2 | 7.8 ± 3.5 | 0.216 |
| Non-AKI (mean ± SD) | 4.1 ± 2.9 | 4.9 ± 2.3 | −0.279 |
| Score Performances | Derivation | Validation | Standardized Difference | ||
|---|---|---|---|---|---|
| AKI/Total | % (95%CI) | AKI/Total | (95%CI) | ||
| AuROC | 86.9 (82.5–85.2) | 74.5 (65.2–83.8) | 0.245 | ||
| Positive predictive value | |||||
| Low (0.0–2.5) | 14/1212 | 1.2 (0.6–1.9) | 1/36 | 2.8 (0.7–1.5) | 0.181 |
| Moderate (3.0–8.5) | 142/1557 | 9.1 (7.7–10.7) | 21/141 | 14.9 (9.5–21.9) | 0.169 |
| High (9.0–11.5) | 95/264 | 36.0 (30.2–42.1) | 9/17 | 52.9 (27.8–77.0) | 0.167 |
| Very high (12.0–16.5) | 45/87 | 51.7 (40.8–2.6) | 6/7 | 85.7 (42.1–99.6) | 0.375 |
| Total | 296/3029 | 9.8 (8.7–10.9) | 37/201 | 18.4 (13.3–24.5) | 0.198 |
| Threshold Probability for AKI (%) | Derivation | Validation | ||||
|---|---|---|---|---|---|---|
| Treat All * | Net Benefit | Reduced Number of Overtreatment per 100 Patients | Treat All * | Net Benefit | Reduced Number of Overtreatment per 100 Patients | |
| 5 | 0.050 | 0.067 | 31.542 | 0.141 | 0.143 | 5.655 |
| 10 | −0.003 | 0.051 | 48.516 | 0.093 | 0.094 | 1.818 |
| 15 | −0.062 | 0.041 | 58.261 | 0.040 | 0.078 | 21.515 |
| 20 | −0.128 | 0.031 | 63.420 | −0.020 | 0.060 | 31.236 |
| 25 | −0.203 | 0.023 | 67.712 | −0.088 | 0.061 | 44.238 |
| 30 | −0.289 | 0.016 | 71.199 | −0.166 | 0.048 | 49.464 |
| 35 | −0.388 | 0.009 | 73.710 | −0.255 | 0.051 | 55.863 |
| 40 | −0.504 | 0.003 | 76.080 | −0.360 | 0.036 | 59.480 |
| 45 | −0.641 | −0.001 | 78.321 | −0.483 | 0.035 | 63.381 |
| 50 | −0.805 | 0.001 | 80.555 | −0.632 | 0.030 | 66.169 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trongtrakul, K.; Patumanond, J.; Phairatwet, P.; Sawawiboon, C.; Chitsomkasem, A.; Kurathong, S.; Prommoon, S.; Trakarnvanich, T.; Phinyo, P. External Validation of the Acute Kidney Injury Risk Prediction Score for Critically Ill Surgical Patients Who Underwent Major Non-Cardiothoracic Surgery. Healthcare 2021, 9, 209. https://doi.org/10.3390/healthcare9020209
Trongtrakul K, Patumanond J, Phairatwet P, Sawawiboon C, Chitsomkasem A, Kurathong S, Prommoon S, Trakarnvanich T, Phinyo P. External Validation of the Acute Kidney Injury Risk Prediction Score for Critically Ill Surgical Patients Who Underwent Major Non-Cardiothoracic Surgery. Healthcare. 2021; 9(2):209. https://doi.org/10.3390/healthcare9020209
Chicago/Turabian StyleTrongtrakul, Konlawij, Jayanton Patumanond, Piyarat Phairatwet, Chaiwut Sawawiboon, Anusang Chitsomkasem, Sathit Kurathong, Surasee Prommoon, Thananda Trakarnvanich, and Phichayut Phinyo. 2021. "External Validation of the Acute Kidney Injury Risk Prediction Score for Critically Ill Surgical Patients Who Underwent Major Non-Cardiothoracic Surgery" Healthcare 9, no. 2: 209. https://doi.org/10.3390/healthcare9020209
APA StyleTrongtrakul, K., Patumanond, J., Phairatwet, P., Sawawiboon, C., Chitsomkasem, A., Kurathong, S., Prommoon, S., Trakarnvanich, T., & Phinyo, P. (2021). External Validation of the Acute Kidney Injury Risk Prediction Score for Critically Ill Surgical Patients Who Underwent Major Non-Cardiothoracic Surgery. Healthcare, 9(2), 209. https://doi.org/10.3390/healthcare9020209






