Abstract
Background: Healthcare-associated infections (HAIs) cause increases in length of stay, mortality, and healthcare costs. A previous study conducted in Taiwan obtained similar results to those reported in Korea and Japan in 2015. Changes in microorganisms have been noted in recent years. Understanding the recent condition of HAIs in intensive care units (ICUs) can enable healthcare providers to develop effective infection control protocols to reduce HAIs. Methods: We used the Taiwan Nosocomial Infection Surveillance System to evaluate the incidence densities of HAIs, the proportions of causative pathogens, and the proportions of antimicrobial resistance (AMR). The Poisson regression model was constructed to incidence density, and the chi-square test was used to assess proportion. Results: The incidence density of HAIs decreased 5.7 to 5.4 per 1000 person-days. However, the proportions of Klebsiella pneumoniae and Enterococcus faecium significantly increased. In addition, the proportions of carbapenem-resistant K. pneumoniae and vancomycin-resistant Enterococcus faecium significantly increased over time. Conclusion: Analysis of the microorganisms involved in HAIs in ICUs showed elevated proportions of K. pneumoniae and E. faecium with AMR. Infection control protocols have been implemented for several years and require improvements regarding environmental cleanliness and medical staff prevention.
1. Introduction
Healthcare-associated infections (HAIs) generally refer to infections acquired after two days of hospitalization by patients entering the healthcare setting to undergo surgery or other medical treatment. HAIs can also occur after receiving home care or ambulatory care, up to six weeks after discharge, and from 30 days to up to 1 year for device-related infections. In other words, HAIs are infections that patients acquire while they are receiving care for other illnesses [1]. There were 1.7 million HAIs in the United States, and HAIs most commonly include bloodstream infections (BSIs), urinary tract infections (UTIs), and healthcare-acquired pneumonia (HAP), surgical site infections (SSIs), and infections from other parts of the body [2]. Escherichia coli, Staphylococcus aureus, and Klebsiella spp. are the most frequently isolated microorganisms [3]. All these types of infections exhibit various percentages of antimicrobial resistance (AMR), involving pathogens such as extended-spectrum beta-lactamase generating strains of Enterobacter spp., Escherichia coli, and K. pneumoniae [4], methicillin-resistant S. aureus (MRSA) [5], carbapenem-resistant K. pneumoniae (CRKP), and vancomycin-resistant Enterococcus faecium (VREfm) [6], which can complicate treatment [4,5,6]. The inappropriate use of antibiotics causes AMR [7].
HAIs involve infectious opportunistic pathogens, weakened hosts, and implants and prostheses with cross-contamination [8]. The patient’s conditions associated with HAIs include more serious hospitalization in intensive care units (ICUs). The percentage of HAIs in ICUs, approximately 37%, is significantly higher than that in general wards at approximately 5–15% [9]. The most common types of HAIs in ICUs are central line-associated BSIs (CLABSIs), catheter-associated UTIs (CAUTIs), and ventilation-associated pneumonia (VAP). HAIs increase length of stay, cost, and mortality. Additionally, they increase patients’ disease severity [10].
Infection control monitoring and prevention procedures are important in preventing HAIs. In addition, the identification of resistant pathogens in HAIs is essential. The AMR of HAIs can be studied to understand the local epidemiology and improve local infection control practices (ICPs). Current control and prevention practices include antimicrobial use, ICPs, environmental cleaning, and efforts to reduce community burden. Trends in the incidence of HAIs, device-induced HAIs (DIHAIs), and AMR have been objectively described and compared with those in other countries [11].
A recent study on HAIs in ICUs from the Taiwan Nosocomial Infection Surveillance System (TNIS) found similar percentages to those reported in Korea and Japan in 2015 [12]. An understanding of recent conditions regarding different microorganisms and their AMR could provide the government with a disease control policy. We investigated the trends in HAIs in ICUs since 2015 to understand the changes in Taiwan.
We aimed to identify recent pathogens of HAIs and AMR in the ICUs of medical centers and regional hospitals in Taiwan. We investigated the trends in HAIs, DIHAIs, and AMR to reveal the changes in HAIs. The prevention of HAIs is a priority; the incidences of HAIs involving different microorganisms and the proportions of AMR need to be recognized to adopt ICPs for HAI reduction in ICUs.
2. Materials and Methods
The TNIS was established in 2007 to monitor the occurrence of HAIs, and we examined publicly available annual summary data on HAIs reported by medical centers and regional hospitals in Taiwan [13]. However, the data did not include HAI data from local hospitals. We used demographic data on HAIs and patient-specific cultures and antimicrobial susceptibility results from reporting hospitals in the TNIS from 2015 to 2019.
We included the following HAIs: BSIs, UTIs, HAP, SSIs, and infections at other sites. We collected data on the overall incidence, causative pathogens, and AMR of HAIs in the ICUs of medical centers and regional hospitals. Information was obtained from 104 hospitals in this study, accounting for over 21.5% of Taiwan’s hospitals.
This study did not require ethical approval because it was based on information that was freely available in the public domain and involved the analysis of open-source datasets, in which the data have been properly anonymized.
2.1. Definitions
The incidence density of HAIs was calculated as the overall number of HAI episodes divided by overall number of hospitalization patient-days (persons/1000 patient-days). The proportion of microorganisms was the number of individual microorganisms divided by the overall number of microorganisms every year. The incidence density of DIHAIs was calculated as the overall number of DIHAI episodes divided by the overall number of hospitalization DIHAIs patient-days. The device use rate was calculated as the number of device-used person-days divided by the number of hospitalization person-days. The proportion of antimicrobial-resistant microorganisms of HAIs was calculated as the number of AMR microorganisms divided by the total number of individual microorganisms every year.
2.2. Statistical Analyses
The Poisson regression model with log-linear regression for count data was used to assess trends in HAI and DIHAI incidence densities. The distributions of pathogens and their AMR over time were determined. Differences between years in the proportions of microorganisms and AMR microorganisms were examined using the Mantel–Haenszel chi-square for linear trends. The significant level was set at p < 0.05. The analysis was conducted using IBM SPSS version 22. (Asia Analytics Taiwan Ltd., Taipei, Taiwan).
3. Results
The incidence density of HAIs in Taiwanese ICUs significantly decreased from 5.7% to 5.4% from 2015 to 2019 (p < 0.001). No significant change was found in the incidence density of BSI (p = 0.134). The incidence densities of UTIs and HAP significantly decreased over time (p < 0.001) (Figure 1). In the ICUs of medical centers, the most common HAIs were BSIs (44.4%) and UTIs (32.9%). In regional hospitals, UTIs (39.5%) and BSIs (36.3%) were predominant. The numbers of episodes involving K. pneumoniae (1045 to 1156) and E. faecium (711 to 867) significantly increased between 2015 and 2019, whereas the numbers of episodes involving Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii significantly decreased. The incidence densities and percentages of DIHAIs in ICUs significantly decreased over time (Table 1). The percentages of K. pneumoniae (9.4% to 11.1%) and E. faecium (6.4% to 8.3%) significantly increased over time. In contrast, the proportions of HAIs involving Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus significantly decreased. The proportion of Candida albicans and E. coli in HAIs of ICUs decreased, but the decreases were insignificant (Figure 2).
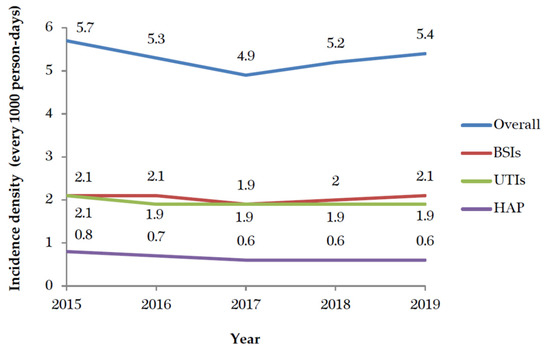
Figure 1.
The trends of healthcare-associated infections from 2015 to 2019. BSIs: bloodstream infections; UTIs: urinary tract infections; HAP: hospital-acquired pneumonia.

Table 1.
Healthcare-associated infections were compared between 2015 and 2019.
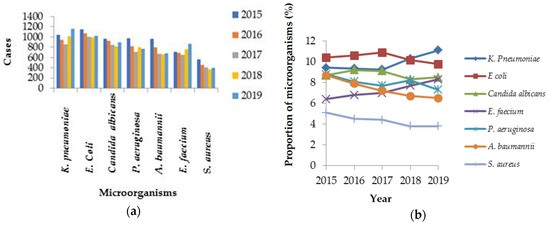
Figure 2.
The numbers (a) and proportions (b) of different microorganisms in healthcare-associated infections from 2015 to 2019.
In 2019, the incidence densities of HAIs were higher than the average incidence density of HAIs (5.4‰) in the medical ICU (MICU) (5.9‰) (p < 0.001), and surgical ICU (SICU) (6.5‰) (p < 0.001). The incidence densities of HAIs in the pediatric ICU (PICU) and SICU were significantly decreased compared with 2015, decreasing from 2.6‰ to 2.1‰ (p < 0.001) and from 6.9‰ to 6.5‰ (p = 0.015), respectively. The incidence density of CLABSIs was higher than the average (3.18 ‰) of CLABSIs in the MICU (3.7‰) (p < 0.001). Furthermore, the incidence density of CAUTIs in MICU (3.1‰) (p = 0.001) was higher than the average (2.73‰). The incidence densities of VAP in the SICU (0.85‰) (p = 0.018) and GICU (0.98‰) (p < 0.001) were higher than the average (0.7‰) (Table 2).

Table 2.
The incidence densities of device-induced healthcare-associated infections in different types of intensive care units during 2019.
The relative percentages of microorganisms of HAIs were compared between 2015 and 2019. K. pneumoniae was the most common pathogen of bloodstream HAIs in ICUs, increasing from 9.6% to 11.9% of the total, with an absolute 2.3% increase. A. baumanni was the second most common pathogen in bloodstream HAIs in ICUs and underwent an absolute 1.9%, decreasing from 10.4% to 8.5%. The third most common microorganism in bloodstream HAIs in ICUs was E. faecium, which increased from 7.2% to 8.5%, an absolute 1.3% increase. Candida otherwise specified was the fourth most common pathogen in bloodstream HAIs in ICUs, it increased from 5.9% to 7.9% over time, with an absolute 2% increase. E. coli was the fifth most common microorganism among bloodstream HAIs.
E. coli was the most common microorganism in urinary tract HAIs in ICUs and underwent an absolute 1.7% reduction over time, decreasing from 19.8% to 18.1%. The second common pathogen in ICU urinary tract HAIs was C. albicans. E. faecium was the third most common pathogen in these HAIs and increased from 8.5% to 10.1%, with an absolute 1.6% increase. K. pneumoniae was the fourth most common pathogen in urinary tract HAIs in ICUs; it increased from 7.3% to 9.6%, with an absolute 2.3% increase. The fifth most common pathogen in ICU urinary tract HAIs was Candida otherwise specified; it increased from 7.2% to 9.3%, with an absolute 2.1% increase.
P. aeruginosa was the most common pathogen in HAP in ICUs, followed by K. pneumoniae. A. baumannii was the third most common microorganism in HAP infection in ICUs, accounting for 18% to 14.2% of cases, with an absolute 3.8% reduction. S. aureus and Enterobacter species were the fourth and fifth most common HAP pathogens in ICUs. Among the SSIs in ICUs, P. aeruginosa was the most common pathogen, followed by K. pneumoniae, E. coli, and E. faecium were the third most common pathogens in ICU SSIs. E. faecium was increased from 4.2% to 8.4%, with an absolute 4.2% increase. A. baumannii was the fifth most common pathogen in ICU SSIs.
K. pneumoniae ranked second to first in BSIs, third to second in the HAP, and fifth to fourth in the UTIs from 2015 to 2019; E. faecium ranked in third position in BSIs, UTIs, and SSIs in 2019 (Table 3).

Table 3.
The distributions of the top five causative pathogens and sites of infection in 2015 and 2019.
The incidence densities of DIHAIs showed decreasing trends from 2015 to 2019 (p < 0.001) (Figure 3). The percentage of central line use rate in CLABSIs was reduced from 78.3% to 73.1%. The percentage of urinary catheter use rate in CAUTIs was reduced from 90.3% to 87.9%. The percentage of ventilation use rate in VAP was reduced from 70.2% to 54.9% from 2015 to 2019 (p < 0.001). There were significant increases in the number of isolates of CRKP and vancomycin-resistant Enterococcus (VRE) from 2015 to 2019. The percentage of CRKP isolates increased from 23.2% to 37.5%, representing 61.6% (absolute 14.3%) (p < 0.001). The percentage of VRE isolates increased from 35.7% to 47.6%, representing a 33.3% increase (absolute 11.9%), and that of VREfm isolates increased from 57.9% to 69.7%, representing a 20.4% increase (absolute 11.8%) (p < 0.001). The percentage of carbapenem-resistant E. coli isolates increased by 90.5% from 2.1% to 4%, corresponding to an absolute increase of 1.9% (p = 0.008). The percentage of MRSA isolates decreased from 70% to 62.2%, representing an 11.1% decrease (absolute 7.8%); this decreasing trend was insignificant (p = 0.107). The percentage of carbapenem-resistant P. aeruginosa isolates increased from 17.8% to 21.5%, representing an insignificant increase of 20.8% (absolute 3.7%) (p = 0.055). The percentage of carbapenem-resistant A. baumanni isolates increased from 71% to 74.3%, representing an insignificant increase of 4.6% (absolute 3.3%) (p = 0.147) (Figure 4).
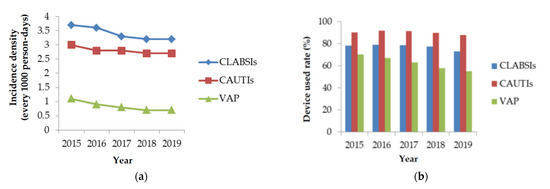
Figure 3.
The trend (a) and device use rate (b) of device-induced healthcare-associated infections from 2015 to 2019. CLABSI: central line-associated bloodstream infections; CAUTIs: catheter-associated urinary tract infections; VAP: ventilator-associated pneumonia.
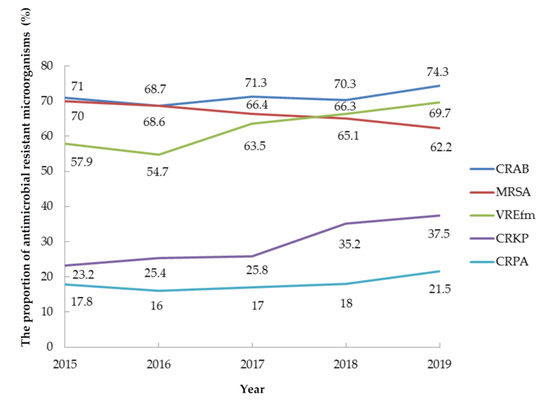
Figure 4.
The proportions of antimicrobial-resistant microorganisms in hospital-associated infections from 2015 to 2019. CRAB: carbapenem-resistant A. baumannii; MRSA: methicillin-resistant S. aureus; VREfm: vancomycin-resistant E. faecium; CRKP: carbapenem-resistant Klebsiella pneumoniae; CRPA: carbapenem-resistant P. aeruginosa.
4. Discussion
The total proportion of K. pneumoniae and E. faecium in HAIs in ICUs increased. The changes were driven by an increase in K. pneumoniae and E. faecium in HAIs representing BSIs, UTIs, and SSIs. The AMR of CRKP and VREfm significantly increased, which may have contributed to these trends. Although ICPs and antimicrobial stewardship have been implemented for years [7], improvements and aggressive procedures for HAIs in Taiwanese ICUs remain needed.
The TNIS can be used to evaluate the epidemiologic trends of HAIs and internationally comparable surveillance indicators. The most frequent type of infection in hospitals in the United States are UTIs (36%), followed by SSIs (20%) and BSIs and pneumonia (both 11%) [8]. BSIs rather than UTIs were the HAIs with the highest frequency in Taiwan, in contrast to the pattern in the USA. In Iranian ICUs, the incidence of BSIs, UTIs, and HAP has been reported to be 2.21, 3.32, and 2.55, respectively, every 1000 person-days [14]. The incidence densities of BSIs, UTIs, and HAP in the ICUs of Taiwan were 2.1, 1.9, and 0.6, respectively, every 1000 person-days. The HAIs in Taiwan have a lower incidence density than those in Iran. In addition, the values for the MICU and general ICU in Taiwan are higher than those in Iran [14]. The most common HAIs of Taiwanese ICUs occurred in the SICU, followed by the MICU. Although the incidence densities have decreased over time in the SICU and PICU; however, aggressive ICPs and monitoring are needed in the SICU.
E. coli (18%), S. aureus (12%), and Klebsiella spp (9%) were the most frequent HAI pathogens reported in Atlanta of the United States [3]. In Ukraine, K. pneumoniae was the most common pathogen reported, accounting for 21.8% of HAIs, followed by A. baumannii (14.3%), P. aeruginosa (12.4%), and E. coli (9.4%) [15]. The most common causative bacteria identified in Thailand were Gram-negative bacteria, of which K. pneumoniae (18.5%) was the most common, followed by A. baumannii (17.8%) and P. aeruginosa (12.6%) [16]. Our study found that K. pneumoniae was the most common microorganism of HAIs in Taiwan, similar to results in Ukraine and Thailand, but different from those in the United States and Europe, where P. aeruginosa has been identified as the most common microorganism [17].
In a 2007 study in Europe, the most frequently isolated microorganisms were P. aeruginosa in ICU-HAP episodes, coagulase-negative staphylococci in ICU-BSIs, and E. coli in ICU-UTIs [18]. These findings are similar to the results for HAP and UTIs in our study. In our study, the most common microorganism of BSIs in ICUs was K. pneumoniae, different from the pattern in Europe. The percentage of BSIs involving E. faecium was found to be 5.4% in 2010 in the USA [19]. Our study revealed a higher prevalence (8.5%). The percentage of UTIs due to E. faecium was 39.9% in a 2014 study in Australia [20]. Our study identified a lower prevalence (10.1%).
The occurrence of HAIs is associated with invasive procedures, and monitoring for CLABSI, CAUTI, and VAP is needed. Infectious disease control requires appropriate ventilation devices, catheters, central lines, timely catheter removal, regular education programs following infection control guilds, monitoring of water and diet quality, and focused care of patients with AMR infections [21]. Our study revealed that DIHAIs were significantly reduced after ICP promotion in recent years in Taiwan.
The proportions of K. pneumoniae and E. faecium among HAIs increased from 2015 to 2019, whereas those of P. aeruginosa, A. baumannii, and S. aureus decreased. In addition, the proportion of CRKP and VREfm significantly increased. The proportions of CRPA, CRAB, and MRSA were insignificantly decreased. Although CR E. coli significantly increased, it increased only to 4%; this percentage is lower than the percentage of E. coli related HAIs, which changed from 10.4% to 9.8% (p = 0.098).
Carbapenem antibiotics are used to treat extended-spectrum β-lactamase bacterial infections. The mortality of CRKP infection is higher, with a value of 27.3% reported in a previous study [22]. Compared with carbapenem-susceptible K. pneumoniae, CRKP in BSI is associated with significantly worse 2-week survival [23]. Difficult-to-treat Gram-negative BSI and more severe lower respiration tract infection are associated with increased in-hospital mortality within one month and appropriate empirical antibiotics related to lower mortality [24]. In Europe, carbapenem resistance was reported in 15% of Klebsiella spp. isolates, 26% of P. aeruginosa isolates, and 64% of A. baumannii isolates. In Ukraine, 29.3% of isolates were found to be CRKP [15], and the percentage of CR Enterobacter isolates in a USA study was 26.7% [25]. Our study revealed that carbapenem resistance among A. baumannii (74.3%) and K. pneuminae (37.5%) was higher in 2019 than in 2015. KPC-2 and OXA-48 have been the most common genes associated with resistance over the last 20 years in Taiwan [26]. The risk factors for CRKP include ICU hospitalization, device use, and antibiotics use [27]. Ceftazidime/avibactam can treat CRKP. The incidence of newly acquired VRE during ICU stay in Taiwan University Hospital was 21.9 per 1000 patient-days in 2009 [28]. The proportion of VRE in Taiwan is 47.6% higher than that (17.3%) in Saudi Arabia [29] and 33.6% in the USA [25]. MLST 414 is the most predominant VRE strain. The increased VRE prevalence is due to cross-transmission of VRE clones ST 414, 78, and 18 by undetected VRE carriers. E. faecium caused 49.1% of enterococcal BSIs in a study in Australia [20]. A previous study in Taiwan found that VREfm increased from 0.3% in 2004 to 24.9% in 2010 [30]. The frequency of VREfm was 75.6% in the United States during 2014 [31], 19.0% in Europe in 2018 [32], and 59.1% in Switzerland with frequent tourism [33]. The risk factors for VREfm in Germany, with a frequency of 26.1% in 2017, were found to be the hospitalization in southwest and southeast areas, age 40–59 years vs. lower, specialist care, and prevention and rehabilitation care [34]. Our study found that the proportion of VREfm was similar to the proportion in the USA, but higher than that in Europe. The most common area of CRKP (52.2%) and VREfm (79.4%) was North Taiwan; the reason might be the higher proportion of medical centers in North Taiwan than in other regions. Regarding the onset of VREfm BSI in the ICU, a 30-day mortality rate of 13.2% has been reported, with an odds ratio of 4.2 (95% confidence interval 1.7–10) [35]. CRKP and VRE are threats to public health. The increasing trends of CRKP and VRE may reflect the extensive use of carbapenem and vancomycin. Effective monitoring, feedback regarding antimicrobial stewardship programs, and aggressive ICPs are needed to reduce CRKP and VRE.
The most frequent route of transmission of HAIs is direct contact. K. pneumoniae and E. faecium can form biofilms with adaptations to hospital conditions that serve as vehicles of transmission and dissemination in the hospital setting. K. pneumoniae can remain on a surface for two hours to two and a half years, and E. faecium can persist on a surface for five days to four months [36]. Cross-transmission of infections causes the contamination of equipment, bed linens, and air droplets, and infections are spread by medical staff and visitors. VRE is the most common multiresistant microorganism isolated in contaminated rooms [37]. It is essential to reduce infection carriage and spread. A clean environment is important in HAIs prevention. The use of nonflammable alcohol vapor in carbon dioxide, H2O2, and ultraviolet C for surface decontamination has been shown in previous studies to reduce environmental microorganisms [38,39,40,41,42]. Environmental improvement, screening for CRKP and VREfm carriage, interinstitutional infection control measures, universal health education, and suitable antimicrobial agent use can lead to reduced harm from infectious diseases.
Three aspects of infection are the ease with which an agent can infect a host with dysfunctional immunity, the infectious agent, and the infection routes. Clean care is safer care. It is important to implement hand washing to maintain hand hygiene. Every healthcare staff member needs to wear facial and oral masks, protective clothing, and gloves. The risk factors for HAIs include age older than 65 years, hospitalization from the emergency room, ICU admission with higher risk, induced devices, surgical procedure, immunocompromised status, and underlying diseases. One-third of HAIs are considered preventable. Hand cleaning, appropriate antibiotic use, patient isolations, the use of appropriate personal protective equipment, and environmental cleaning with disinfection procedures are performed by personnel for infection control [43].
This study used national surveillance data to evaluate the trends of HAIs of ICUs in Taiwan, and the results support recent data from elsewhere in Asia. There are some limitations to this study. First, it included data only from regional hospitals and medical centers, representing approximately 21.5% of all hospitals in Taiwan, and data from local hospitals need to be accessed in the future. However, only 13.6% of all ICU hospitalizations resulted in patients admitted to the ICU at local hospitals in Taiwan [44]. The HAIs in this study cover 86% of ICU admissions to medical centers and regional hospitals and seem representative of Taiwan. Second, data on the patients’ clinical conditions and antibiotic effects were not available; the past study found that appropriate antibiotic therapy did not affect mortality due to CRKP bacteriuria in ICUs, yielding controversial conclusions [45]. A registered study could address this limitation. Third, the proposed association between AMR and microorganisms is speculative, and future studies are needed to confirm the hypothesis.
5. Conclusions
This study found that K. pneumoniae and E. faecium in HAIs exhibited significant increases over a five-year period. The AMR of CRKP and VRE is the most important factor influencing cross-transmission. During the COVID-19 outbreak in March 2020, the prevention of HAIs and subsequent complications due to infection was essential for patient survival. ICPs need to be implemented in accordance with recent findings. Target preventative measures with clean environments and the appropriate use of antibiotics to reduce AMR need to be implemented. It is important to understand the latest condition of HAIs in different countries. These present findings can provide other countries with alerts to these conditions and allow them to develop strategies to prevent HAIs in ICUs.
Author Contributions
Conceptualization, C.-G.C. and C.-A.C.; Data curation, C.-P.Y.; Formal analysis, C.-A.C.; Funding acquisition, Y.-R.L.; Investigation, Y.-Y.L.; Project administration, Y.-R.L. and C.-P.Y.; Resources, C.-P.Y.; Software, C.-P.Y.; Supervision, C.-G.C. and C.-A.C.; Validation, Y.-Y.L. and Y.-S.Y.; Visualization, Y.-Y.L. and Y.-S.Y.; Writing—original draft preparation, Y.-R.L.; Writing—review and editing, C.-G.C. and C.-A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethical approval as it involved information freely available in the public domain, and the analysis of open-source datasets, in which the data have been properly anonymized.
Informed Consent Statement
Patient consent was waived due to the use of open-access datasets.
Data Availability Statement
Annul reports of Taiwan Nosocomial Infections Surveillance System: https://www.cdc.gov.tw/Category/MPage/4G8HuDdUN1k4xaBJhbPzKQ (accessed on 1 June 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to prevent healthcare-associated infections: A narrative overview. Risk Manag. Healthc. Policy 2020, 13, 1765. [Google Scholar] [CrossRef]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L., Jr.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galani, I.; Souli, M.; Daikos, G.L.; Chrysouli, Z.; Poulakou, G.; Psichogiou, M.; Panagea, T.; Argyropoulou, A.; Stefanou, I.; Plakias, G.; et al. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J. Chemother. 2012, 24, 191–194. [Google Scholar] [CrossRef]
- Kumari, J.; Shenoy, S.M.; Baliga, S.; Chakrapani, M.; Bhat, G.K. Healthcare-Associated Methicillin-Resistant Staphylococcus aureus: Clinical characteristics and antibiotic resistance profile with emphasis on macrolide-lincosamide-streptogramin B resistance. Sultan Qaboos Univ. Med. J. 2016, 16, e175–e181. [Google Scholar] [CrossRef]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 707330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.-T.; Yang, Y.-Q.; Cao, N.-X.; Yin, Y.-P.; Chen, X.-S. Novel education-based intervention to reduce inappropriate antibiotic prescribing for treatment of gonorrhoea in China: Protocol for a cluster randomised controlled trial. BMJ Open 2020, 10, e037549. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, J.L. Nosocomial infections in adult intensive-care units. Lancet 2003, 361, 2068–2077. [Google Scholar] [CrossRef]
- Saint, S.; Greene, M.T.; Fowler, K.E.; Ratz, D.; Patel, P.K.; Meddings, J.; Krein, S.L. What US hospitals are currently doing to prevent common device-associated infections: Results from a national survey. BMJ Qual. Saf. 2019, 28, 741–749. [Google Scholar] [CrossRef]
- Rock, C.; Small, B.A.; Thom, K.A. Innovative methods of hospital disinfection in prevention of healthcare-associated infections. Curr. Treat. Options Infect. Dis. 2018, 10, 65–77. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Pan, S.-C.; Yang, T.-S.; Matsuda, K.; Bin Kim, H.; Choi, Y.H.; Hori, S.; Wang, J.-T.; Sheng, W.-H.; Chen, Y.-C.; et al. Healthcare-associated infections in intensive care units in Taiwan, South Korea, and Japan: Recent trends based on national surveillance reports. Antimicrob. Resist. Infect. Control 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taiwanese CDC. Taiwan Nosocomial Infection Surveillance System 2021. Available online: https://www.cdc.gov.tw/En/Category/Page/J63NmsvevBg2u3I2qYBenw (accessed on 1 June 2021).
- Izadi, N.; Eshrati, B.; Mehrabi, Y.; Etemad, K.; Hashemi-Nazari, S.-S. The national rate of intensive care units-acquired infections, one-year retrospective study in Iran. BMC Public Health 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Salmanov, A.; Vozianov, S.; Kryzhevsky, V.; Litus, O.; Drozdova, A.; Vlasenko, I. Prevalence of healthcare-associated infections and antimicrobial resistance in acute care hospitals in Kyiv, Ukraine. J. Hosp. Infect. 2019, 102, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Moolasart, V.; Manosuthi, W.; Thienthong, V.; Vachiraphan, A.; Judaeng, T.; Rongrungrueng, Y.; Vanprapar, N.; Danchaivijitr, S. Prevalence and risk factors of healthcare-associated infections in Thailand 2018: A point-prevalence survey. J. Med. Assoc. Thail. 2019, 102, 1309–1316. [Google Scholar]
- Plachouras, D.; Lepape, A.; Suetens, C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018, 44, 2216–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European CDC. Healthcare-Associated Infections Acquired in Intensive Care Units. 2021. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections-acquired-intensive-care-units (accessed on 1 June 2021).
- Mendes, R.E.; Castanheira, M.; Farrell, D.J.; Flamm, R.K.; Sader, H.S.; Jones, R.N. Longitudinal (2001–14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010–13) analysis of oritavancin in vitro potency. J. Antimicrob. Chemother. 2016, 71, 3453–3458. [Google Scholar] [CrossRef] [Green Version]
- Coombs, G.W.; Daley, D.A.; Mowlaboccus, S.; Pang, S. Australian Group on Antimicrobial Resistance (AGAR) Australian Enterococcal Sepsis Outcome Programme (AESOP) Annual Report 2019. Commun. Dis. Intell. 2020, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J.; Moody, J. Prevention of device-related healthcare-associated infections. F1000Research 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-F.; Chuang, C.; Lin, Y.-T.; Chan, Y.-J.; Lin, J.-C.; Lu, P.-L.; Wang, J.-T.; Siu, L.K.; Fung, C.-P. Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: A multicenter study in Taiwan. Eur. J. Clin. Micro-350 Biol. Infect. Dis. 2018, 37, 651–659. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Chuang, Y.C.; Wang, J.T.; Chen, Y.C.; Hsieh, S.M. Healthcare-associated carbapenem-resistant Klebsiella pneumoniae bloodstream infections: Risk factors, mortality, and antimicrobial susceptibility, 2017–2019. J. Formos. Med. Assoc. 2021, 120, 1994–2002. [Google Scholar] [CrossRef]
- Huh, K.; Chung, D.R.; Ha, Y.E.; Ko, J.-H.; Kim, S.-H.; Kim, M.-J.; Huh, H.J.; Lee, N.Y.; Cho, S.Y.; Kang, C.-I.; et al. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: Retrospective analysis of nationwide surveillance data. Clin. Infect. Dis. 2020, 71, e487–e496. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, J.A.; Hatfield, K.M.; Wolford, H.; Nelson, R.E.; Olubajo, B.; Reddy, S.C.; McCarthy, N.; Paul, P.; McDonald, L.C.; Kallen, A.; et al. Multidrug-resistant bacterial infections in US hospitalized patients, 2012–2017. N. Engl. J. Med. 2020, 382, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Yu, W.-L. Klebsiella pneumoniae Harboring Carbapenemase Genes in Taiwan: Its Evolution over 20 Years, 1998–2019. Int. J. Antimicrob. Agents 2021, 58, 106354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-M.; Yuan, Z.; Zhou, H.-Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.C.; Wang, J.T.; Chen, Y.C.; Chang, Y.Y.; Chen, M.L.; Chang, S.C. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS ONE 2012, 7, e47297. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Al-Saafin, M. Overview of prevalence, characteristics, risk factors, resistance, and virulence of vancomycin-resistant enterococci in Saudi Arabia. Microb. Drug Resist. 2019, 25, 350–358. [Google Scholar] [CrossRef]
- Wang, J.T.; Chang, S.C.; Wang, H.Y.; Chen, P.C.; Shiau, Y.R.; Lauderdale, T.L. High rates of multidrug resistance in Enterococcus faecalis and E. faecium isolated from inpatients and outpatients in Taiwan. Diagn. Microbiol. Infect Dis. 2013, 75, 406–411. [Google Scholar] [CrossRef]
- Zhou, X.; Willems, R.J.; Friedrich, A.W.; Rossen, J.W.; Bathoorn, E. Enterococcus faecium: From microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob. Resist. Infect. Control 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Piezzi, V.; Gasser, M.; Atkinson, A.; Kronenberg, A.; Vuichard-Gysin, D.; Harbarth, S.; Marschall, J.; Buetti, N. Increasing proportion of vancomycin resistance among enterococcal bacteraemias in Switzerland: A 6-year nation-wide surveillance, 2013 to 2018. Eurosurveillance 2020, 25, 1900575. [Google Scholar] [CrossRef]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, L.C.; Chang, Y.J.; Chang, C.Y. Clinical and microbiological characteristics of vancomycin-resistant Enterococcus faecium bloodstream infection in Central Taiwan. Medicine 2017, 96, e9000. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kecik Bosnak, V.; Namiduru, M.; Karaoglan, I.; Ozlem Mete, A. Evaluation of compliance in control and prevention study of vancomycin resistant enterococcus outbreak. Sci. World J. 2013, 2013, 252469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totaro, M.; Casini, B.; Profeti, S.; Tuvo, B.; Privitera, G.; Baggiani, A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 2020, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.T.; Imai, T.; Teeka, J.; Sekine, M.; Kanno, A.; Van Le, T.; Higuchi, T.; Phummala, K.; Yamamoto, K. Comparison of disinfection effect of pressurized gases of CO2, N2O, and N2 on Escherichia coli. Water Res. 2013, 47, 4286–4293. [Google Scholar] [CrossRef]
- Yang, J.-H.; Wu, U.-I.; Tai, H.-M.; Sheng, W.-H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef]
- Ramos, C.C.R.; Roque, J.L.A.; Sarmiento, D.B.; Suarez, L.E.G.; Sunio, J.T.P.; Tabungar, K.I.B.; Tengco, G.S.C.; Rio, P.C.; Hilario, A.L. Use of ultraviolet-C in environmental sterilization in hospitals: A systematic review on efficacy and safety. Int. J. Health Sci. 2020, 14, 52. [Google Scholar]
- Dang, L.T.; Imai, T.; Le, T.V.; Nishihara, S.; Higuchi, T.; Nguyen, M.K.; Kanno, A.; Yamamoto, K.; Sekine, M. Effects of pressure and pressure cycling on disinfection of Enterococcus sp. in seawater using pressurized carbon dioxide with different content rates. J. Environ. Sci. Health Part A 2016, 51, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Accardi, R.; Castaldi, S.; Marzullo, A.; Ronchi, S.; Laquintana, D.; Lusignani, M. Prevention of healthcare associated infections: A descriptive study. Ann. Ig. 2017, 29, 101–115. [Google Scholar] [PubMed]
- Taiwanese Ministry of Health and Welfare. The ICU Hospitalization Patients of Different Levels Hospitals in 2019. Available online: https://dep.mohw.gov.tw/DOS/lp-5099-113.html (accessed on 1 June 2021).
- Chuang, C.; Su, C.-F.; Lin, J.-C.; Lu, P.-L.; Huang, C.-T.; Wang, J.-T.; Chuang, Y.-C.; Siu, L.K.; Fung, C.-P.; Lin, Y.-T. Does antimicrobial therapy affect mortality of patients with carbapenem-resistant klebsiella pneumoniae bacteriuria? A nationwide multicenter study in Taiwan. Microorganisms 2020, 8, 2035. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).