Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Intervention
2.3.1. Real-Time Feedback
2.3.2. EMG–FES Interface Training
2.3.3. General Physical Therapy
2.4. Outcome Measurement
2.5. Data Analysis
3. Results
3.1. Lower-Limb Function
3.2. Balance
3.3. Gait Ability
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merkel, C.; Hausmann, J.; Hopf, J.M.; Heinze, H.J.; Buentjen, L.; Schoenfeld, M.A. Active prosthesis dependent functional cortical reorganization following stroke. Sci. Rep. 2017, 7, 8680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, R.B.; Everaert, D.G.; Thompson, A.K.; Chong, S.L.; Whittaker, M.; Robertson, J.; Kuether, G. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil. Neural Repair 2010, 24, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.J.; Yarossi, M. Weight transfer analysis in adults with hemiplegia using ankle foot orthosis. Prosthet. Orthot. Int. 2011, 35, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, A.M.; Alshehri, M.M.; Alothman, S.; Rucker, J.; Dunning, K.; D’Silva, L.J.; Kluding, P.M. Functional reach, depression scores, and number of medications are associated with number of falls in people with chronic stroke. PM R 2018, 10, 806–816. [Google Scholar] [CrossRef]
- Bang, D.H.; Shin, W.S.; Kim, S.Y.; Choi, J.D. The effects of action observational training on walking ability in chronic stroke patients: A double-blind randomized controlled trial. Clin. Rehabil. 2013, 27, 1118–1125. [Google Scholar] [CrossRef]
- Bergmann, J.; Krewer, C.; Bauer, P.; Koenig, A.; Riener, R.; Müller, F. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 397–407. [Google Scholar]
- Krishnamoorthy, V.; Hsu, W.L.; Kesar, T.M.; Benoit, D.L.; Banala, S.K.; Perumal, R.; Sangwan, V.; Binder-Macleod, S.A.; Agrawal, S.K.; Scholz, J.P. Gait training after stroke: A pilot study combining a gravity-balanced orthosis, functional electrical stimulation, and visual feedback. J. Neurol. Phys. Ther. 2008, 32, 192–202. [Google Scholar] [CrossRef]
- Sharif, F.; Ghulam, S.; Malik, A.N.; Saeed, Q. Effectiveness of functional electrical stimulation (fes) versus conventional electrical stimulation in gait rehabilitation of patients with stroke. J. Coll. Phys. Surg. Pak. 2017, 27, 703–706. [Google Scholar]
- Jonsdottir, J.; Thorsen, R.; Aprile, I.; Galeri, S.; Spannocchi, G.; Beghi, E.; Bianchi, E.; Montesano, A.; Ferrarin, M. Arm rehabilitation in post stroke subjects: A randomized controlled trial on the efficacy of myoelectrically driven fes applied in a task-oriented approach. PLoS ONE 2017, 12, e0188642. [Google Scholar] [CrossRef]
- Rong, W.; Tong, K.Y.; Hu, X.L.; Ho, S.K. Effects of electromyography-driven robot-aided hand training with neuromuscular electrical stimulation on hand control performance after chronic stroke. Disabil. Rehabil. Assist. Technol. 2015, 10, 149–159. [Google Scholar] [CrossRef]
- Kapadia, N.M.; Nagai, M.K.; Zivanovic, V.; Bernstein, J.; Woodhouse, J.; Rumney, P.; Popovic, M.R. Functional electrical stimulation therapy for recovery of reaching and grasping in severe chronic pediatric stroke patients. J. Child Neurol. 2014, 29, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Morita, A.; Hara, Y. Effect of dual therapy with botulinum toxin a injection and electromyography-controlled functional electrical stimulation on active function in the spastic paretic hand. J. Nippon. Med. Sch. 2016, 83, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasso, E.; Gemma, M.; Agosta, F.; Filippi, M.; Gatti, R. Action observation training to improve motor function recovery: A systematic review. Arch. Physiother. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.J.; Yoo, E.Y.; Jung, M.Y.; Park, S.H.; Park, J.H.; Lee, J. Effects of mental practice with action observation training on occupational performance after stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Action observation in the modification of postural sway and gait: Theory and use in rehabilitation. Gait Posture 2017, 58, 115–120. [Google Scholar] [CrossRef]
- Begg, R.K.; Tirosh, O.; Said, C.M.; Sparrow, W.A.; Steinberg, N.; Levinger, P.; Galea, M.P. Gait training with real-time augmented toe-ground clearance information decreases tripping risk in older adults and a person with chronic stroke. Front. Hum. Neurosci. 2014, 8, 243. [Google Scholar] [CrossRef]
- Rostamian, S.; Mahinrad, S.; Stijnen, T.; Sabayan, B.; de Craen, A.J. Cognitive impairment and risk of stroke: A systematic review and meta-analysis of prospective cohort studies. Stroke 2014, 45, 1342–1348. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.A.; Docherty, C.L. Validity of clinical outcome measures to evaluate ankle range of motion during the weight-bearing lunge test. J. Sci. Med. Sport 2017, 20, 618–621. [Google Scholar] [CrossRef]
- Ben-Shabat, E.; Palit, M.; Fini, N.A.; Brooks, C.T.; Winter, A.; Holland, A.E. Intra-and interrater reliability of the modified tardieu scale for the assessment of lower limb spasticity in adults with neurologic injuries. Arch. Phys. Med. Rehabil. 2013, 94, 2494–2501. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Li, X. Test-retest reliability and inter-rater reliability of the modified tardieu scale and the modified ashworth scale in hemiplegic patients with stroke. Eur. J. Phys. Rehabil. Med. 2014, 50, 9–15. [Google Scholar]
- Manji, A.; Amimoto, K.; Matsuda, T.; Wada, Y.; Inaba, A.; Ko, S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci. Lett. 2018, 662, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Si Tou, J.I.; Tse, M.M.; Ng, S.S. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazani, A.; Mohammadi, H.; Shahidi, G.A.; Habibi, S.A.H.; Forogh, B.; Ahadi, T.; Eftekharsadat, B. Reliability and validity of the persian translation of berg balance scale in parkinson disease. Aging Clin. Exp. Res. 2017, 29, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.; Jasani, H.; Poon, V.; Inness, E.L.; McIlroy, W.E.; Mansfield, A. Inter- and intra-rater reliability of the gaitrite system among individuals with sub-acute stroke. Gait Posture 2014, 40, 259–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilkar, R.; Ramanujam, A.; Nolan, K.J. Alterations in spectral attributes of surface electromyograms after utilization of a foot drop stimulator during post-stroke gait. Front. Neurol. 2017, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.; Park, S.I.; Jang, Y.Y.; Lee, B.H. Effects of brain-computer interface-based functional electrical stimulation on balance and gait function in patients with stroke: Preliminary results. J. Phys. Ther. Sci. 2015, 27, 513–516. [Google Scholar] [CrossRef]
- Kluding, P.M.; Santos, M. Effects of ankle joint mobilizations in adults poststroke: A pilot study. Arch. Phys Med. Rehabil. 2008, 89, 449–456. [Google Scholar] [CrossRef]
- Hara, Y.; Obayashi, S.; Tsujiuchi, K.; Muraoka, Y. The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clin. Neurophysiol. 2013, 124, 2008–2015. [Google Scholar] [CrossRef]
- Samuelides, M.; Doyon, B.; Cessac, B.; Quoy, M.; Dauce, E. Self-organization and dynamics reduction in recurrent networks: Stimulus presentation and learning. Neural. Netw. 1998, 11, 521–533. [Google Scholar]
- Rydahl, S.J.; Brouwer, B.J. Ankle stiffness and tissue compliance in stroke survivors: A validation of myotonometer measurements. Arch. Phys. Med. Rehabil. 2004, 85, 1631–1637. [Google Scholar] [CrossRef]
- Zhu, M.-H.; Wang, J.; Gu, X.-D.; Shi, M.-F.; Zeng, M.; Wang, C.-Y.; Chen, Q.-Y.; Fu, J.-M. Effect of action observation therapy on daily activities and motor recovery in stroke patients. Int. J. Nurs. Sci. 2015, 2, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Yom, C.; Cho, H.Y.; Lee, B. Effects of virtual reality-based ankle exercise on the dynamic balance, muscle tone, and gait of stroke patients. J. Phys. Ther. Sci. 2015, 27, 845–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, K.; Levine, P.; Schmitt, L.; Israel, S.; Fulk, G. An ankle to computer virtual reality system for improving gait and function in a person 9 months poststroke. Top. Stroke Rehabil. 2008, 15, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Mayer, N.H.; Esquenazi, A. Muscle overactivity and movement dysfunction in the upper motoneuron syndrome. Phys. Med. Rehabil. Clin. N. Am. 2003, 14, 855–883. [Google Scholar] [CrossRef]
- Karthikbabu, S.; Chakrapani, M.; Ganesan, S.; Ellajosyula, R.; Solomon, J.M. Efficacy of trunk regimes on balance, mobility, physical function, and community reintegration in chronic stroke: A parallel-group randomized trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 1003–1011. [Google Scholar] [CrossRef]
- Osaka, H.; Shinkoda, K.; Watanabe, S.; Fujita, D.; Kobara, K.; Yoshimura, Y.; Ito, T.; Suehiro, T. Association between trunk acceleration during walking and clinically assessed balance in patients with stroke. NeuroRehabilitation 2017, 41, 783–790. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar]
- Park, E.C.; Hwangbo, G. The effects of action observation gait training on the static balance and walking ability of stroke patients. J. Phys. Ther. Sci. 2015, 27, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.A.; Eng, J.J.; Hung, C. The effect of functional electrical stimulation on balance function and balance confidence in community-dwelling individuals with stroke. Physiother. Can. 2010, 62, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Barclay-Goddard, R.; Stevenson, T.; Poluha, W.; Moffatt, M.E.; Taback, S.P. Force platform feedback for standing balance training after stroke. Cochrane Database Syst. Rev. 2004, 4, Cd004129. [Google Scholar] [CrossRef]
- Genthe, K.; Schenck, C.; Eicholtz, S.; Zajac-Cox, L.; Wolf, S.; Kesar, T.M. Effects of real-time gait biofeedback on paretic propulsion and gait biomechanics in individuals post-stroke. Top. Stroke Rehabil. 2018, 25, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.P.; Chan, C.C.; Lee, T.M.; Hui-Chan, C.W. Mental imagery for promoting relearning for people after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.J.; Milner, C.E. Real-time kinematic, temporospatial, and kinetic biofeedback during gait retraining in patients: A systematic review. Phys. Ther. 2010, 90, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
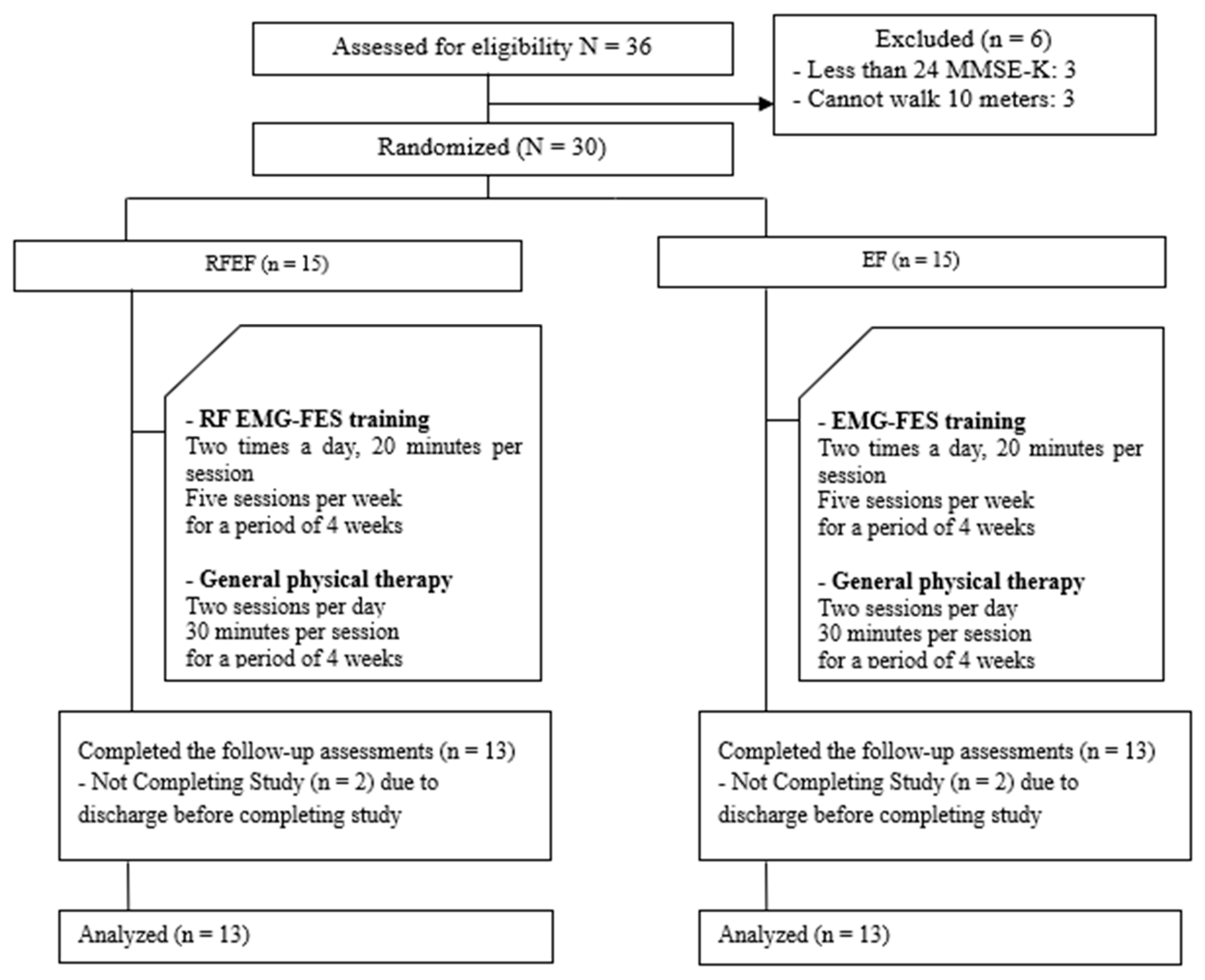
| Categories | RFEF Group (n = 13) | EF Group (n = 13) | x2/t (p) |
|---|---|---|---|
| Gender (Male/Female) | 13 (5/8) | 13 (5/8) | 0.000 (1.000) |
| Age (year) | 57.69 ± 9.49 | 49.15 ± 12.80 | 1.931 (0.066) |
| Height (cm) | 163.69 ± 10.26 | 162.46 ± 11.98 | 0.281 (0.781) |
| Weight (kg) | 60.23 ± 11.27 | 60.85 ± 9.88 | −0.148 (0.884) |
| BMI (kg/m2) | 22.41 ± 3.28 | 23.03 ± 2.44 | −0.541 (0.594) |
| Diagnosis | |||
| Cerebral Hemorrhage | 9 (69.2%) | 8 (61.5%) | 0.170 (0.680) |
| ICH/SAH | 9/0 | 6/2 | |
| Cerebral Infarction | 4 (30.8%) | 5 (38.5%) | |
| MCA/SC/CR | 3/1/0 | 3/0/2 | |
| Affected Side | |||
| Right | 5 (38.5%) | 7 (53.8%) | 0.619 (0.431) |
| Left | 8 (61.5%) | 6 (46.2%) | |
| Onset Time (day) | 272.31 ± 90.29 | 274.92 ± 105.09 | −0.068 (0.946) |
| MMSE-K (score) | 27.54 ± 2.47 | 27.77 ± 2.58 | −0.233 (0.818) |
| Parameters | Trials | RFEF Group (n = 13) | EF Group (n = 13) | t (p) |
|---|---|---|---|---|
| WBLT (cm) | pretest | 3.30 ± 1.70 a | 3.65 ± 1.67 | 1.896 (0.070) |
| posttest | 5.23 ± 2.35 | 4.38 ± 1.98 | ||
| pre–post | 1.92 ± 1.44 | 0.73 ± 0.78 | −2.623 (0.015) | |
| t (p) | −4.811 (0.000) | −3.376 (0.000) | ||
| Tardieu Scale (score) | pretest | 1.35 ± 0.51 | 1.17 ± 0.58 | 0.695 (0.493) |
| posttest | 0.79 ± 0.63 | 1.06 ± 0.69 | ||
| pre–post | −0.55 ± 0.51 | −0.11 ± 0.60 | 59.500 (0.025) | |
| t (p) | −2.940 (0.003) | −0.357 (0.721) |
| Parameters | Trials | RFEF Group (n = 13) | EF Group (n = 13) | t (p) |
|---|---|---|---|---|
| TUG (s) | pretest | 36.54 ± 25.25 a | 20.84 ± 15.93 | 1.896 (0.070) |
| posttest | 28.73 ± 19.13 | 18.49 ± 13.51 | ||
| pre–post | −7.81 ± 7.84 | −2.35 ± 3.51 | −2.290 (0.035) | |
| t (p) | 3.593 (0.004) | 2.417(0.033) | ||
| BBS (score) | pretest | 37.38 ± 9.70 | 40.69 ± 8.01 | −0.947 (0.353) |
| posttest | 44.53 ± 8.51 | 44.69 ± 8.04 | ||
| pre–post | 7.15 ± 3.93 | 4.00 ± 3.08 | 2.275 (0.032) | |
| t (p) | −6.557 (0.000) | −4.679 (0.001) |
| Parameters | Trials | RFEF Group (n = 13) | EF Group (n = 13) | t (p) |
|---|---|---|---|---|
| Gait velocity (cm/s) | pretest | 38.85 ± 32.38 a | 52.47 ± 25.55 | −1.190 (0.246) |
| posttest | 49.73 ± 37.91 | 56.81 ± 26.49 | ||
| pre–post | 10.88 ± 9.39 | 4.34 ± 5.90 | 2.126 (0.044) | |
| t (p) | −4.176 (0.001) | −2.651 (0.021) | ||
| Cadence (steps/min) | pretest | 69.63 ± 25.99 | 75.62 ± 22.74 | −0.626 (0.537) |
| posttest | 76.60 ± 27.16 | 78.68 ± 23.20 | ||
| pre–post | 6.97 ± 10.26 | 3.05 ± 4.71 | 1.249 (0.229) | |
| t (p) | −2.450 (0.031) | −2.337 (0.038) | ||
| Step length (affected) (cm) | pretest | 31.83 ± 15.21 | 38.81 ± 10.47 | −1.363 (0.185) |
| post test | 37.69± 17.02 | 40.49 ± 11.33 | ||
| pre–post | 5.86 ± 4.11 | 1.68 ± 4.09 | 2.595 (0.016) | |
| t (p) | −5.135 (0.000) | −1.479 (0.165) | ||
| Stride length (affected) (cm) | pretest | 60.01 ± 29.25 | 78.67 ± 21.51 | −1.853 (0.076) |
| posttest | 70.35 ± 33.35 | 82.17 ± 22.31 | ||
| pre–post | 10.34 ± 6.69 | 3.50 ± 7.30 | 2.487 (0.020) | |
| t (p) | −5.566 (0.000) | −1.729 (0.109) | ||
| Stance phase (affected) (%) | pretest | 74.70 ± 7.83 | 72.12 ± 5.03 | 0.999 (0.328) |
| posttest | 70.30 ± 9.50 | 71.06 ± 4.69 | ||
| pre–post | −4.40 ± 5.20 | −1.05 ± 2.29 | −2.120 (0.050) | |
| t (p) | 3.050 (0.010) | 1.659 (0.123) | ||
| Swing phase (affected) (%) | pretest | 25.28 ± 7.83 | 27.88 ± 5.03 | −1.005 (0.325) |
| post test | 29.70 ± 9.50 | 28.94 ± 4.70 | ||
| pre–post | 4.41 ± 5.20 | 1.05 ± 2.28 | 2.127 (0.049) | |
| t (p) | −3.056 (0.010) | −1.669 (0.121) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Lee, J.; Lee, B.-H. Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis. Healthcare 2020, 8, 292. https://doi.org/10.3390/healthcare8030292
Bae S, Lee J, Lee B-H. Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis. Healthcare. 2020; 8(3):292. https://doi.org/10.3390/healthcare8030292
Chicago/Turabian StyleBae, Subeen, Jin Lee, and Byoung-Hee Lee. 2020. "Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis" Healthcare 8, no. 3: 292. https://doi.org/10.3390/healthcare8030292
APA StyleBae, S., Lee, J., & Lee, B.-H. (2020). Effect of an EMG–FES Interface on Ankle Joint Training Combined with Real-Time Feedback on Balance and Gait in Patients with Stroke Hemiparesis. Healthcare, 8(3), 292. https://doi.org/10.3390/healthcare8030292






