Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. SensewearTM Activity Armband
2.3. SF-36 Questionnaire: Physical Activity Subscale
2.4. Cardiopulmonary Exercise Testing
2.5. Statistical Analysis
3. Results
Patient Clinical Data
4. Discussion
4.1. Physical Activity Questionnaires in Previous ME/CFS Studies:
4.2. Number of Steps in Previous ME/CFS Studies
4.3. Cardiopulmonary Exercise Test in Previous ME/CFS Studies
4.4. Summary of Previous ICC Clinical Severity Category Grading
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Carruthers, B.M.; van de Sande, M.I.; DE Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Arnett, S.V.; Alleva, L.M.; Korossy-Horwood, R.; Clark, I.A. Chronic fatigue syndrome—A neuroimmunological model. Med. Hypotheses 2011, 77, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Pietrangelo, T.; Mancinelli, R.; Saggini, R.; Fano, G. Specific correlations between muscle oxidative stress and chronic fatigue syndrome: A working hypothesis. J. Muscle Res. Cell Motil. 2007, 28, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, T.R.; Papanicolaou, D.A.; Amsterdam, J.D.; Bingham, S.; Grossman, A.; Hedrick, T.; Herberman, R.B.; Krueger, G.; Levine, S.; Mohagheghpour, N.; et al. Immunologic aspects of chronic fatigue syndrome. Report on a Research Symposium convened by The CFIDS Association of America and co-sponsored by the US Centers for Disease Control and Prevention and the National Institutes of Health. Neuroimmunomodulation 2004, 11, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Oktayoglu, P. Central nervous system abnormalities in fibromyalgia and chronic fatigue syndrome: New concepts in treatment. Curr. Pharm. Des. 2008, 14, 1274–1294. [Google Scholar] [CrossRef]
- Jones, D.E.; Hollingsworth, K.G.; Taylor, R.; Blamire, A.M.; Newton, J.L. Abnormalities in pH handling by peripheral muscle and potential regulation by the autonomic nervous system in chronic fatigue syndrome. J. Intern. Med. 2010, 267, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Klimas, N.G.; Salvato, F.R.; Morgan, R.; Fletcher, M.A. Immunologic abnormalities in chronic fatigue syndrome. J. Clin. Microbiol. 1990, 28, 1403–1410. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Cho, T.A. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin. Neurol. 2011, 31, 325–337. [Google Scholar] [CrossRef]
- McCully, K.K.; Smith, S.; Rajaei, S.; Leigh, J.S., Jr.; Natelson, B.H. Blood flow and muscle metabolism in chronic fatigue syndrome. Clin. Sci. (Lond.) 2003, 104, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Naess, H.; Sundal, E.; Myhr, K.M.; Nyland, H.I. Postinfectious and chronic fatigue syndromes: Clinical experience from a tertiary-referral centre in Norway. In Vivo 2010, 24, 185–188. [Google Scholar] [PubMed]
- Okamoto, L.E.; Raj, S.R.; Peltier, A.; Gamboa, A.; Shibao, C.; Diedrich, A.; Black, B.K.; Robertson, D.; Biaggioni, I. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin. Sci. (Lond.) 2012, 122, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernandez, O.D.; Shoenfeld, Y. Infection, vaccination, and autoantibodies in chronic fatigue syndrome, cause or coincidence? Ann. N. Y. Acad. Sci. 2009, 1173, 600–609. [Google Scholar] [CrossRef]
- Stewart, J.M. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000, 48, 218–226. [Google Scholar] [CrossRef]
- Wong, R.; Lopaschuk, G.; Zhu, G.; Walker, D.; Catellier, D.; Burton, D.; Teo, K.; Collins-Nakai, R.; Montague, T. Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest 1992, 102, 1716–1722. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Visser, F.C. The Effect of Curcumin in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Disparate Responses in Different Disease Severities. Pharmacovigil. Pharmacoepidemiol. 2019, 2, 22–27. [Google Scholar] [CrossRef]
- Viehoff, P.B.; van Genderen, F.R.; Wittink, H. Upper limb lymphedema 27 (ULL27): Dutch translation and validation of an illness-specific health-related quality of life questionnaire for patients with upper limb lymphedema. Lymphology 2008, 41, 131–138. [Google Scholar]
- van Campen, C.L.M.C.; Visser, F.C. Validity of 2-day cardiopulmonary exercise testing in female patients with myalgic encephalomyelitis/chronic fatigue syndrome. Int. J. Curr. Res. 2020, 12, 10436–10442. [Google Scholar] [CrossRef]
- Liu, X. Classification accuracy and cut point selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
- Hoo, Z.H.; Candlish, J.; Teare, D. What is an ROC curve? Emerg. Med. J. 2017, 34, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.; Saez-Francas, N.; Castro-Marrero, J.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Gender differences in chronic fatigue syndrome. Reum. Clin. 2016, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.L.M.C.; Rowe, P.C.; Verheugt, F.W.A.; Visser, F.C. Physical activity measures in patients with myalgic encephalomyalitis/chronic fatigue syndrome: Correlations between peak oxygen consumption, the physical functioning scale of the SF-36 scale, and the number of steps from an activity meter. J. Transl. Med. 2020, 18, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Dugan, S.A.; Everson-Rose, S.A.; Karavolos, K.; Sternfeld, B.; Wesley, D.; Powell, L.H. The impact of physical activity level on SF-36 role-physical and bodily pain indices in midlife women. J. Phys. Act. Health 2009, 6, 33–42. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Matcham, F.; Scott, I.C.; Rayner, L.; Hotopf, M.; Kingsley, G.H.; Norton, S.; Scott, D.L.; Steer, S. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 123–130. [Google Scholar] [CrossRef]
- Gu, M.; Cheng, Q.; Wang, X.; Yuan, F.; Sam, N.B.; Pan, H.; Li, B.; Ye, D. The impact of SLE on health-related quality of life assessed with SF-36: A systemic review and meta-analysis. Lupus 2019, 28, 371–382. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Gu, C.; Gu, Z.; Li, L.; Li, Z.; Dong, C.; Zhu, J.; Fu, T.; Gao, J. The impact of systemic lupus erythematosus on health-related quality of life assessed using the SF-36: A systematic review and meta-analysis. Psychol. Health Med. 2019, 24, 978–991. [Google Scholar] [CrossRef]
- Yang, X.; Fan, D.; Xia, Q.; Wang, M.; Zhang, X.; Li, X.; Cai, G.; Wang, L.; Xin, L.; Xu, S.; et al. The health-related quality of life of ankylosing spondylitis patients assessed by SF-36: A systematic review and meta-analysis. Qual. Life Res. 2016, 25, 2711–2723. [Google Scholar] [CrossRef]
- Li, L.; Cui, Y.; Chen, S.; Zhao, Q.; Fu, T.; Ji, J.; Li, L.; Gu, Z. The impact of systemic sclerosis on health-related quality of life assessed by SF-36: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2018, 21, 1884–1893. [Google Scholar] [CrossRef]
- Wyld, M.; Morton, R.L.; Hayen, A.; Howard, K.; Webster, A.C. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012, 9, e1001307. [Google Scholar] [CrossRef] [PubMed]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: A systematic review and meta-analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef] [PubMed]
- Filbay, S.; Pandya, T.; Thomas, B.; McKay, C.; Adams, J.; Arden, N. Quality of Life and Life Satisfaction in Former Athletes: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.; Brown, M.; Evans, M.; Anderson, V.; Lerch, A.; Brown, A.; Hunnell, J.; Porter, N. Measuring substantial reductions in functioning in patients with chronic fatigue syndrome. Disabil. Rehabil. 2011, 33, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.; McManimen, S.; Gleason, K.; Stoothoff, J.; Newton, J.L.; Strand, E.B.; Jason, L.A. Assessing current functioning as a measure of significant reduction in activity level. Fatigue 2016, 4, 175–188. [Google Scholar] [CrossRef]
- An, H.S.; Jones, G.C.; Kang, S.K.; Welk, G.J.; Lee, J.M. How valid are wearable physical activity trackers for measuring steps? Eur. J. Sport Sci. 2017, 17, 360–368. [Google Scholar] [CrossRef]
- Wahl, Y.; Duking, P.; Droszez, A.; Wahl, P.; Mester, J. Criterion-Validity of Commercially Available Physical Activity Tracker to Estimate Step Count, Covered Distance and Energy Expenditure during Sports Conditions. Front. Physiol. 2017, 8, 725. [Google Scholar] [CrossRef]
- Almeida, G.J.; Wasko, M.C.; Jeong, K.; Moore, C.G.; Piva, S.R. Physical activity measured by the SenseWear Armband in women with rheumatoid arthritis. Phys. Ther. 2011, 91, 1367–1376. [Google Scholar] [CrossRef]
- Avesani, C.M.; Trolonge, S.; Deleaval, P.; Baria, F.; Mafra, D.; Faxen-Irving, G.; Chauveau, P.; Teta, D.; Kamimura, M.A.; Cuppari, L.; et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transplant. 2012, 27, 2430–2434. [Google Scholar] [CrossRef]
- Hill, K.; Dolmage, T.E.; Woon, L.; Goldstein, R.; Brooks, D. Measurement properties of the SenseWear armband in adults with chronic obstructive pulmonary disease. Thorax 2010, 65, 486–491. [Google Scholar] [CrossRef]
- Tanhoffer, R.A.; Tanhoffer, A.I.; Raymond, J.; Hills, A.P.; Davis, G.M. Comparison of methods to assess energy expenditure and physical activity in people with spinal cord injury. J. Spinal Cord Med. 2012, 35, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.; Camillo, C.A.; Vitorasso, R.; Sant’anna, T.; Hernandes, N.A.; Probst, V.S.; Pitta, F. Obesity and Physical Activity in the Daily Life of Patients with COPD. Lung 2012, 190, 403–410. [Google Scholar] [CrossRef]
- Calabro, M.A.; Welk, G.J.; Eisenmann, J.C. Validation of the SenseWear Pro Armband algorithms in children. Med. Sci. Sports Exerc. 2009, 41, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Khorana, N.; Jason, L.A. The role of changes in activity as a function of perceived available and expended energy in nonpharmacological treatment outcomes for ME/CFS. J. Clin. Psychol. 2011, 67, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Evering, R.M.; Tonis, T.M.; Vollenbroek-Hutten, M.M. Deviations in daily physical activity patterns in patients with the chronic fatigue syndrome: A case control study. J. Psychosom. Res. 2011, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ickmans, K.; Clarys, P.; Nijs, J.; Meeus, M.; Aerenhouts, D.; Zinzen, E.; Aelbrecht, S.; Meersdom, G.; Lambrecht, L.; Pattyn, N. Association between cognitive performance, physical fitness, and physical activity level in women with chronic fatigue syndrome. J. Rehabil. Res. Dev. 2013, 50, 795–810. [Google Scholar] [CrossRef]
- Jason, L.A.; King, C.P.; Frankenberry, E.L.; Jordan, K.M.; Tryon, W.W.; Rademaker, F.; Huang, C.F. Chronic fatigue syndrome: Assessing symptoms and activity level. J. Clin. Psychol. 1999, 55, 411–424. [Google Scholar] [CrossRef]
- Kop, W.J.; Lyden, A.; Berlin, A.A.; Ambrose, K.; Olsen, C.; Gracely, R.H.; Williams, D.A.; Clauw, D.J. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005, 52, 296–303. [Google Scholar] [CrossRef]
- Solomon-Moore, E.; Jago, R.; Beasant, L.; Brigden, A.; Crawley, E. Physical activity patterns among children and adolescents with mild-to-moderate chronic fatigue syndrome/myalgic encephalomyelitis. BMJ Paediatr. Open 2019, 3, e000425. [Google Scholar] [CrossRef]
- Wyller, V.B.; Vitelli, V.; Sulheim, D.; Fagermoen, E.; Winger, A.; Godang, K.; Bollerslev, J. Altered neuroendocrine control and association to clinical symptoms in adolescent chronic fatigue syndrome: A cross-sectional study. J. Transl. Med. 2016, 14, 121. [Google Scholar] [CrossRef]
- De Becker, P.; Roeykens, J.; Reynders, M.; McGregor, N.; De Meirleir, K. Exercise capacity in chronic fatigue syndrome. Arch. Intern. Med. 2000, 160, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, K.Y.; White, P.D. Strength and physiological response to exercise in patients with chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 2000, 69, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Hodges, L.D.; Nielsen, T.; Baken, D. Physiological measures in participants with chronic fatigue syndrome, multiple sclerosis and healthy controls following repeated exercise: A pilot study. Clin. Physiol. Funct. Imaging 2018, 38, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Jammes, Y.; Steinberg, J.G.; Mambrini, O.; Bregeon, F.; Delliaux, S. Chronic fatigue syndrome: Assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J. Intern. Med. 2005, 257, 299–310. [Google Scholar] [CrossRef]
- Keller, B.A.; Pryor, J.L.; Giloteaux, L. Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO(2)peak indicates functional impairment. J. Transl. Med. 2014, 12, 104. [Google Scholar] [CrossRef]
- Sargent, C.; Scroop, G.C.; Nemeth, P.M.; Burnet, R.B.; Buckley, J.D. Maximal oxygen uptake and lactate metabolism are normal in chronic fatigue syndrome. Med. Sci. Sports Exerc. 2002, 34, 51–56. [Google Scholar] [CrossRef]
- Sisto, S.A.; LaManca, J.; Cordero, D.L.; Bergen, M.T.; Ellis, S.P.; Drastal, S.; Boda, W.L.; Tapp, W.N.; Natelson, B.H. Metabolic and cardiovascular effects of a progressive exercise test in patients with chronic fatigue syndrome. Am. J. Med. 1996, 100, 634–640. [Google Scholar] [CrossRef]
- Snell, C.R.; Stevens, S.R.; Davenport, T.E.; Van Ness, J.M. Discriminative Validity of Metabolic and Workload Measurements to Identify Individuals With Chronic Fatigue Syndrome. Phys. Ther. 2013. [Google Scholar] [CrossRef]
- Vanness, J.M.; Snell, C.R.; Stevens, S.R. Diminished cardiopulmonary capacity during post-exertional malaise. J. Chronic Fatigue Syndr. 2007, 14, 77–85. [Google Scholar] [CrossRef]
- Vermeulen, R.C.; Kurk, R.M.; Visser, F.C.; Sluiter, W.; Scholte, H.R. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J. Transl. Med. 2010, 8, 93. [Google Scholar] [CrossRef]
- Vermeulen, R.C.; Vermeulen van Eck, I.W. Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J. Transl. Med. 2014, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Wallman, K.E.; Morton, A.R.; Goodman, C.; Grove, R. Physiological responses during a submaximal cycle test in chronic fatigue syndrome. Med. Sci. Sports Exerc. 2004, 36, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.D.; Atkinson, G.; Atkinson, J.M.; Batterham, A.M. Peak Oxygen Uptake in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Meta-Analysis. Int. J. Sports Med. 2019, 40, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cureton, K.; Bishop, P.; Hutchinson, P.; Newland, H.; Vickery, S.; Zwiren, L. Sex difference in maximal oxygen uptake. Effect of equating haemoglobin concentration. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 54, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Pina, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef]
- Fomin, A.; Ahlstrand, M.; Schill, H.G.; Lund, L.H.; Stahlberg, M.; Manouras, A.; Gabrielsen, A. Sex differences in response to maximal exercise stress test in trained adolescents. BMC Pediatr. 2012, 12, 127. [Google Scholar] [CrossRef]
- Higginbotham, M.B.; Morris, K.G.; Coleman, R.E.; Cobb, F.R. Sex-related differences in the normal cardiac response to upright exercise. Circulation 1984, 70, 357–366. [Google Scholar] [CrossRef]
- Sharma, H.B.; Kailashiya, J. Gender Difference in Aerobic Capacity and the Contribution by Body Composition and Haemoglobin Concentration: A Study in Young Indian National Hockey Players. J. Clin. Diagn. Res. 2016, 10, CC09–CC13. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Snyder, E.M.; Johnson, B.D.; Olson, T.P. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus 2014, 3, 445. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Two-day cardiopulmonary exercise testing in females with a severe grade of myalgic encephalomylitis/chornic fatigue syndrome: Comparison with patients with a mild and moderate disease. Healthcare 2020, 8, 192. [Google Scholar] [CrossRef]
- Martina, J.R.; Westerhof, B.E.; van Goudoever, J.; de Beaumont, E.M.; Truijen, J.; Kim, Y.S.; Immink, R.V.; Jobsis, D.A.; Hollmann, M.W.; Lahpor, J.R.; et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R). Anesthesiology 2012, 116, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. (1985) 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.; Koch, B.; Ittermann, T.; Schaper, C.; Dorr, M.; Felix, S.B.; Volzke, H.; Ewert, R.; Hansen, J.E. Influence of age, sex, body size, smoking, and beta blockade on key gas exchange exercise parameters in an adult population. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 469–476. [Google Scholar] [CrossRef] [PubMed]
| Males (n = 51) | Females (n = 238) | p-Value | |
|---|---|---|---|
| Age (years) | 42 (11) | 39 (11) | 0.14 |
| BMI (kg/m2) | 24.9 (3.9) | 24.5 (5.3) | 0.64 |
| Disease duration (years) | 11 (8) | 12 (9) | 0.69 |
| Disease severity: mild/moderate/severe (%) * | 23/15/13 (45/29/26%) | 98/83/57 (41/29/20%) | Chi-square 0.75 |
| Heart rate at rest (bpm) | 89 (16) | 89 (19) | 0.84 |
| SBP at rest (mmHg) | 125 (16) | 130 (16) | 0.18 |
| DBP at rest (mmHg) | 85 (20) | 84 (11) | 0.85 |
| SF-36 PAS | 48 (23) | 48 (24) | 0.96 |
| Number of steps/day | 5768 (2511) | 5687 (2713) | 0.84 |
| VT VO2 (ml/kg/min) | 12 (4) | 11 (3) | 0.09 |
| %VT VO2 | 38 (14) | 40 (11) | 0.33 |
| peak VO2 (ml/kg/min) | 23 (8) | 20 (6) | 0.005 |
| %peak VO2 | 72 (24) | 71 (19) | 0.79 |
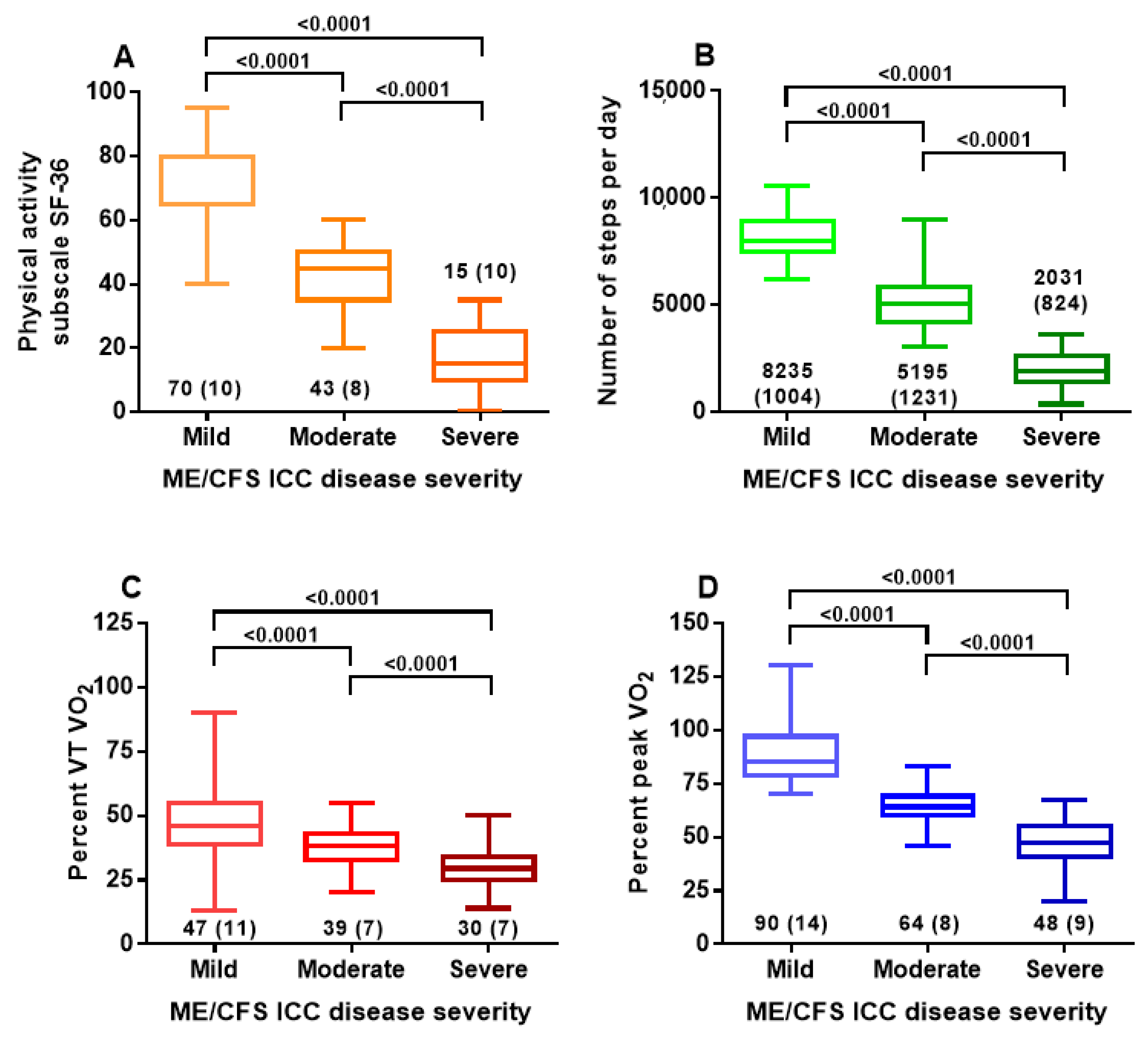
| Group 1: Mild | Group 2: Moderate | Group 3: Severe | One-Way ANOVA and Post-Hoc Tukey’s Test | |
|---|---|---|---|---|
| Number | 121 | 98 | 70 | |
| Male/female | 23/98 | 15/83 | 13/57 | Chi-square 0.75 (3 × 2 table) |
| Age (years) | 43 (11) | 38 (11) | 35(11) | F (2, 286) = 12.1; p < 0.0001. Post-hoc tests: 1 vs. 2 p = 0.008; 1 vs. 3 p < 0.0001 and 2 vs. 3 p = 0.12 |
| Disease duration (years) | 12 (9) | 12 (8) | 12 (8) | F (2, 286) = 0.06; p = 0.94 |
| SF-36 PAS | 70 (11) | 43 (8) | 15 (10) | F (2, 286) = 717.6; p < 0.0001. Post-hoc tests: 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001 |
| Number of steps/day | 8235 (1004) | 5195 (1231) | 2031 (824) | F (2, 286) = 792.4; p < 0.0001. Post-hoc tests: 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001 |
| %VT VO2 | 47 (11) | 38 (7) | 30 (7) | F (2, 286) = 85.7; p < 0.0001. Post-hoc tests: 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001 |
| %peak VO2 | 90 (14) | 64 (8) | 48 (9) | F (2, 286) = 355.0; p < 0.0001. Post-hoc tests: 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001 |
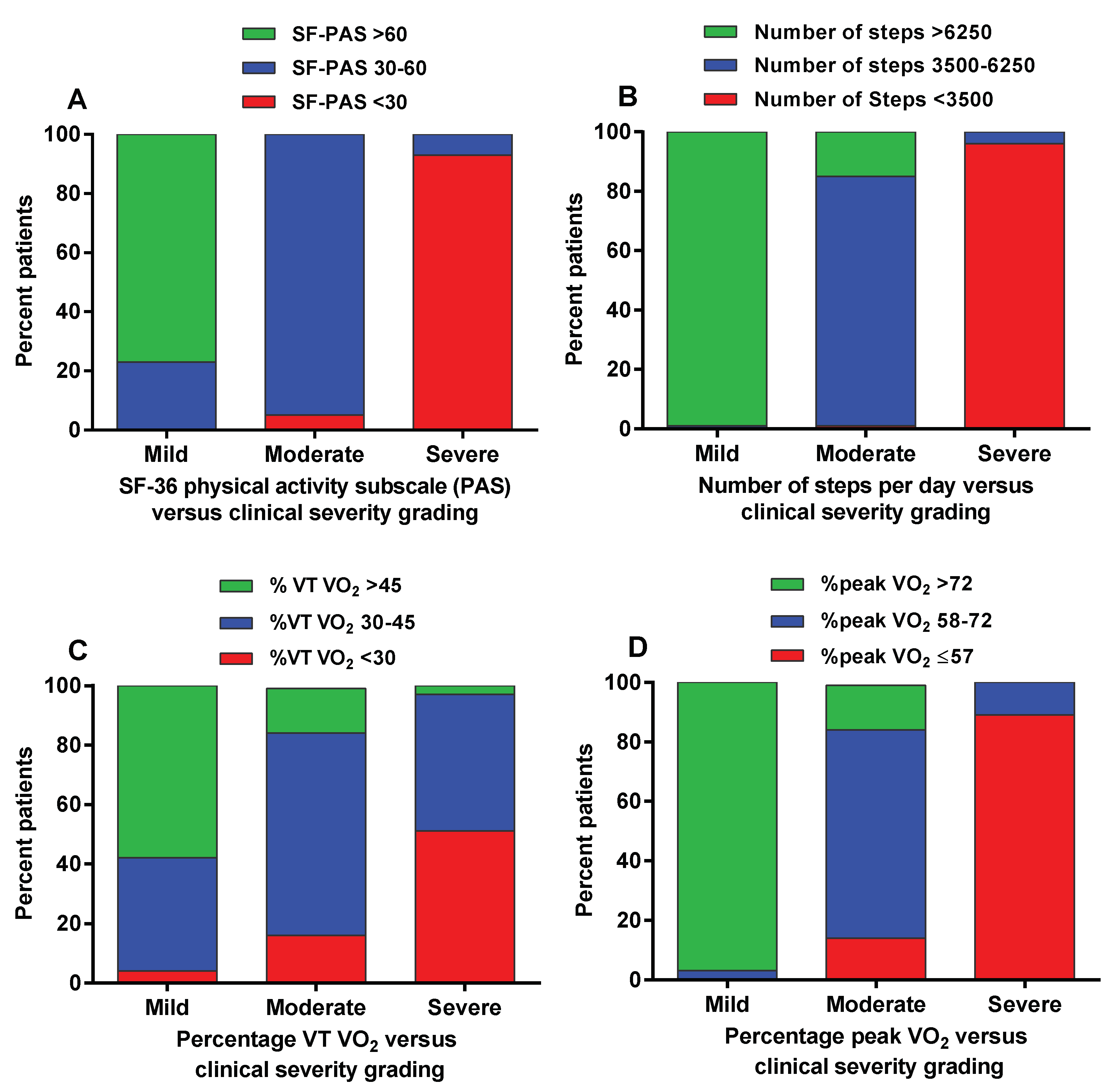
| Group 1 Mild | Group 2 Moderate | Group 3 Severe | Measure of Agreement (Kappa) | |
|---|---|---|---|---|
| Physical Activity subscale of the SF-36 (PAS) (panel A Figure 2) | ||||
| >60 | 93 (77%) | 0 (0%) | 0 (0%) | Kappa 0.80 (95%CI: 0.742–0.859) |
| 30–60 | 28 (23%) | 93 (95%) | 5 (7%) | |
| <30 | 0 (0%) | 5 (5%) | 65 (93%) | |
| Number of steps (panel B Figure 2) | ||||
| >6250 | 120 (99%) | 15 (15%) | 0 (0%) | Kappa 0.89 (95% CI: 0.848–0.938). |
| 3500–6250 | 1 (1%) | 82 (84%) | 3 (4%) | |
| <3500 | 0 (0%) | 1 (1%) | 67 (96%) | |
| %predicted oxygen consumption at the ventilatory threshold (panel C Figure 2) | ||||
| >56% | 70 (58%) | 15 (15%) | 2 (3%) | Kappa 0.39 (95% CI: 0.303–0.473). |
| 30–45% | 46 (38%) | 68 (69%) | 32 (46%) | |
| <30% | 5 (4%) | 15 (16%) | 36 (51%) | |
| %predicted peak oxygen consumption (panel D Figure 2) | ||||
| >72% | 117 (97%) | 15 (15%) | 0 (0%) | Kappa 0.78 (95% CI: 0.721–0.843). |
| 58–72% | 4 (3%) | 70 (71%) | 8 (11%) | |
| ≤57% | 0 (0%) | 13 (14%) | 62 (89%) | |
| Group 1: Mild | Group 2: Moderate | Group 3: Severe | Two-Way Mixed ANOVA with Post Hoc Tukey Test | |
|---|---|---|---|---|
| Female/Male ME/CFS patients | ||||
| Number of males/females | 23/98 | 15/83 | 13/57 | |
| Male SF-36 PAS | 67 (13) | 43 (7) | 20 (11) | F (2, 283) = 3.55; p = 0.030. Post-hoc tests: female patients 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001; male patients 1 vs. 2 p < 0.0001; 1 vs. 3 p < 0.0001 and 2 vs. 3 p < 0.0001 |
| Female SF-36 PAS | 71 (10) | 44 (9) | 14 (10) | |
| Male Number of steps/day | 8067 (1152) | 5056 (892) | 2523 (860) | F (2, 283) = 2.51; p = 0.083 |
| Female Number of steps/day | 8274 (969) | 5220 (1286) | 1919 (780) | |
| Male %VT VO2 | 48 (15) | 33 (5) | 26 (6) | F (2, 283) = 2.11; p = 0.12. |
| Female %VT VO2 | 47 (10) | 39 (7) | 30 (8) | |
| Male %peak VO2 | 93 (15) | 60 (9) | 46 (9) | F (2, 283) = 2.46; p = 0.088 |
| Female %peak VO2 | 89 (13) | 65 (7) | 48 (9) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Campen, C.M.C.; Rowe, P.C.; Visser, F.C. Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare 2020, 8, 273. https://doi.org/10.3390/healthcare8030273
van Campen CMC, Rowe PC, Visser FC. Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare. 2020; 8(3):273. https://doi.org/10.3390/healthcare8030273
Chicago/Turabian Stylevan Campen, C. (Linda) M. C., Peter C. Rowe, and Frans C. Visser. 2020. "Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36" Healthcare 8, no. 3: 273. https://doi.org/10.3390/healthcare8030273
APA Stylevan Campen, C. M. C., Rowe, P. C., & Visser, F. C. (2020). Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare, 8(3), 273. https://doi.org/10.3390/healthcare8030273





