1. Introduction
Bone and soft tissue sarcomas are rare, accounting for approximately 1% of all malignancies. In the United Kingdom, 3298 new soft tissue sarcomas and 531 bone sarcomas were diagnosed in 2010 [
1]. They comprise a heterogeneous group of tumours, with over 100 different histological subtypes [
2]. The diagnosis can often be difficult and delayed; hence a large proportion of patients will require palliative intervention during the course of their disease. Approximately 50% of patients with high grade soft tissue sarcoma will develop metastatic disease requiring palliative treatment [
3,
4,
5], most commonly with systemic chemotherapy. In addition, radiotherapy may have a role in the palliation of symptoms. A review of sarcoma services in Australia demonstrated that 36% of patients with metastatic disease were offered palliative radiotherapy [
6].
Sarcomas are commonly regarded as being relatively radio-resistant, with doses of 60–70 Gy in 2 Gy per fraction required in the radical setting to control microscopic disease. Therefore, it could be hypothesized that achieving good palliation with radiation may require larger doses than are used for more common solid tumour types. To date, limited data is available in the published literature regarding appropriate palliative radiotherapy dose fractionation schedules for sarcoma. Furthermore, there is evidence that different histological subtypes of sarcoma have different response rates to commonly prescribed chemotherapeutic agents [
7,
8] and therefore it seems likely that the same is true for these tumours inherent radio-sensitivity. Some histological subtypes may potentially require larger doses to achieve good palliation compared to others. The rarity and heterogeneity of sarcomas makes this very difficult to research and therefore limited data is currently available to guide the appropriate palliative radiotherapy dose fractionation schedules for the different subtypes of bone and soft tissue sarcoma. In addition, these tumours can present as primary or secondary tumours at any anatomical site, adding further complexities when prescribing palliative radiotherapy as the organs at risk and their normal tissue tolerances can vary greatly.
Symptoms requiring palliation in patients with metastatic sarcoma are no different to those suffered by patients with other metastatic solid tumour types and include pain (both from bony and soft tissue tumours), neurological symptoms from brain or spinal metastases, bleeding or superior vena cava obstruction. A previously published case series considered 17 patients with symptomatic metastatic sarcoma requiring rapid palliation [
9]. They were treated with a hypofractionated regime with 39 Gy in 13 fractions, treating daily Monday to Friday and reported that this was well tolerated. At a median follow-up of approximately 6 months, the majority achieved durable pain control. Similarly, a review of four patients treated with palliative radiotherapy for metastatic spinal cord compression secondary to soft tissue sarcoma revealed a successful rate of pain control with standard palliative doses of radiation (20 Gy in 5 fractions or 30 Gy in 10 fractions), however the effect on preservation of motor function was less successful and the authors concluded that upfront neurosurgery is required to have any benefit on neurological function [
10].
The aim of this case series was to assess the effectiveness of different radiotherapy dose and fractionation regimens in providing symptomatic improvement in patients with advanced and metastatic sarcomas at one institution.
2. Materials and Methods
Patients receiving radiotherapy for sarcoma with palliative intent, at University Hospitals Birmingham Birmingham, United Kingdom between July 2010 and April 2019 were retrospectively identified from a prospective database. Patients were included if they had a histologically confirmed soft tissue or bone sarcoma and had provided informed consent for palliative radiotherapy.
Data collected included demographics, bone or soft tissue sarcoma status, histological subtype of sarcoma, indication for radiotherapy, site treated and details of the dose fractionation schedule of radiation offered and delivered. Dose fractionation schedules were selected by the treating clinician based upon the histological subtype of sarcoma, with the aim of giving a higher biologically effective dose for less radiosensitive subtypes. Furthermore, the indication for radiotherapy also influenced the chosen dose fractionation schedule, with a higher biological effective dose (BED) often used for patients with a better prognosis. Radiotherapy was planned with either simulation, virtual simulation or a conformal planning technique. Radiotherapy was delivered using a single field, parallel opposed fields, a conformal plan, an intensity modulated radiotherapy plan or a stereotactic ablative body radiotherapy (SABR) plan. The majority of patients were treated with 6-MV or 10-MV photons delivered using a linear accelerator; however, a small number of patients were treated with SABR (CyberKnife).
2.1. Outcomes
The primary outcome was documented symptomatic improvement following radiotherapy. Clinical and electronic patient records were retrospectively reviewed considering the baseline symptoms and the main indication for radiotherapy, as well as documented improvement in these symptoms following radiotherapy. Symptomatic improvement was defined by clear documentation that the symptom had either resolved or improved in the three months after radiotherapy. Given the retrospective nature of this study no attempt was made to further quantify the degree of improvement of the symptoms as this could not be accurately measured. Secondary outcome was overall survival.
2.2. Statistics
Overall survival was calculated from the time of completion of radiotherapy to the time of death or last follow-up. An alpha beta ratio of 4 was used for the tumour, as per previous publications [
9] to calculate the biological effective dose of each dose fractionation schedule used, to allow a comparison of these schedules. The study was registered with the hospital clinical governance committee (reference number: CARMS-15316).
3. Results
A total of 105 patients were identified as having received palliative radiotherapy between July 2010 and April 2019 with a total of 137 sites treated. Baseline tumour characteristics are shown in
Table 1. Of the sites treated 114 sites were soft tissue sarcomas and 23 were bone sarcomas. The median age at the time radiotherapy was deliveredwas 54 (range 8–90) years.
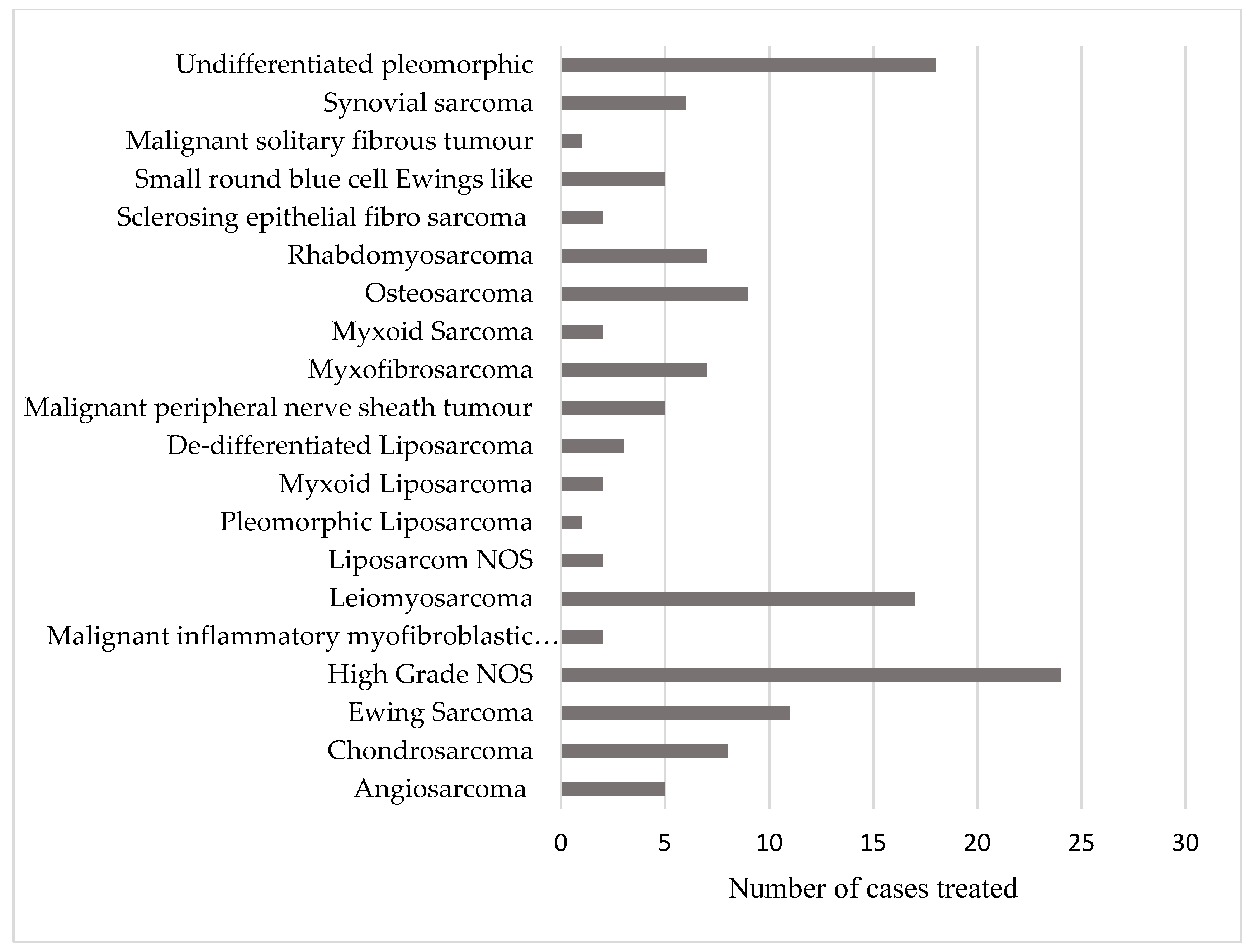
The patient population included 17 subtypes of soft tissue sarcoma and 3 subtypes of bone sarcoma (
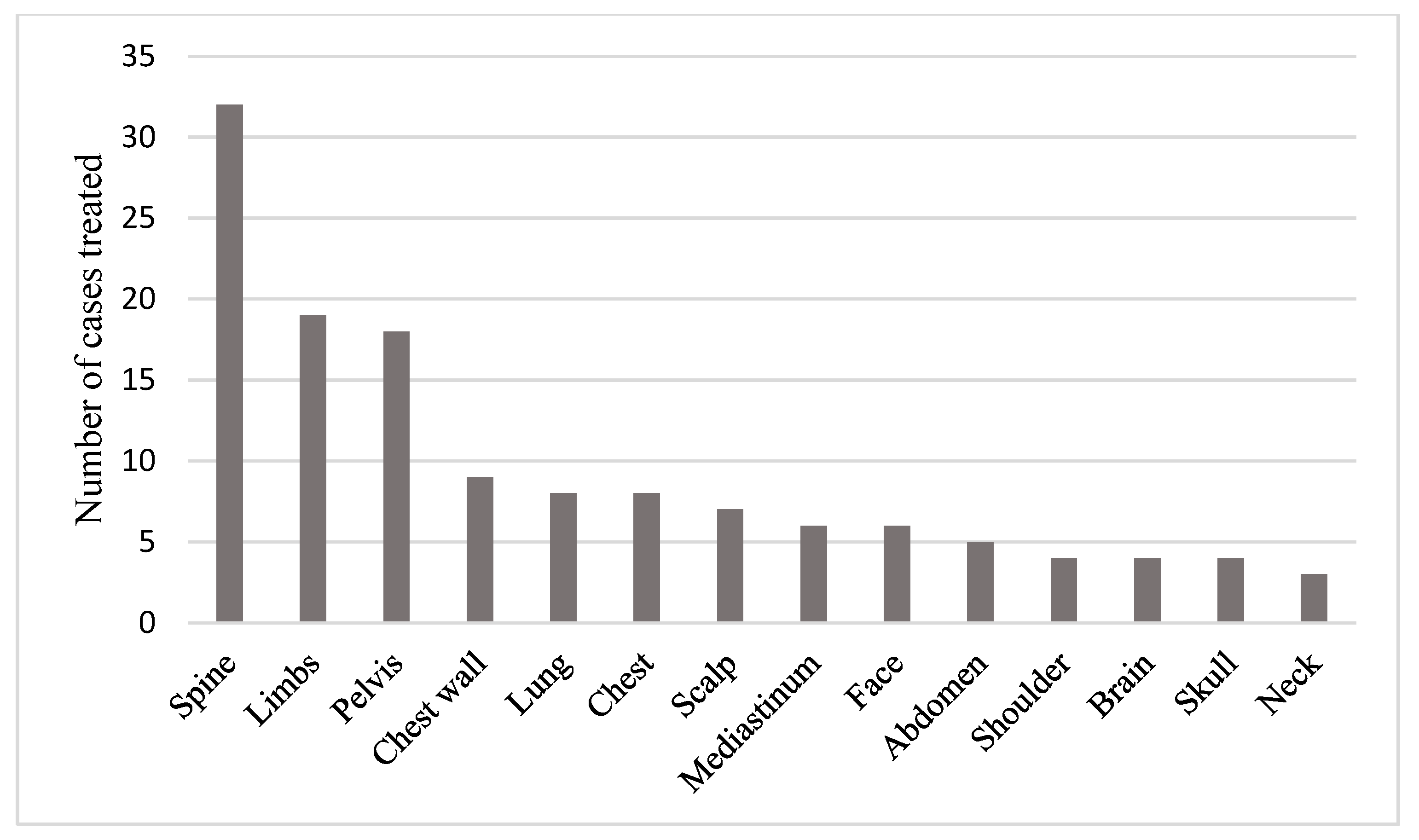
Figure 1). A total of 15 different anatomical sites were treated with palliative radiotherapy during the time period studied. These are shown in
Figure 2.
Table 2 and
Table 3 shows the details of the radiotherapy dose fractionation schedules delivered, and the radiotherapy techniques used. A total of 25 different dose fractionation schedules were used.
The commonest indication for treatment was pain (n = 74). Other indications included breathlessness (n = 5), fungating tumour (n = 7), metastatic spinal cord compression (n = 6), bleeding (n = 4), neurological dysfunction (n = 3), symptomatic brain metastases (n = 2), cough (n = 1), and superior vena cava obstruction (n = 1). Several patients were given a higher dose of palliative radiotherapy for local control (n = 23).
Data on symptomatic improvement was available in 56% of the soft tissue sarcomas and 67% of the bone sarcomas treated. A total of 70% of soft tissue and 55% of bone sarcoma patients reported symptomatic improvement. Twenty Gy in 5 fractions was the most commonly used schedule with 50% of patients with soft tissue sarcomas reporting an improvement in symptoms, whilst 67% of patients with bone sarcoma reported an improvement.
Because of the large variation in dose and fractionations used, each schedule was converted to biological effective dose (BED) to allow comparison between schedules. An alpha beta ratio (α/β) of 4 for the tumour was used as this has been used in a previous case series [
9].
Table 4 illustrates the BED calculation and
Figure 3 demonstrates the symptomatic response compared to the BED.
Because of the small numbers of patients in each histological subtype it is difficult to analyse the response to radiotherapy based on subtype. However, response rates were higher for soft tissue sarcomas with all patients with angiosarcoma and leiomyosarcoma reporting an improvement in symptoms. Response was lowest in osteosarcomas and chondrosarcomas with only 25% of patients reporting an improvement in symptoms.
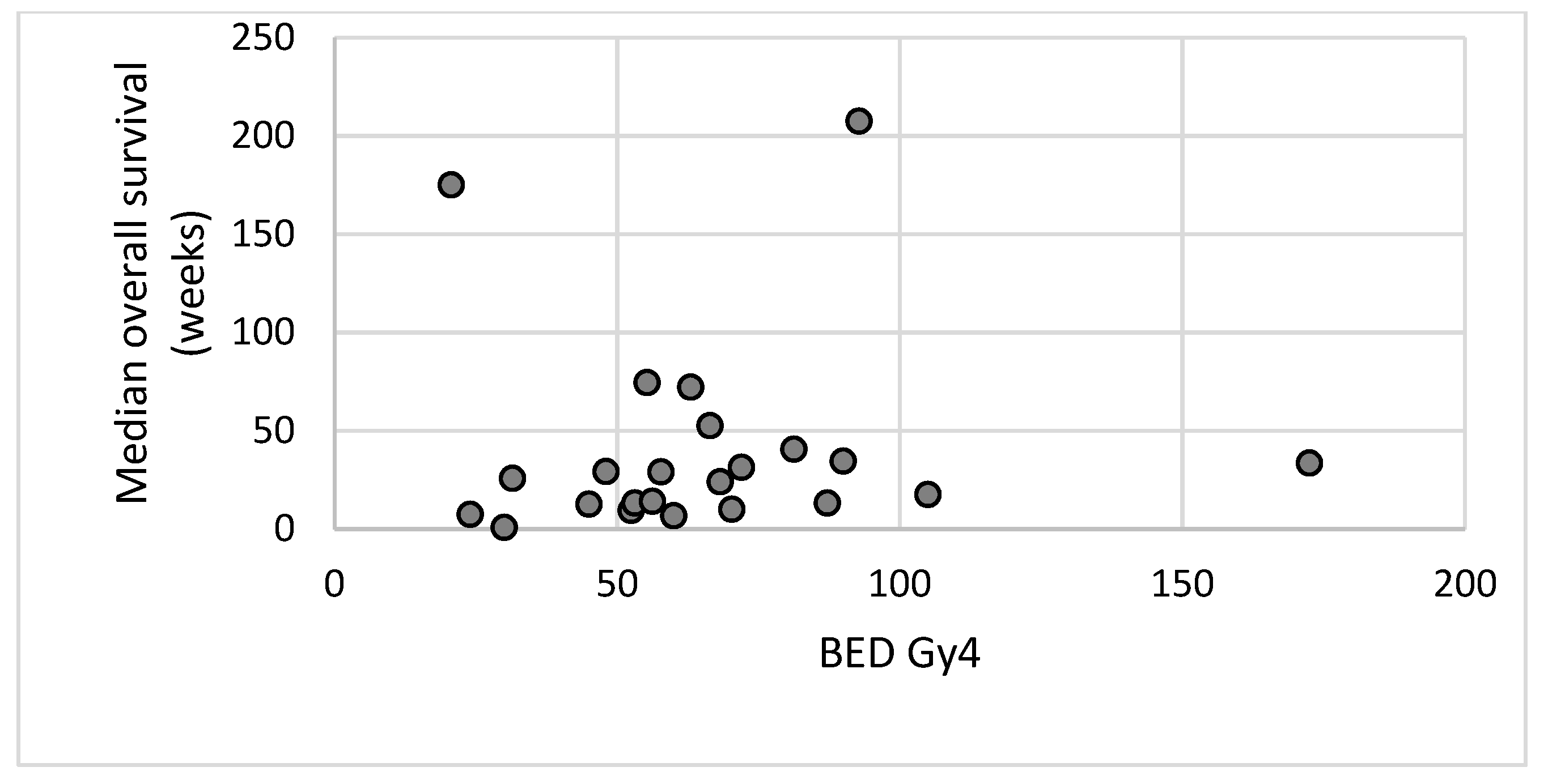
Survival data was available for 129 sites treated. This was calculated from the date of completion of radiotherapy to the date of death or last follow-up.
Figure 4 illustrates median overall survival versus the biological effective dose of the radiotherapy delivered.
4. Discussion
There is little published literature considering the role of palliative radiotherapy in metastatic sarcoma, although it is recommended as a treatment option for palliation by both the UK bone and soft tissue sarcoma guidelines [
7,
8] and the European Society of Medical Oncology (ESMO) guidelines [
11,
12]. To the authors’ knowledge this is the largest retrospective study published to date reviewing the use of palliative radiotherapy for sarcoma. The results confirm that palliative radiotherapy can successfully provide symptomatic benefit to patients with metastatic sarcoma. This study is limited by being retrospective such that the assessment of symptomatic improvement could only be assessed from documentation in medical records. It was therefore not possible to quantify the amount of symptomatic improvement seen or indeed to be certain of the accuracy of the results. The aim of this review, however, was to confirm that radiotherapy does have a role in the palliation of advanced sarcomas and to guide future prospective studies to gain more accurate evidence of the use of palliative radiotherapy in this setting. In addition, this study was limited by the heterogeneous population studied. Both soft tissue and bone sarcomas of multiple types were included. The radiobiology of the different subtypes of sarcoma has not been well studied, but it is accepted that these tumours tend to be relatively radio-resistant, with a high alpha beta ratio. There have been several publications considering the role of palliative radiotherapy for other, more common solid tumour types. A meta-analysis has shown that when treating bone metastases from a number of different primary tumour sites and histology’s, there appears to be little short-term symptomatic benefit in a fractionated course of radiotherapy, compared to a single fraction [
13]. Most oncologists therefore advocate the use of a single fraction to control pain secondary to bone metastases [
14]. With longer follow-up, however, it seems that patients treated with a fractionated course of palliative radiotherapy are less likely to need retreatment than those given a single fraction [
13]. This study demonstrates that selected patients with advanced sarcoma can benefit from palliative radiotherapy provided their prognosis is long enough for the benefits from radiotherapy, in terms of symptomatic improvement, to outweigh the inconvenience of undergoing radiotherapy and the associated side effects that they may suffer.
As sarcomas are felt to be intrinsically radio-resistant, it can be hypothesized that a single fraction of palliative radiotherapy may not be sufficient to offer adequate symptomatic benefit. The results presented here suggest a higher symptomatic response rate with a biological effective dose (BED) of 50 or greater.
Figure 3b demonstrates an increase in response rate with increasing BED up to 50 Gy
4. Beyond this point the response is maintained but does not appear to increase further, suggesting very high doses of palliative radiotherapy may not be necessary to achieve a good symptomatic response in this patient group. The one apparent outlier in
Figure 3b is the 100% symptomatic response in patients receiving a BED of 30–39.9, this is likely to be an outlier as there was only one patient in this group.
Because of the limited evidence available in the use of radiotherapy for palliation in sarcoma the Royal College of Radiologists Radiotherapy dose fractionation guidance (3rd edition) [
15] recommends several different dose fractionation schedules (
Table 5). Excluding 8 Gy single fraction and 20 Gy in 5 fractions which are commonly used schedules for all solid tumours; the remaining recommended dose fractionation schedules all have a BED of greater than 50 Gy
4. Although this case series has demonstrated that very high doses of radiotherapy may not provide additional short-term symptomatic benefit, longer courses of radiotherapy may be considered in patients with a good performance status and prognosis in an attempt to provide a longer period of symptomatic benefit without the need for retreatment. A previously published case series of 17 patients who received palliative radiotherapy for sarcoma using 39 Gy in 13 fractions over two and a half weeks demonstrated this was well tolerated and provided high rates of durable pain control [
9]. Collection of survival data may help to assess if the correct patients are being offered higher dose, longer courses of radiotherapy.
Figure 4 however does not appear to show any correlation between the BED of the radiotherapy delivered and overall survival. Interpretation of this data is extremely limited because of the heterogeneity of the patient population with multiple different histological subtypes and the small numbers of patients treated with each dose and fractionation schedule.
To gain a better understanding of the most appropriate dose and fractionation schedules for different sarcoma subtypes large volume, multicentre, prospective data collections is required using a small number of different dose and fractionation schedules, ideally those outlined by the Royal College of Radiologists (
Table 5) [
15].
5. Conclusions
Palliative radiotherapy offers symptomatic improvement for sarcoma patients. These results are limited by the heterogeneous study population which includes different sarcoma subtypes with different radio-sensitivities, treated with different radiotherapy schedules. Despite this radiotherapy appears to be an effective treatment for symptom control with two-thirds of patients reporting symptomatic improvement. Further multicentre prospective data collection is needed considering the sarcoma subtype radio-sensitivity, to determine appropriate palliative dose fractionation schedules.
Author Contributions
Conceptualization, H.T. and J.S.; investigation, H.T., D.S., D.P. and J.S.; methodology, H.T. and J.S.; supervision, D.S., D.P. and J.S.; visualization, H.T.; writing—original draft, H.T. and J.S.; writing—review and editing, H.T. and J.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Cancer Intelligence Network (NCIN). Bone and Soft Tissue Sarcomas, UK Incidence and Survival, 1996 to 2010, Version 2.0. November 2013. Available online: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/sarcomas/ (accessed on 20 June 2019).
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. (Eds.) WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC: Lyon, France, 2013. [Google Scholar]
- Blay, J.-Y.; van Glabbeke, M. Advanced soft-tissue sarcoma: A disease that is potentially curable for a subset of patients treated with chemotherapy. Eur. J. Cancer 2003, 39, 64–69. [Google Scholar] [CrossRef]
- Van Glabbeke, M.; van Oosterom, A.T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2185 patients treated with anthracyclines containing first-line regimens—An European organization for research and treatment of cancer soft tissue and bone sarcoma group study. J. Clin. Oncol. 1999, 17, 150–157. [Google Scholar] [PubMed]
- Judson, I.; Verweij, J. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Bae, S.; Crowe, P. Patterns of care for patients with advanced soft tissue sarcoma: Experience from Australian sarcoma services. Clin. Sarcoma Res. 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Dangoor, A.; Seddon, B. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Gerrand, C.; Athansou, N.; BSG. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Soyfer, V.; Corn, B.W. Radiation therapy for palliation of sarcoma metastases: A unique and uniform hypofractionation experience. Sarcoma 2010, 2010, 927972. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Bolm, L. Palliative Radiation Therapy for Spinal Cord Compression from Metastatic Soft Tissue Sarcoma. In Vivo 2016, 30, 529–531. [Google Scholar] [PubMed]
- Casali, P.G.; Bielack, S. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; On Behalf of the ESMO Guidelines Committee and EURACAN; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv51–iv67. [Google Scholar] [CrossRef] [PubMed]
- Sze, W.M.; Shelley, M.D. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy—A systematic review of randomised trials. Clin. Oncol. (R. Coll. Radiol.) 2003, 15, 345–352. [Google Scholar] [CrossRef]
- Royal College of Radiologists. Chapter 18 Bone metastases. In Radiotherapy Dose Fractionation, 3rd ed.; Royal College of Radiologists: London, UK, 2019. [Google Scholar]
- Royal College of Radiologists. Chapter 14 Sarcoma. In Radiotherapy Dose Fractionation, 3rd ed.; Royal College of Radiologists: London, UK, 2019. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).