Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults
Abstract
1. Introduction
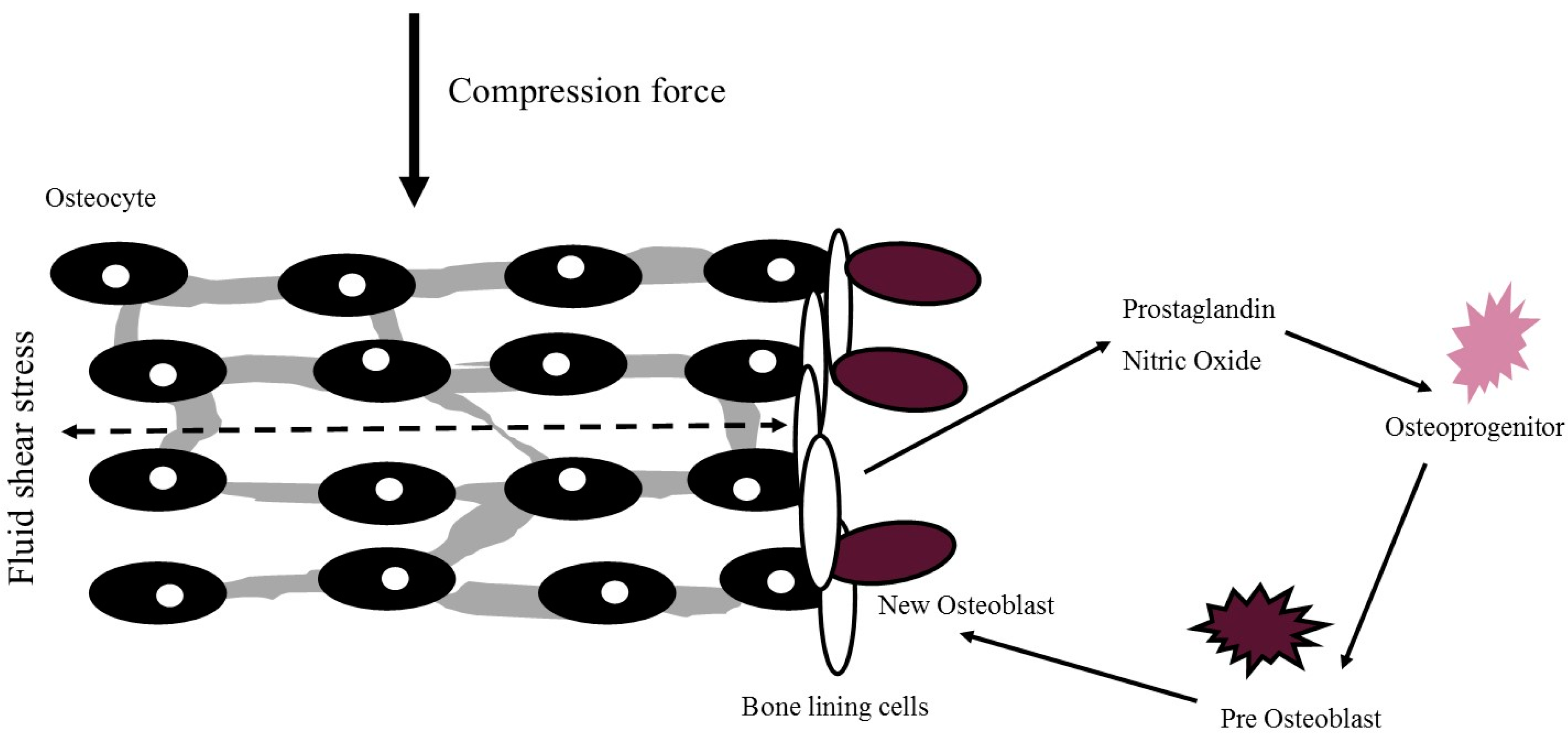
2. Mechanism of PA and Exercise Effects on Bone Health
3. Modalities of PA and Exercise
3.1. Progressive Resistance Training
3.2. Ambulatory Activity
3.3. High-Impact Activity
3.4. Whole-Body Vibration Therapy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sallis, R. Exercise is medicine: A call to action for physicians to assess and prescribe exercise. Phys. Sportsmed. 2015, 43, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.M.; Beresford, S.A.A.; Larson, E.B.; LaCroix, A.Z.; Wagner, E.H. Effects of Physical Activity on Health Status in Older Adults II: Intervention Studies. Annu. Rev. Public Health 1992, 13, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.M. Physical activity and quality of life in older adults. J. Am. Med. Assoc. 1997, 277, 64–66. [Google Scholar] [CrossRef]
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk. Diabetes Care 2008, 31, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Caserotti, P.; Patel, K.V.; Matthews, C.E.; Berrigan, D.; van Domelen, D.R.; Brychta, R.J.; Chen, K.Y.; Harriset, T.B. Association of Sedentary time with mortality independent of moderate to vigorous physical activity. PLoS ONE 2012, 7, e37696. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Velasco, S.; Villarejo-Galende, A.; Contador, I.; Lora Pablos, D.; Hernandez-Gallego, J.; Bermejo-Pareja, F. Physical activity and long-term mortality risk in older adults: A prospective population based study (NEDICES). Prev. Med. Rep. 2016, 4, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Gianoudis, J.; Bailey, C.A.; Daly, R.M. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos. Int. 2014, 26, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Australian National Preventative Health Agency. Obesity: Prevalence and Trends in Australia; Australian National Preventative Health Agency: Canberra, Australia, 2014.
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sports Med. 2009, 43, 1–2. [Google Scholar] [PubMed]
- Chastin, S.F.M.; Mandrichenko, O.; Helbostadt, J.L.; Skelton, D.A. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone 2014, 64, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wilks, D.C.; Winwood, K.; Gilliver, S.F.; Kwiet, A.; Chatfield, M.; Michaelis, I.; Sun, L.W.; Ferretti, J.L.; Sargeant, A.J.; Felsenberg, D.; et al. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: A pQCT study. Bone 2009, 45, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wills, K.; Laslett, L.L.; Oldenburg, B.; Jones, G.; Winzenberg, T. Moderate-to-Vigorous Physical Activity But Not Sedentary Time Is Associated With Musculoskeletal Health Outcomes in a Cohort of Australian Middle-Aged Women. J. Bone Miner. Res. 2016, 32, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.J.; Deere, K.C.; Hartley, A.; Al-Sari, U.A.; Clark, E.M.; Fraser, W.D.; Tobias, J.H. Habitual levels of higher, but not medium or low, impact physical activity are positively related to lower limb bone strength in older women: Findings from a population-based study using accelerometers to classify impact magnitude. Osteoporos. Int. 2016, 28, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Nordstrom, A.; Nordstrom, P. Objectively measured physical activity is associated with parameters of bone in 70-year-old men and women. Bone 2015, 81, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Casperson, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Kanis, J.A. Osteoporosis 1990; Royal College of Physicians of London: London, UK, 1990. [Google Scholar]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Harvey, N.C.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Ebeling, P.R.; Kyer, C.S. The Asia-Pacific Regional Audit: Epidemiology, costs & burden of osteoporosis in 2013: A report of International Osteoporosis Foundation. Indian J. Endocrinol. Metab. 2014, 18, 449–454. [Google Scholar] [PubMed]
- Khosla, S.; Shane, E. A Crisis in the Treatment of Osteoporosis. J. Bone Miner. Res. 2016, 31, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Turner, C.H. Mechanotransduction and the Functional Response of Bone to Mechanical Strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Pavalko, F.M. Mechanotransduction and functional response of the skeleton to physical stress: The mechanisms and mechanics of bone adaptation. J. Orthop. Sci. 1998, 3, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone “Mass” and the “Mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [PubMed]
- Warden, S.J.; Hurst, J.A.; Sanders, M.S.; Turner, C.H.; Burr, D.B.; Li, J. Bone Adaptation to a Mechanical Loading Program Significantly Increases Skeletal Fatigue Resistance. J. Bone Miner. Res. 2004, 20, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Layne, J.E.; Nelson, M.E. The effects of progressive resistance training on bone density: A review. Med. Sci. Sports Exerc. 1999, 31, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Krahl, H.; Michaelis, U.; Pieper, H.-G.; Quack, G.; Montag, M. Stimulation of Bone Growth Through Sports. Am. J. Sports Med. 1994, 22, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Korpelainen, R.; Keinänen-Kiukaanniemi, S.; Heikkinen, J.; Väänänen, K.; Korpelainen, J. Effect of impact exercise on bone mineral density in elderly women with low BMD: A population-based randomized controlled 30-month intervention. Osteoporos. Int. 2006, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Horan, S.A.; Beck, B.R. Heavy resistance training is safe and improves bone, function, and stature in postmenopausal women with low to very low bone mass: Novel early findings from the LIFTMOR trial. Osteoporos. Int. 2015, 26, 2889–2894. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Nigh, P.; Thyfault, J. Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: A 12-month randomized, clinical trial. Bone 2015, 79, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.A.; Ackland, T.; Maslen, B.; Morton, A.; Prince, R.L. Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J. Bone Miner. Res. 2001, 16, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gianoudis, J.; Bailey, C.A.; Ebeling, P.R.; Nowson, C.A.; Sanders, K.M.; Hill, K.; Daly, R.M. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: A community-based randomized controlled trial. J. Bone Miner. Res. 2014, 29, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, M.; Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos. Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Bemben, D.A.; Fetters, N.L.; Bemben, M.G.; Nabavi, N.; Koh, E.T. Musculoskeletal responses to high-and low-intensity resistance training in early postmenopausal women. Med. Sci. Sport Exerc. 2000, 32, 1949–1957. [Google Scholar] [CrossRef]
- Fjeldstad, C.; Palmer, I.J.; Bemben, M.G.; Bemben, D.A. Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas 2009, 63, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, J.; Ackland, T.R.; Dhaliwal, S.S.; James, A.P.; Woodhouse, J.J.; Price, R.; Kerr, D.A. Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos. Int. 2010, 21, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Notelovitz, M.; Martin, D.; Tesar, R.; Khan, F.Y.; Probart, C.; Fields, C.; McKenzie, L. Estrogen therapy and variable-resistance weight training increase bone mineral in surgically menopausal women. J. Bone Miner. Res. 1991, 6, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Wanderley, F.; Machado, L.; Sousa, F.; Viana, J.L.; Moreira-Gonçalves, D.; Moreira, P.; Mota, J.; Carvalho, J. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 2011, 46, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Suominen, H.; Rahkila, P. Bone mineral density of the calcaneus in 70-to 81-year-old male athletes and a population sample. Med. Sci. Sport Exerc. 1991, 23, 1227–1233. [Google Scholar]
- Karlsson, M.K.; Johnell, O.; Obrant, K.J. Bone mineral density in weight lifters. Calcif. Tissue Int. 1993, 52, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Fiatarone Singh, M.A.; Morganti, C.M.; Trice, I.; Greenberg, R.; Evans, W.J. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: A randomized controlled trial. JAMA 1994, 272, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, V.; Ivaska, K.K.; Kiviranta, R.; Bucci, M.; Lipponen, H.; Sandboge, S.; Raiko, J.; Eriksson, J.G.; Parkkola, R.; Iozzo, P.; et al. Bone mineral density is increased after a 16-week resistance training intervention in elderly women with decreased muscle strength. Eur. J. Endocrinol. 2016, 175, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Bolam, K.A.; Van Uffelen, J.G.Z.; Taaffe, D.R. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos. Int. 2013, 24, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Hong, A.; Lau, E.; Lynn, H. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing 2007, 36, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Treuth, M.; Rubin, M.; Miller, J.; Nicklas, B.; Landis, D.; Pratley, R.E.; Libanati, C.R.; Gundberg, C.M.; Hurley, B.F. Effects of strength training on bone mineral density: Hormonal and bone turnover relationships. J. Appl. Physiol. 1994, 77, 1678–1684. [Google Scholar] [PubMed]
- Kukuljan, S.; Nowson, C.A.; Bass, S.L.; Sanders, K.M.; Nicholson, G.C.; Seibel, M.J.; Salmon, J.; Daly, R.M. Effects of a multi-component exercise program and calcium-vitamin-D3-fortified milk on bone mineral density in older men: A randomised control trial. Osteoporos. Int. 2009, 20, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Daly, R.M.; Singh, M.A.F.; Taaffe, D.R. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 2017, 20, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Karinkanta, S.; Kannus, P.; Uusi-Rasi, K.; Heinonen, A.; Sievänen, H. Combined resistance and balance-jumping exercise reduces older women’s injurious falls and fractures: 5-year follow-up study. Age Ageing 2015, 44, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R.; Beck, B.R.; Bennell, K.L.; Brosseau, L.; Costa, L.; Cramp, F.; Cup, E.; et al. Consensus on Exercise Reporting Templater (CERT): Modified Delphi Study. Phys. Ther. 2016, 96, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, R.M.; Smith, J.J.; Morgan, P.J.; Barnett, L.M.; Hallal, P.C.; Colyvas, K.; Lubansa, D.R. Global participation in sport and leisure-time physical activities: A systematic review and meta-analysis. Prev. Med. 2016, 95, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, J.; Omasu, F.; Nakahara, Y. Effect of daily walking steps on ultrasound parameters of the calcaneus in elderly Japanese women. Osteoporos. Int. 2003, 14, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Hitchcock, C.L.; Kingwell, E.J.; Davison, K.S.; Berger, C.; Forsmo, S.; Zhou, W.; Kreiger, N.; Prior, J.C. Physical activity, body mass index and bone mineral density-associations in a prospective population-based cohort of women and men: The Canadian Multicentre Osteoporosis Study (CaMos). Bone 2012, 50, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D. Walking and Leisure-Time Activity and Risk of Hip Fracture in Postmenopausal Women. JAMA 2002, 288, 2300. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Weissman, J.; Wolf, J.; Mumford, K.; Contant, C.K.; Hwang, W.T. Comparing GPS, log, survey, and accelerometry to measure physical activity. Am. J. Health Behav. 2016, 40, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.; Quinn, S.; Jones, G. Pedometer determined ambulatory activity and bone mass: A population-based longitudinal study in older adults. Osteoporos. Int. 2010, 21, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Wavell, K.; Jones, P.R.M.; Hardman, A.E. Influence of Brisk Walking on Bone Quality of Healthy Elderly Women. Bone 1996, 18, 2–3. [Google Scholar] [CrossRef]
- Brooke-Wavell, K.; Jones, P.R.M.; Hardman, A.E. Brisk walking reduces calcaneal bone loss in post-menopausal women. Clin. Sci. 1997, 92, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Thompson, P.W.; Baskaran, V.; Evans, K. Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Ageing 1997, 26, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Wavell, K.; Jones, P.R.M.; Hardman, A.E.; Tsuritani, I.; Yamada, Y. Commencing, continuing and stopping brisk walking: Effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos. Int. 2001, 12, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Palombaro, K.M. Effects of walking-only interventions on bone mineral density at various skeletal sites: A meta-analysis. J. Geriatr. Phys. Ther. 2005, 28, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Hasegawa, A.; Adachi, H.; Shínozaki, A.; Hayashi, R.; Okano, H.; Mizunuma, H.; Murata, K. The effects of walking at the anaerobic threshold level on vertebral bone loss in postmenopausal women. Calcif. Tissue Int. 1993, 52, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Notelovitz, M. Effects of aerobic training on bone mineral density of postmenopausal women. J. Bone Miner. Res. 1993, 8, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Bonaiuti, D.; Shea, B.; Iovine, R.; Negrini, S.; Welch, V.; Kemper, H.; Wells, G.; Tugwell, P.; Cranney, A. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2002, 3, CD000333. [Google Scholar] [CrossRef]
- Vainionpää, A.; Korpelainen, R.; Sievänen, H.; Vihriälä, E.; Leppäluoto, J.; Jämsä, T. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 2007, 40, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Welsh, L.; Rutherford, O.M. Hip bone mineral density is improved by high-impact aerobic exercise in postmenopausal women and men over 50 years. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, M.; Zhang, L. Efficiency of jumping exercise in improving bone mineral density among premenopausal women: A meta-analysis. Sport Med. 2014, 44, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, O.O.; Forsyth, J.J.; Gidlow, C.J. A meta-analysis of brief high-impact exercises for enhancing bone health in premenopausal women. Osteoporos. Int. 2012, 23, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.J.; Hartley, A.; Coulson, J.; Moss, C.; Edwards, M.H.; Dennison, E.; Gaysin, T.; Cooper, R.; Wong, A.; McPhee, J.S.; et al. A novel accelerometer-based method to describe day-to-day exposure to potentially osteogenic vertical impacts in older adults: Findings from a multi-cohort study. Osteoporos. Int. 2017, 28, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Multanen, J.; Rantalainen, T.; Kautiainen, H.; Ahola, R.; Jämsä, T.; Nieminen, M.T.; Lammentausta, E.; Häkkinen, A.; Kiviranta, I.; Heinonen, A. Effect of progressive high-impact exercise on femoral neck structural strength in postmenopausal women with mild knee osteoarthritis: A 12-month RCT. Osteoporos. Int. 2017, 28, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.D.F.; de Oliveira, M.L.; Lirani-Galvão, A.P.; Marin-Mio, R.V.; dos Santos, R.N.; Lazaretti-Castro, M. Physical exercise and osteoporosis: Effects of different types of exercises on bone and physical function of postmenopausal women. Arq. Bras. Endocrinol. Metabol. 2014, 58, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J.; Folland, J.P.; Rennie, W.J.; Summers, G.D.; Brooke-Wavell, K. High impact exercise increased femoral neck bone mineral density in older men: A randomised unilateral intervention. Bone 2013, 53, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.K.; Beck, B.R. The BPAQ: A bone specific physical activity assessment instrument. Osteoporos. Int. 2008, 888, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Bolam, K.A.; Beck, B.R.; Adlard, K.N.; Skinner, T.L.; Cormie, P.; Galvão, D.A.; Spry, N.; Newton, R.U.; Taaffe, D.R. The relationship between BPAQ-derived physical activity and bone density of middle-aged and older men. Osteoporos. Int. 2014, 25, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.J.; Deere, K.C.; Worrall, S.; Hartley, A.; Tobias, J.H. Characterization of vertical accelerations experienced by older people attending an aerobics class designed to produce high impacts. J. Aging Phys. Act. 2016, 24, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Deere, K.C.; Hannam, K.J.; Coulson, J.; Ireland, A.; McPhee, J.S.; Moss, C.; Edwards, M.H.; Dennison, E.; Cooper, C.; Sayers, A.; et al. Quantifying habitual levels of physical activity according to impact in older people: Accelerometry protocol for the VIBE study. J. Aging Phys. Act. 2016, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, A.; Korpelainen, R.; Vihriälä, E.; Rinta-Paavola, A.; Leppäluoto, J.; Jämsä, T. Intensity of exercise as associated with bone density change in premenopausal women. Osteoporos. Int. 2006, 17, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.H.; Gould, V.; Brunton, L.; Deere, K.C.; Rittweger, J.; Lipperts, M.; Grimm, B. Physical activity and bone: May the force be with you. Front. Endocrinol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bassey, E.J.; Rothwell, M.C.; Littlewood, J.J.; Pye, D.W. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J. Bone Miner. Res. 1998, 13, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.A.; Londeree, B.R. Bone density in post-menopausal women: High impact vs. low impact exercise. Med. Sci. Sport Exerc. 1992, 24, 1190–1194. [Google Scholar]
- Bassey, E.J.; Ramsdale, S.J. Weight-bearing exercise and ground reaction forces: A 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone 1995, 16, 469–476. [Google Scholar] [CrossRef]
- Leung, K.S.; Li, C.Y.; Tse, Y.K.; Choy, T.K.; Leung, P.C.; Hung, V.W.Y.; Chan, S.Y.; Leung, A.H.; Cheung, W.H. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly—A cluster-randomized controlled trial. Osteoporos. Int. 2014, 25, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Prisby, R.D.; Lafage-Proust, M.H.; Malaval, L.; Belli, A.; Vico, L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res. Rev. 2008, 7, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Falcai, M.J.; Zamarioli, A.; Okubo, R.; de Paula, F.J.A.; Volpon, J.B. The osteogenic effects of swimming, jumping, and vibration on the protection of bone quality from disuse bone loss. Scand. J. Med. Sci. Sports 2015, 25, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.; Giangregorio, L.M. Mechanical stimuli and bone health: What is the evidence? Curr. Opin. Rheumatol. 2012, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Bovenzi, M.; Griffin, M.J. Haemodynamic changes in ipsilateral and contralateral fingers caused by acute exposures to hand transmitted vibration. Occup. Environ. Med. 1997, 54, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Von Stengel, S.; Kemmler, W.; Bebenek, M.; Engelke, K.; Kalender, W.A. Effects of whole-body vibration training on different devices on bone mineral density. Med. Sci. Sports Exerc. 2011, 43, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.M.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-Month Whole Body Vibration Training on Hip Density, Muscle Strength, and Postural Control in Postmenopausal Women: A Randomized Controlled Pilot Study. J. Bone Miner. Res. 2003, 19, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Gusi, N.; Raimundo, A.; Leal, A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC Musculoskelet. Disord. 2006, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Torode, M.; Climstein, M.; Naughton, G.; Greene, D.; Baker, M.K.; Fiatarone Singh, M.A. A Randomized Controlled Trial of Whole Body Vibration Exposure on Markers of Bone Turnover in Postmenopausal Women. J. Osteoporos. 2011, 2011, 1–10. [Google Scholar]
- Totosy de Zepetnek, J.O.; Giangregorio, L.M.; Craven, B.C. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: A review. J. Rehabil. Res. Dev. 2009, 46, 529. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.; Butler, M.; Shamliyan, T.; Kane, R.L. Whole-body vibration therapy for osteoporosis: State of the science. Ann. Intern. Med. 2011, 155, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Oliveira, R.G.; Pires-Oliveira, D.A.A. Effects of whole body vibration on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2913–2933. [Google Scholar] [CrossRef] [PubMed]
- Karakiriou, S.K.; Douda, H.T.; Smilios, I.G.; Volaklis, K.A.; Tokmakidis, S.P. Effects of vibration and exercise training on bone mineral density and muscle strength in post-menopausal women. Eur. J. Sport Sci. 2012, 12, 81–88. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H. Reduction of Vertebral Fracture Risk in Postmenopausal Women With Osteoporosis Treated With Raloxifene. JAMA 2009, 282, 637–646. [Google Scholar] [CrossRef]
- Silverman, S.L.; Schousboe, J.T.; Gold, D.T. Oral bisphosphonate compliance and persistence: A matter of choice? Osteoporos. Int. 2011, 22, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Forwood, M.R.; Larsen, J.A. Exercise recommendations for osteoporosis. Aust. Fam. Physician 2000, 29, 761–764. [Google Scholar] [PubMed]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br. J. Sports Med. 2016. [Google Scholar] [CrossRef]
- Duckham, R.L.; Masud, T.; Taylor, R.; Kendrick, D.; Carpenter, H.; Iliffe, S.; Morris, R.; Gage, H.; Skelton, D.A.; Dinan-Young, S.; et al. Randomised controlled trial of the effectiveness of community group and home-based falls prevention exercise programmes on bone health in older people: The ProAct65+ bone study. Age Ageing 2015, 44, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.B.; Armstrong, J.J.; Adachi, J.D.; MacDermid, J.C. Facilitators and barriers to exercise adherence in patients with osteopenia and osteoporosis: A systematic review. Osteoporos. Int. 2016, 28, 735–745. [Google Scholar] [CrossRef] [PubMed]
| Population | Intervention | Length | Intervention Group BMD Change | Control Instructions | Control group BMD Change | |
|---|---|---|---|---|---|---|
| Korpelainen et al. [28] | Elderly women (n = 160) | 20 min daily unsupervised & 1 h intermittent supervision | 30 months | ↔Femoral neck | Daily PA | ↓Femoral neck ↓Trochanter |
| Welsh et al. [67] | Men & women (n = 30) | Supervised exercises sessions 2–3/week | 12 months | ↑ Femoral neck | Daily PA | ↓Femoral neck |
| Bassey et al. [82] | Postmenopausal women (n = 44) | 50 ‘heel drops’ daily | 12 months | ↔ Lumbar spine ↔Femoral neck | Weekly exercise class | ↔ Lumbar spine ↔Femoral neck |
| Allison et al. [73] | Older men (n = 50) | 50 unilateral hops/day | 12 months | ↑ Femoral neck ↑ L4 | Daily PA | ↓Femoral neck |
| Population | Modality | Frequency | Length | Exclusions | Outcomes | BMD Change | |
|---|---|---|---|---|---|---|---|
| Von Stengel et al. [88] | Postmenopausal women (n = 108) | Vertical: 35 Hz Rotational: 12.5 Hz | 3x/week | 12 months | Diseases or medication affecting bone | DXA BMD Isometric strength | ↑Lumbar spine |
| Leung et al. [83] | Women ≥ 60 years (n = 710) | Vertical: 35 Hz | 5x/week | 18 months | Disease or medication affecting bone | Falls & fracture DXA BMD | ↔Hip |
| Verschueren et al. [89] | Postmenopausal women (n = 70) | Vertical: 35–40 Hz | 3x/week | 6 months | Osteoporosis or Medication affecting bone | DXA BMD C-Telopeptide Osteocalcin | ↑Hip |
| Gusi et al. [90] | Postmenopausal women (n = 28) | Lateral: 12.6 Hz | 3x/week | 8 months | Osteoporosis or medication affecting bone High Impact PA | DXA BMD | ↑Femoral neck |
| Turner et al. [91] | Postmenopausal women (n = 46) | Vertical: 12 Hz | 1x/week & 3x/week | 8 weeks | WBVT contraindications Bone disease other than osteoporosis | Alkaline phosphatase N-telopeptide | Not reported |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMillan, L.B.; Zengin, A.; Ebeling, P.R.; Scott, D. Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults. Healthcare 2017, 5, 85. https://doi.org/10.3390/healthcare5040085
McMillan LB, Zengin A, Ebeling PR, Scott D. Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults. Healthcare. 2017; 5(4):85. https://doi.org/10.3390/healthcare5040085
Chicago/Turabian StyleMcMillan, Lachlan B., Ayse Zengin, Peter R. Ebeling, and David Scott. 2017. "Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults" Healthcare 5, no. 4: 85. https://doi.org/10.3390/healthcare5040085
APA StyleMcMillan, L. B., Zengin, A., Ebeling, P. R., & Scott, D. (2017). Prescribing Physical Activity for the Prevention and Treatment of Osteoporosis in Older Adults. Healthcare, 5(4), 85. https://doi.org/10.3390/healthcare5040085







