Quality of Life and Its Psychosocial Predictors among Patients with Disorders of Gut–Brain Interaction: A Comparison with Age- and Sex-Matched Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure
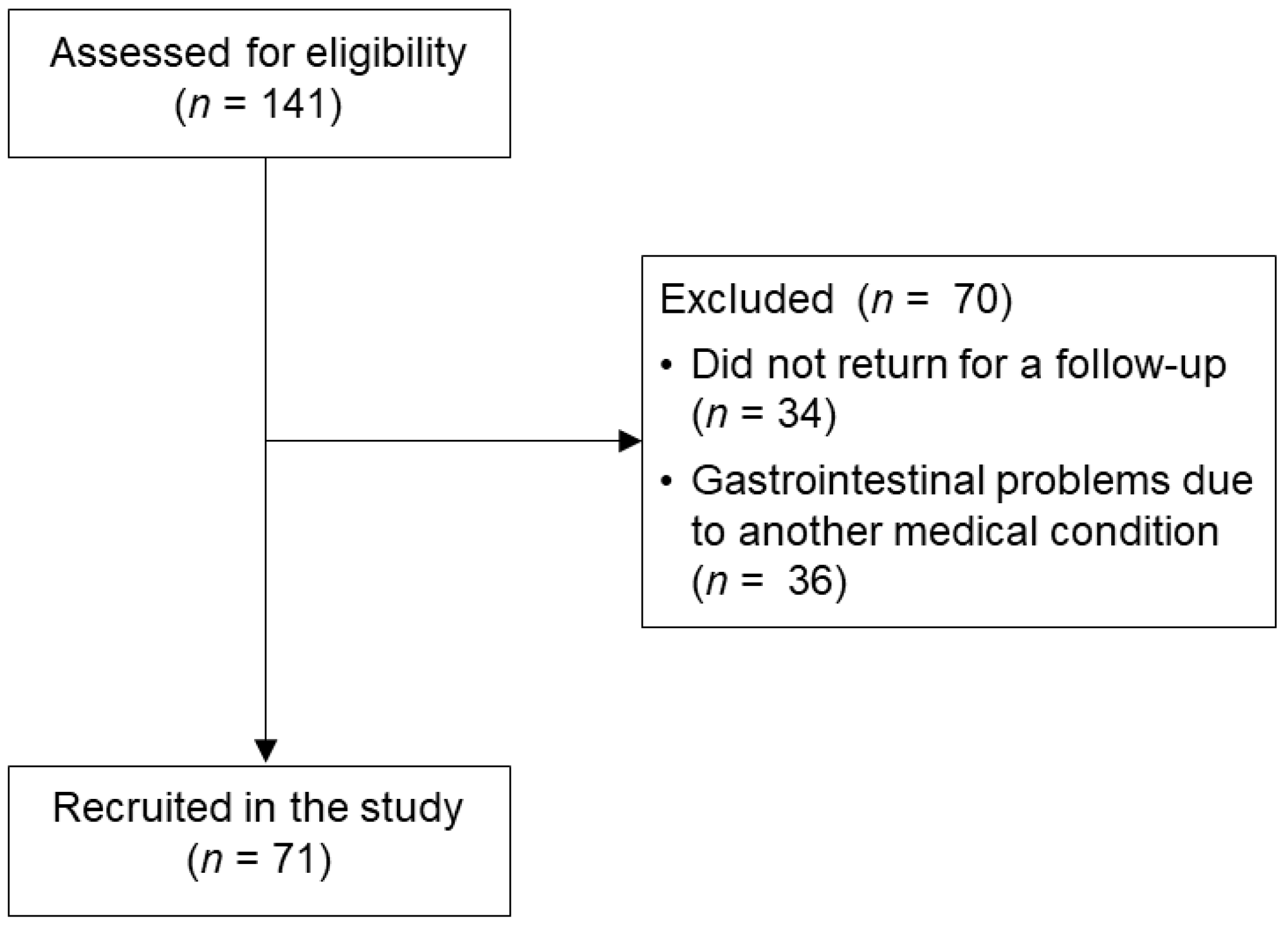
2.2. Participants
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Between-Group Differences in Sociodemographic and Clinical Data
3.2. Computing the Latent Variable “Quality of Life” (QoL)
3.3. Between-Group Differences in Psychological Variables (Aim 1)
3.4. Psychological Predictors of QoL among Patients with DGBIs and Healthy Controls (Aim 2)
4. Discussion
4.1. Between-Group Differences in Psychological Variables
4.2. Psychological Predictors of QoL among Patients with DGBIs and Healthy Controls
4.3. Limitations
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.; Holtmann, G.; Talley, N.J. Is there a causal link between psychological disorders and functional gastrointestinal disorders? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. Epidemiology and health care seeking in the functional GI disorders: A population-based study. Am. J. Gastroenterol. 2002, 97, 2290–2299. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Veldhuyzen Van Zanten, S.J.; Feeny, D.H.; Patrick, D.L. Measuring quality of life in clinical trials: A taxonomy and review. CMAJ Can. Med. Assoc. J. = J. L’association Medicale Can. 1989, 140, 1441–1448. [Google Scholar]
- Addante, R.; Naliboff, B.; Shih, W.; Presson, A.P.; Tillisch, K.; Mayer, E.A.; Chang, L. Predictors of Health-related Quality of Life in Irritable Bowel Syndrome Patients Compared With Healthy Individuals. J. Clin. Gastroenterol. 2019, 53, e142–e149. [Google Scholar] [CrossRef]
- Halder, S.L.S.; Locke III, G.R.; Talley, N.J.; Fett, S.L.; Zinsmeister, A.R.; Melton III, L.J. Impact of functional gastrointestinal disorders on health-related quality of life: A population-based case–control study. Alimentary Pharmacol. Ther. 2004, 19, 233–242. [Google Scholar] [CrossRef]
- Koloski, N.A.; Talley, N.J.; Boyce, P.M. The impact of functional gastrointestinal disorders on quality of life. Am. J. Gastroenterol. 2000, 95, 67–71. [Google Scholar] [CrossRef]
- Trindade, I.A.; Melchior, C.; Törnblom, H.; Simrén, M. Quality of life in irritable bowel syndrome: Exploring mediating factors through structural equation modelling. J. Psychosom. Res. 2022, 159, 110809. [Google Scholar] [CrossRef]
- Cassar, G.E.; Youssef, G.J.; Knowles, S.; Moulding, R.; Austin, D.W. Health-Related Quality of Life in Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Gastroenterol. Nurs. 2020, 43, E102–E122. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Olden, K.; Bjorkman, D. Health-related quality of life among persons with irritable bowel syndrome: A systematic review. Aliment. Pharmacol. Ther. 2002, 16, 1171–1185. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D.; Levy, R.L. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367. [Google Scholar] [CrossRef]
- Wong, R.K.M.; Drossman, D.A. Quality of life measures in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 277–284. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Li, Y.; Liu, C.; Yang, H.; Yang, C. The role of psychological factors in functional gastrointestinal disorders: A systematic review and meta-analysis. Int. J. Color. Dis. 2023, 38, 65. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Berens, S.; Schaefert, R.; Baumeister, D.; Gauss, A.; Eich, W.; Tesarz, J. Does symptom activity explain psychological differences in patients with irritable bowel syndrome and inflammatory bowel disease? Results from a multi-center cross-sectional study. J. Psychosom. Res. 2019, 126, 109836. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, M.; Yao, L.; Wang, Y.; Wang, E.; Yuan, J.; Wang, F.; Yang, K.; Bian, Z.; Zhong, L.L.D. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: A network meta-analysis. BMC Gastroenterol. 2021, 21, 23. [Google Scholar] [CrossRef]
- Torun, F.; Koç, G.; Ocak Serın, S.; Dılek Torun, S. Psychiatric symptoms and relationship of disease with stress and traumatic experiences in patients with irritable bowel syndrome. Riv. Di Psichiatr. 2020, 55, 292–296. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; Demyttenaere, K.; Tack, J.; Aziz, Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 663–680. [Google Scholar] [CrossRef]
- Agostini, A.; Moretti, M.; Calabrese, C.; Rizzello, F.; Gionchetti, P.; Ercolani, M.; Campieri, M. Attachment and quality of life in patients with inflammatory bowel disease. Int. J. Color. Dis. 2014, 29, 1291–1296. [Google Scholar] [CrossRef]
- Gerson, C.D.; Gerson, M.J.; Chang, L.; Corazziari, E.S.; Dumitrascu, D.; Ghoshal, U.C.; Porcelli, P.; Schmulson, M.; Wang, W.A.; Zali, M. A cross-cultural investigation of attachment style, catastrophizing, negative pain beliefs, and symptom severity in irritable bowel syndrome. Neurogastroenterol. Motil. 2015, 27, 490–500. [Google Scholar] [CrossRef]
- Kano, M.; Muratsubaki, T.; Yagihashi, M.; Morishita, J.; Mugikura, S.; Dupont, P.; Takase, K.; Kanazawa, M.; Van Oudenhove, L.; Fukudo, S. Insula Activity to Visceral Stimulation and Endocrine Stress Responses as Associated With Alexithymia in Patients With Irritable Bowel Syndrome. Psychosom. Med. 2020, 82, 29–38. [Google Scholar] [CrossRef]
- Porcelli, P.; De Carne, M.; Leandro, G. The role of alexithymia and gastrointestinal-specific anxiety as predictors of treatment outcome in irritable bowel syndrome. Compr. Psychiatry 2017, 73, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.N.; Wei, J.; Ke, M.Y.; Hong, X.; Li, T.; Zhu, L.M.; Sha, Y.; Jiang, J.; Fischer, F. Illness Perception of Patients with Functional Gastrointestinal Disorders. Front. Psychiatry 2018, 9, 122. [Google Scholar] [CrossRef]
- Carrozzino, D.; Porcelli, P. Alexithymia in Gastroenterology and Hepatology: A Systematic Review. Front. Psychol. 2018, 9, 334457. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, P.; De Carne, M.; Leandro, G. Alexithymia and gastrointestinal-specific anxiety in moderate to severe irritable bowel syndrome. Compr. Psychiatry 2014, 55, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Afshar, H.; Weinland, S.; Mohammadi, N.; Adibi, P. Alexithymia and functional gastrointestinal disorders (FGID). Med. Arh. 2012, 66, 28–32. [Google Scholar] [CrossRef]
- Yanartaş, Ö.; Kani, H.T.; Kani, A.S.; Akça, Z.N.D.; Akça, E.; Ergün, S.; Tezcan, N.; Atug, Ö.; İmeryüz, N.; Sayar, K. Depression and anxiety have unique contributions to somatic complaints in depression, irritable bowel syndrome and inflammatory bowel diseases. Psychiatry Clin. Psychopharmacol. 2019, 29, 418–426. [Google Scholar] [CrossRef]
- Lackner, J.M.; Gudleski, G.D.; Firth, R.; Keefer, L.; Brenner, D.M.; Guy, K.; Simonetti, C.; Radziwon, C.; Quinton, S.; Krasner, S.S.; et al. Negative aspects of close relationships are more strongly associated than supportive personal relationships with illness burden of irritable bowel syndrome. J. Psychosom. Res. 2013, 74, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.M.; Gurtman, M.B. Patterns of interpersonal problems in irritable bowel syndrome patients: A circumplex analysis. J. Psychosom. Res. 2005, 58, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Musso, M.; Viterbori, P.; Bonci, F.; Del Mastro, L.; Garrone, O.; Venturini, M.; Morasso, G. Detecting psychological distress in cancer patients: Validity of the Italian version of the Hospital Anxiety and Depression Scale. Support. Care Cancer 1999, 7, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kulich, K.R.; Calabrese, C.; Pacini, F.; Vigneri, S.; Carlsson, J.; Wiklund, I.K. Psychometric validation of the italian translation of the gastrointestinal symptom-rating scale and quality of life in reflux and dyspepsia questionnaire in patients with gastro-oesophageal reflux disease. Clin. Drug Investig. 2004, 24, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 1998, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Balzarotti, S.; John, O.P.; Gross, J.J. An Italian adaptation of the Emotion Regulation Questionnaire. Eur. J. Psychol. Assess. 2010, 26, 61–67. [Google Scholar] [CrossRef]
- Gross, J.J.; John, O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Personal. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef]
- Barsky, A.J.; Wyshak, G.; Klerman, G.L. The somatosensory amplification scale and its relationship to hypochondriasis. J. Psychiatr. Res. 1990, 24, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bernini, O.; Berrocal Montiel, C.; Ciaramella, A.; Poli, P.; Guazzelli, M. Reliability and validity of the Italian version of the Somatosensory Amplification Scale. Psychol. Health 2008, 23, 65. [Google Scholar]
- Brugnera, A.; Zarbo, C.; Farina, B.; Picardi, A.; Greco, A.; Lo Coco, G.; Tasca, G.A.; Carlucci, S.; Auteri, A.; Greco, F.; et al. Psychometric properties of the Italian version of the Experience in Close Relationship Scale 12 (ECR-12): An exploratory structural equation modeling study. Res. Psychother. 2019, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, M.-F.; Brassard, A.; Lussier, Y.; Valois, P.; Shaver, P.R.; Johnson, S.M. Selecting the best items for a short-form of the Experiences in Close Relationships questionnaire. Eur. J. Psychol. Assess. 2016, 32, 140–154. [Google Scholar] [CrossRef]
- Lo Coco, G.; Mannino, G.; Salerno, L.; Oieni, V.; Di Fratello, C.; Profita, G.; Gullo, S. The Italian Version of the Inventory of Interpersonal Problems (IIP-32): Psychometric Properties and Factor Structure in Clinical and Non-clinical Groups. Front. Psychol. 2018, 9, 293546. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, L.M.; Alden, L.E.; Wiggins, J.S.; Pincus, A.L. Inventory of Interpersonal Problems (IIP-32/IIP-64); Psychological Corporation: London, UK, 2000. [Google Scholar]
- Bagby, R.M.; Parker, J.D.A.; Taylor, G.J. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Bressi, C.; Taylor, G.; Parker, J.; Bressi, S.; Brambilla, V.; Aguglia, E.; Allegranti, I.; Bongiorno, A.; Giberti, F.; Bucca, M.; et al. Cross validation of the factor structure of the 20-item Toronto Alexithymia Scale: An Italian multicenter study. J. Psychosom. Res. 1996, 41, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Mulaik, S.A. Foundations of Factor Analysis, 2nd ed.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 7th ed.; Pearson: New York, NY, USA, 2019. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Grossi, E.; Groth, N.; Mosconi, P.; Cerutti, R.; Pace, F.; Compare, A.; Apolone, G. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S). Health Qual. Life Outcomes 2006, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Wright, B.J.; Kent, S. Psychosocial predictors of irritable bowel syndrome diagnosis and symptom severity. J. Psychosom. Res. 2013, 75, 467–474. [Google Scholar] [CrossRef]
- Sandler, R.S.; Drossman, D.A.; Nathan, H.P.; McKee, D.C. Symptom complaints and health care seeking behavior in subjects with bowel dysfunction. Gastroenterology 1984, 87, 314–318. [Google Scholar] [CrossRef]
- Chang, L. Review article: Epidemiology and quality of life in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2004, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, A.M.; Kanter, J.W.; Robinaugh, D.J. Differential associations between interpersonal variables and quality-of-life in a sample of college students. Qual. Life Res. 2020, 29, 127–139. [Google Scholar] [CrossRef]
- Mattila, A.K.; Saarni, S.I.; Salminen, J.K.; Huhtala, H.; Sintonen, H.; Joukamaa, M. Alexithymia and Health-Related Quality of Life in a General Population. Psychosomatics 2009, 50, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Boudabbous, M.; Issa, A.B.; Feki, I.; Gdoura, H.; Chtourou, L.; Moalla, M.; Sallemi, R.; Mnif, l.; Amouri, A.; Masmoudi, J.; et al. Alexithymia impairs quality of life in irritable bowel syndrome. Future Sci. OA 2023, 9, FSO881. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Roohafza, H.R.; Mohammadi, M.; Afshar, H. The structural model of pain, cognitive strategies, and negative emotions in functional gastrointestinal disorders. J. Res. Med. Sci. 2016, 21, 107. [Google Scholar] [CrossRef]
- Belokrylov, I.; Rasskazova, E.; Semikov, S.; Tkhostov, A.; Yavorovskaya, A. Cognitive and behavioral factors of quality of life in patients with somatoform disorders. Eur. Psychiatry 2021, 64, S192–S193. [Google Scholar] [CrossRef]
- Köteles, F.; Witthöft, M. Somatosensory amplification—An old construct from a new perspective. J. Psychosom. Res. 2017, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ponizovsky, A.M.; Drannikov, A. Contribution of attachment insecurity to health-related quality of life in depressed patients. World J Psychiatry 2013, 3, 41–49. [Google Scholar] [CrossRef]
- Maassen, G.H.; Bakker, A.B. Suppressor variables in path models: Definitions and interpretations. Sociol. Methods Res. 2001, 30, 241–270. [Google Scholar] [CrossRef]
- Porcelli, P.; Michael Bagby, R.; Taylor, G.J.; De Carne, M.; Leandro, G.; Todarello, O. Alexithymia as Predictor of Treatment Outcome in Patients with Functional Gastrointestinal Disorders. Psychosom. Med. 2003, 6, 911–918. [Google Scholar] [CrossRef]
| DGBI | HC | |||
|---|---|---|---|---|
| Variable(s) | Frequency (%) | Frequency (%) | Test Statistic | p-Value |
| Age, mean (SD) | 41.49 (17.23) | 40.45 (16.38) | U = 2448.50 | 0.769 |
| Sex, female | 47 (66.2%) | 47 (66.2%) | χ2(1) = 0 | 1 |
| Education | χ2(2) = 4.616 | 0.099 | ||
| University or Ph.D. | 18 (25.4%) | 30 (42.3%) | ||
| High Schools | 40 (53.3%) | 32 (45.1%) | ||
| Middle or Primary Schools | 13 (18.3%) | 9 (12.7%) | ||
| Civil Status | χ2(3) = 0.179 | 0.981 | ||
| Widowed | 2 (2.8%) | 2 (2.8%) | ||
| Divorced | 5 (7.0%) | 6 (8.5%) | ||
| Married | 28 (39.4%) | 26 (36.6%) | ||
| Single | 36 (50.7%) | 37 (52.1%) | ||
| Work Status | χ2(3) = 5.740 | 0.125 | ||
| Student | 9 (13.0%) | 19 (26.8%) | ||
| Unemployed | 5 (7.2%) | 6 (8.5%) | ||
| Part- or Full-Time Worker | 46 (66.7%) | 42 (59.2%) | ||
| Retired | 9 (13.0%) | 4 (5.6%) | ||
| Living Status | χ2(3) = 4.830 | 0.185 | ||
| Alone | 10 (14.1%) | 9 (12.7%) | ||
| With parents | 18 (25.4%) | 16 (22.5%) | ||
| With spouse and/or children | 41 (57.7%) | 37 (52.1%) | ||
| With others | 2 (2.8%) | 9 (12.7%) | ||
| Drinking alcohol | 39 (56.5%) * | 50 (70.4%) | χ2(1) = 2.920 | 0.087 |
| Smoking | 14 (20.3%) * | 19 (26.8%) | χ2(1) = 0.813 | 0.367 |
| DGBI Diagnosis | ||||
| IBS-U (Unspecified) | 10 (14.1%) | / | / | / |
| IBS-C (predominant Constipation) | 17 (23.9%) | / | / | / |
| IBS-D (predominant Diarrhea) | 12 (16.9%) | / | / | / |
| IBS-M (Mixed) | 10 (14.1%) | / | / | / |
| Other DGBI (e.g., functional dyspepsia) | 22 (31.0%) | / | / | / |
| Long-term symptoms onset (>1 year) | 47 (66.2%) | / | / | / |
| History of previous visits for GI symptoms | 40 (56.3%) | / | / | / |
| Presence of any comorbidity | 37 (61.7%) * | / | / | / |
| Currently under pharmacological treatment | 28 (51.9%) * | / | / | / |
| Family history of a GI disease | 6 (10.5%) * | / | / | / |
| Previous surgical treatment for GI problems | 11 (18.6%) * | / | / | / |
| DGBI | HC | |||
|---|---|---|---|---|
| Variable(s) | n | Correlation Coefficient r | n | Correlation Coefficient r |
| GSRS | 66 | −0.079 | 71 | −0.227 |
| SSAS | 68 | −0.256 * | 71 | −0.199 |
| ERQ Cognitive Reappraisal | 68 | 0.188 | 71 | 0.152 |
| ERQ Expressive Suppression | 68 | −0.416 ** | 71 | −0.197 |
| ECR-12 Anxiety | 68 | −0.302 * | 71 | −0.596 ** |
| ECR-12 Avoidance | 67 | −0.181 | 71 | −0.150 |
| IIP-32 | 68 | −0.529 ** | 71 | −0.610 ** |
| TAS-20 Difficulty Describing Feelings | 68 | −0.273 * | 71 | −0.399 ** |
| TAS-20 Difficulty Identifying Feelings | 66 | −0.471 ** | 71 | −0.540 ** |
| TAS-20 Externally Oriented Thinking | 66 | −0.078 | 71 | −0.285 * |
| DGBI | HC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable(s) | α | n | Mean (SD) | n | Mean (SD) | df | t-Value | p-Value | d |
| QoL | 0.77 | 68 | −0.17 (1.00) | 71 | 0.16 (0.99) | 137 | −1.936 | 0.055 | −0.33 |
| GSRS | 0.82 | 69 | 30.96 (9.35) | 71 | 23.48 (8.27) | 138 | 4.734 | <0.001 | 0.86 |
| SSAS | 0.61 | 71 | 14.06 (4.93) | 71 | 13.15 (5.54) | 140 | 1.116 | 0.266 | 0.19 |
| ERQ Cognitive Reappraisal | 0.87 | 71 | 27.91 (8.44) | 71 | 27.87 (8.78) | 140 | 0.023 | 0.981 | 0.004 |
| ERQ Expressive Suppression | 0.64 | 71 | 12.96 (4.54) | 71 | 13.21 (5.79) | 132.14 * | −0.338 | 0.736 | −0.06 |
| ECR-12 Anxiety | 0.86 | 71 | 3.58 (1.46) | 71 | 3.33 (1.82) | 133.79 * | 1.071 | 0.286 | 0.18 |
| ECR-12 Avoidance | 0.89 | 70 | 2.46 (1.14) | 71 | 2.78 (1.49) | 130.55 * | −1.364 | 0.175 | −0.23 |
| IIP-32 | 0.87 | 71 | 33.09 (14.13) | 71 | 35.08 (16.84) | 140 | −0.731 | 0.466 | −0.12 |
| TAS-20 Difficulty Describing Feelings | 0.72 | 71 | 12.10 (4.25) | 71 | 12.42 (5.05) | 136.09 * | −0.414 | 0.680 | −0.07 |
| TAS-20 Difficulty Identifying Feelings | 0.79 | 69 | 17.19 (6.18) | 71 | 14.96 (5.96) | 138 | 2.174 | 0.031 | 0.37 |
| TAS-20 Externally Oriented Thinking | 0.62 | 69 | 17.41 (4.37) | 71 | 18.20 (5.77) | 130.30 * | −0.916 | 0.361 | −0.15 |
| DGBI | HC | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable(s) | Beta | t-Value | p-Value | Partial r | Beta | t-Value | p-Value | Partial r |
| Constant | 0.484 | 0.630 | 3.628 | 0.001 | ||||
| GSRS | −0.040 | −0.380 | 0.706 | −0.053 | 0.057 | 0.559 | 0.578 | 0.072 |
| SSAS | −0.043 | −0.355 | 0.724 | −0.049 | −0.007 | −0.066 | 0.947 | −0.009 |
| ERQ Cognitive Reappraisal | 0.261 | 2.164 | 0.035 | 0.287 | 0.024 | 0.257 | 0.798 | 0.033 |
| ERQ Expressive Suppression | −0.325 | −2.588 | 0.012 | −0.338 | 0.108 | 0.982 | 0.330 | 0.126 |
| ECR-12 Anxiety | 0.147 | 1.065 | 0.292 | 0.146 | −0.305 | −2.722 | 0.008 | −0.332 |
| ECR-12 Avoidance | 0.189 | 1.418 | 0.162 | 0.193 | −0.050 | −0.469 | 0.641 | −0.060 |
| IIP-32 | −0.384 | −2.702 | 0.009 | −0.351 | −0.364 | −3.297 | 0.002 | −0.392 |
| TAS-20 Difficulty Describing Feelings | 0.090 | 0.618 | 0.539 | 0.085 | −0.056 | −0.448 | 0.656 | −0.058 |
| TAS-20 Difficulty Identifying Feelings | −0.391 | −2.643 | 0.011 | −0.344 | −0.328 | −2.547 | 0.013 | −0.312 |
| TAS-20 Externally Oriented Thinking | 0.153 | 1.234 | 0.223 | 0.169 | 0.042 | 0.373 | 0.711 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brugnera, A.; Remondi, C.; La Tona, A.; Nembrini, G.; Lo Coco, G.; Compare, A.; Cardinali, A.; Scollato, A.; Marchetti, F.; Bonetti, M.; et al. Quality of Life and Its Psychosocial Predictors among Patients with Disorders of Gut–Brain Interaction: A Comparison with Age- and Sex-Matched Controls. Healthcare 2024, 12, 757. https://doi.org/10.3390/healthcare12070757
Brugnera A, Remondi C, La Tona A, Nembrini G, Lo Coco G, Compare A, Cardinali A, Scollato A, Marchetti F, Bonetti M, et al. Quality of Life and Its Psychosocial Predictors among Patients with Disorders of Gut–Brain Interaction: A Comparison with Age- and Sex-Matched Controls. Healthcare. 2024; 12(7):757. https://doi.org/10.3390/healthcare12070757
Chicago/Turabian StyleBrugnera, Agostino, Chiara Remondi, Antonino La Tona, Greta Nembrini, Gianluca Lo Coco, Angelo Compare, Alice Cardinali, Alessandra Scollato, Fabio Marchetti, Matteo Bonetti, and et al. 2024. "Quality of Life and Its Psychosocial Predictors among Patients with Disorders of Gut–Brain Interaction: A Comparison with Age- and Sex-Matched Controls" Healthcare 12, no. 7: 757. https://doi.org/10.3390/healthcare12070757
APA StyleBrugnera, A., Remondi, C., La Tona, A., Nembrini, G., Lo Coco, G., Compare, A., Cardinali, A., Scollato, A., Marchetti, F., Bonetti, M., & Pigozzi, M. G. (2024). Quality of Life and Its Psychosocial Predictors among Patients with Disorders of Gut–Brain Interaction: A Comparison with Age- and Sex-Matched Controls. Healthcare, 12(7), 757. https://doi.org/10.3390/healthcare12070757







