Abstract
Neuroimaging studies using autobiographical recall methods investigated the neural correlates of happy autobiographical memories (AMs). The scope of the present activation likelihood estimation (ALE) meta-analysis was to quantitatively analyze neuroimaging studies of happy AMs conducted with autobiographical recall paradigms. A total of 17 studies (12 fMRI; 5 PET) on healthy individuals were included in this meta-analysis. During recall of happy life events, consistent activation foci were found in the frontal gyrus, the cingulate cortex, the basal ganglia, the parahippocampus/hippocampus, the hypothalamus, and the thalamus. The result of this quantitative coordinate-based ALE meta-analysis provides an objective view of brain responses associated with AM recollection of happy events, thus identifying brain areas consistently activated across studies. This extended brain network included frontal and limbic regions involved in remembering emotionally relevant positive events. The frontal gyrus and the cingulate cortex may be responsible for cognitive appraisal processes during recollection of happy AMs, while the subthalamic nucleus and globus pallidus may be involved in pleasure reactions associated with recollection of happy life events. These findings shed light on the neural network involved in recalling positive AMs in healthy individuals, opening further avenues for future research in clinical populations with mood disorders.
1. Introduction
Autobiographical memory (AM) refers to the ability to remember events and lifetime periods from one’s past, which is crucial for a sense of identity, self-continuity, and mental time traveling [1,2,3,4]. AM is considered a subsystem of episodic memory, more generally defined as the conscious recollection of experienced events and meaningful reconstruction of one’s own past [5]. According to Rubin [6,7,8], retrieval of AMs results from the interaction between multiple neural and cognitive systems; specifically, this process includes the recollection of personal life events (i.e., the ability to retrieve, re-experience, and relive a past event), self-referential processing, mental imagery, narrative reasoning, language, and emotion.
AMs are generally characterized by emotional content compared to other types of episodic or semantic memories [1,9,10]. AMs with positive emotional valence are generally marked under the broad category of positive AMs. However, the expressions positive AMs and happy AMs are often used interchangeably in the literature. In the following, we will refer to the concept of happy AMs. Indeed, the majority of the studies included in the current meta-analysis instructed participants to recall happy AMs instead of positive AMs. Noteworthy, happy life events do not constitute a homogenous category: for example, a distinction between hedonic and eudaimonic happy events (hedonic events include life occurrences in which people pursue extrinsically motivated activities to experience enjoyment and pleasure, either sensory or psychological, whereas eudaimonic happy events are life occurrences in which people engage in intrinsically meaningful activities that enable the person to cultivate his or her skills and to develop his or her best potentials [11,12]) has been proposed in recent years by Positive Psychology [13,14,15]. Happy personal events occur frequently and occupy a central position in the life stories of individuals [11,12,16,17]. Empirical research on happy AMs may be particularly relevant since remembering these types of events may induce the re-experience of positive emotions, potentially contributing to psychological well-being and quality of life.
Functional neuroimaging studies of AM increased over the last decade, allowing an enlarged understanding of brain processes and neural underpinning of memory for personal experiences. A distributed network encompassing different subsystems of AMs (e.g., recollection, self-referential processing, emotional component) has been identified. This network operates thanks to the contribution of the following neural areas: the medial temporal lobe and the hippocampus for AMs retrieval [18]; the lateral prefrontal cortex (lPFC) for memory search and controlled processes [19,20]; the medial prefrontal cortex (mPFC) for self-referential processes [21,22,23]; the lateral and medial parietal cortex for orienting attentional resources to internal representations, contributing to the re-experience of AMs [24,25], and visual-processing areas including the occipital cortex, cuneus, and precuneus to evoke vivid sensory details and mental imagery [26,27].
As regards the emotional component of AMs, recollection of emotionally relevant personal events involves frontotemporal regions and limbic areas such as the amygdala [28,29,30], the hippocampus [31], and the inferior frontal gyrus [32]. Concerning the emotional dimensions of AMs, the intensity of the emotion (arousal) affects the degree to which a personal life event is relived during retrieval and memory vividness [33]. In addition to arousal, the valence of an emotional event can influence how likely and how accurately AMs are remembered [34,35]. Accordingly, neuroimaging showed that brain activity during recall of AMs is modulated not only by arousal but also by the valence of emotion [36,37].
Neuroscientific research on AMs with emotional content has developed various methods of mood induction, with autobiographical recall being the most effective compared to other approaches [38]. In autobiographical recall tasks, participants are usually submitted to a pre-scan interview in which they have to select and write down personal events of their lives. Afterward, interviews are reviewed by the experimenter and then presented during the scan session using generic or specific cues to elicit the retrieval (i.e., written instruction, emotionally related words or images, human faces expressing emotions) [38]. Then, participants are guided by the cues to relieve and re-experience their emotional events as vividly and intensively as possible [39].
In 2002, a meta-analysis of neuroimaging studies investigating emotions was conducted [40] including 16 (out of 55) studies using autobiographical recall methods to induce retrieval of personal emotional events (e.g., fear, sadness, happiness, anger, and disgust). Results showed that the anterior cingulate cortex (ACC) and the insula are the brain regions most frequently activated during the recall of AMs, regardless of their valence. Of note, some of these studies highlighted differential brain activations during the recall of AMs corresponding to positive valence. For example, Lane and colleagues [41] showed greater activation of the ventromedial PFC while recalling AMs for happy events compared to AMs for sad events. Accordingly, subsequent studies showed that frontal brain regions (e.g., medial PFC) were more active during retrieval of positive AMs, whereas posterior regions (e.g., right temporal lobe) were more active during retrieval of negative events [36,37].
The interest in understanding the processing of positive emotions such as happiness and joy and their neural correlates has increased [42,43,44,45,46,47]. A meta-analysis of imaging studies on happiness was conducted to identify the neural correlates of three happiness domains: pleasure, engagement, and meaning [47]. A wide range of tasks was used to examine these three domains of happiness across the 64 studies included in the meta-analysis identifying 33 brain regions [47]. A further step would be the identification of the brain areas that are specifically involved in the reliving of positive AMs in healthy individuals. This is crucial for understanding their possible alterations in mood disorders: for example, depressed individuals exhibit impaired memory for positive material [48] and recall less vividly positive AMs [49]. In this direction, a work by Suardi and colleagues [50] reviewed 15 neuroimaging studies (7 fMRI, 8 PET) investigating AMs of happy events in healthy individuals, all of them employing autobiographical recall methods. The PFC, ACC, and insula were the most frequently reported areas associated with remembering happy AMs, suggesting that these may be crucial areas implicated in the recall of positive AMs. However, due to the descriptive nature of the review, it was not possible to quantitatively define consistent and significant activation patterns across studies.
Of note, individual imaging studies, if examined separately, have small sample sizes, thus leading to low statistical power and low reliability [51]. Furthermore, evaluating consistency is important to avoid false-positive rates in the activation locations reported by single studies, which in neuroimaging is relatively high compared to other fields [52]. To overcome these limitations, meta-analysis is a valuable tool for summarizing results and identifying consistently activated brain regions across a set of studies [53]. Therefore, the present study aimed to identify consistent activations across neuroimaging studies of AM for happy life events using Activation Likelihood Estimation (ALE) [54,55], which is one of the most common algorithms used for coordinate-based meta-analysis [53]. To elucidate the neural underpinning of happy AMs, all the included studies were conducted in healthy samples.
The present meta-analysis differs from those previously described [40,47]. First, we investigated the neural correlates of positive AMs rather than happiness as a broader emotion [47]. Second, the present work includes a significantly larger number of studies on happy AMs than the previous meta-analysis conducted by Phan and colleagues in 2002 [40]. Finally, this is the first meta-analysis to focus on autobiographical recall as a specific type of mood induction procedure, which would help to reduce the differences in brain activation related to the experimental paradigm.
2. Materials and Methods
2.1. Information Sources and Search Strategy
This meta-analysis was conducted according to the international guidelines embraced by the Cochrane Collaboration and the “PRISMA” statement to ensure transparent and complete reporting of data selection [56]. A systematic literature search strategy was conducted using the two electronic databases (Web of Science and PubMed/Medline). The search included articles published up to 22 January 2023. Different sets of query terms were adopted:
- -
- “autobiographical memory” OR “autobiographical recall”, AND “positive events”, OR “happy events”, AND “fMRI” OR “functional magnetic resonance imaging”.
- -
- “autobiographical memory” OR “autobiographical recall”, AND “positive events” OR “happy events”, AND “PET” OR “positron emission tomography”.
Additional sources included published reviews and meta-analyses on neural correlates of emotions and specifically on autobiographical memory for emotional events (e.g., [9,40,46,47]). Most of the studies were selected from a previous review paper on the neural correlates of happiness [50].
2.2. Eligibility Criteria
The retrieved papers were analyzed to ascertain that they met the following inclusion criteria: (1) population of healthy adults (2) using autobiographical recall to asses AMs referred to specific events (i.e., episodes lasting between some minutes and one day); (3) including happy AMs and a control condition, such as AM of neutral events or events eliciting emotions with a negative valence (e.g., fear, disgust, sadness); (4) presenting specified neuroimaging acquisition parameters: (a) using whole-brain analysis; (b) reporting the results in Talairach or Montreal Neurological Institute (MNI) coordinates; (5) reporting cerebral activation changes, as assessed by blood-oxygen-level dependent (BOLD) -fMRI or PET.
We excluded studies that employed non-human participants, clinical populations, as well as those not assessing AM using autobiographical recall techniques or not respecting the neuroimaging parameters. The following publication types were excluded: meta-analysis, systematic reviews, case reports or series, and grey literature. Moreover, articles not written in English were excluded. The authors double-checked the fulfillment of the eligibility criteria.
2.3. Coordinate-Based Meta-Analysis
ALE was performed using the random effects algorithm of GingerAle (v.3.0.2, http://brainmap.org, accessed date: 22 January 2023) [57,58,59]. Each focus of every experiment is modeled by the ALE as a Gaussian probability distribution:
where d indicates the Euclidean distance between the voxels and the considered focus, and indicates the spatial uncertainty. The standard deviation is easily obtained through the Full-Width Half-Maximum (FWHM) as follows:
Subsequently, we determined for every experiment a modeled alteration (MA) map as the union of the Gaussian probability distribution of each focus of the experiment. The union of these MA maps provided the final ALE map. The statistical significance of the activation within the ALE map was calculated by cluster-level inference, as suggested by Eickhoff et al. [57,60,61]. Given a particular cluster forming threshold, a null distribution of cluster sizes was obtained by simulating a long series of experiments using the same characteristic of the real data and then by calculating an ALE map. The obtained score histogram was used to assign threshold p values.
2.4. Automated Regional Behavioral Analysis
The cluster obtained from the previous ALE meta-analysis was submitted to an automated regional behavioral analysis [62]. Behavioral analysis software was developed and tested as a plug-in application for the Multi-image Analysis GUI (Mango, v. 4.1) image processing system (Lancaster, Martinez; https://mangoviewer.com/api/edu/uthscsa/ric/mango/package-summary.html, accessed date: 22 January 2023). A primary goal of the behavioral analysis is to determine specific behaviors for each region under investigation. The analysis is performed in several steps. For each location in the cluster image obtained from the ALE analysis, a table of behavior domains and sub-domains within each row of the list of coordinates is created. Then, for each location in a behavior coordinate list, a “one” is added and then an image of activation foci by location is created.
To test for the significance of behaviors, we used the null hypothesis that the observed probability of activation foci was not different from expected, i.e., that po = pe. To determine variance for effect size, we modeled the two possible outcomes of activations (inside or outside of the ROI) using the binomial distribution. In this study, po and pe served as binomial “success” probabilities (probability of activations falling within the ROI) and the number of trials was the whole-brain activation tally (Nb) for a sub-domain. For the binomial distribution, the variance of “p” is calculated as p(1 − p)/N. An effect-size z-score for each behavioral sub-domain was calculated.
3. Results
3.1. Study Selection
A total of 365 articles were retrieved from the literature search and other sources. After removing duplicates (n = 100), we conducted title and abstract screening for the remaining 287 studies. This resulted in the exclusion of 243 studies because they were not related to emotional AMs or because they did not use neuroimaging (i.e., fMRI or PET). The remaining 44 studies were screened at a full-text level. Of these studies, 27 were excluded for reasons including (1) no contrasts for happy AM; (2) no coordinates; (3) region of interest (ROI) analysis; (4) only deactivation foci for happy AM; (5) no healthy control sample; (6) review/meta-analysis. A total of 17 studies (12 fMRI, 5 PET) were finally included in the meta-analysis (see Figure 1).
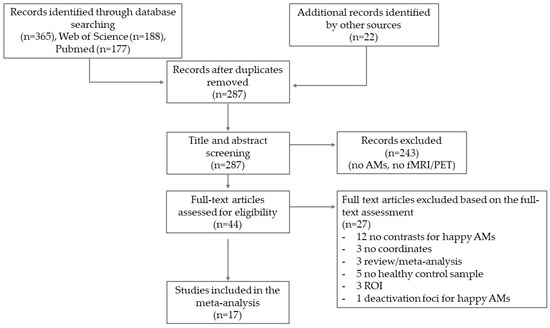
Figure 1.
Flow diagram.
3.2. Characteristics of the Studies
The 17 studies included were published between 1995 and 2019. For each study, the following characteristics are specified in Table 1: the sample size; the neuroimaging technique; the recall induction technique adopted to elicit AMs; the remoteness of the events to remember; which experimental conditions are contrasted to measure brain activation (e.g., happiness vs. neutral); and the number of activation foci (See Table 1).
The total sample comprised 340 healthy participants ranging in age from 18 to 74 years. Although the majority of studies recruited participants of both genders, six studies included only female participants [63,64,65,66,67,68,69] and one study included only male participants [70], resulting in an overall higher number of females (n = 241) than males (n = 99) in the final sample.
In all the studies, participants were required to re-imagine or mentally recall AMs during the MRI scan. The technique adopted to elicit autobiographical recall differed across studies. In most of the studies, participants are required to recall and relive personally experienced emotional events selected prior to the experimental session. The autobiographical events were cued by written keywords or short sentences in nine studies (see Table 1). In four studies [65,71,72,73], events to be recalled were elicited by listening to pre-recorded audio scripts of autobiographical events. In two studies, the events were cued by pictures depicting emotional facial expressions [63,64], whereas in one study [74], emotional pictures from the International Affective Pictures System (IAPS [75]) served as cues. All the studies adopted a measure to assess the effectiveness of the recall procedure by asking participants to evaluate phenomenological features of AMs (e.g., vividness, intensity, sensory details), although these measures varied across studies. Although neural differences have been suggested during the search and the elaboration phases of the memory process [76], none of the studies included a distinction between these two stages of AM retrieval.
Eight out of seventeen studies [30,37,65,66,67,72,73,77] considered the remoteness of the event to recall, by asking the participants to evoke events within defined time periods (e.g., the last 5 years). By contrast, in the rest of the studies, participants were free to evoke AMs, without any temporal delimitation.
In eight studies, cerebral activations referred to the contrast between a happiness condition and a neutral condition consisting of recalling autobiographical events without emotional content [64,65,68,71,72,73,78,79], with two studies additionally including the contrast between happiness and irritability [72,73]. In four studies, the control conditions were AMs with negative valence [37,66,67,77]; in one study, AMs related to sadness [63]; and in two studies, AMs related to disgust [74]. Of note, two studies [30,69] reported activations for a happiness condition contrasted with a resting condition, and one study [70] contrasted a happiness condition with a control task (count backward from 100 by subtracting 4). A total of 282 activation foci were reported for all the studies.

Table 1.
Characteristics of the studies and number of activation foci.
Table 1.
Characteristics of the studies and number of activation foci.
| Year of Publication | First Author and Reference | Neuroimaging | Original Coordinates | Sample | Recall Induction Technique | Remoteness | Contrasted Conditions | Activation Foci |
|---|---|---|---|---|---|---|---|---|
| 1995 | George [63] | PET | Talairach | n = 11 (F, mean age: 33.3, SD: 12.3) | REC/REL Two events for condition cued with pictures of emotional faces | not defined | happiness > sadness | 5 |
| 1996 | George [64] | PET | Talairach | n = 20 (10 F, mean age: 34.5, SD: 12.1; 10 M, mean age: 35.5, SD: 8.8) | REC/REL Two events for condition cued with pictures of emotional faces | not defined | happiness > neutral | 8 |
| 1997 | Lane [65] | PET | Talairach | n = 12 (F, mean age: 23.3, SD: 3.2) | LIST.SCRIPT Three events for condition | last 6 months | happiness > neutral | 4 |
| 2000 | Damasio [78] | PET | Talairach | n = 41 (21 F, 20 M divided into four cohorts, age: from 23 to 42) | REC/REL One event for condition | not defined | happiness > neutral | 20 |
| 2003 | Markowitsch [36] | fMRI | MNI | n = 13 (7 F, 6 M, mean age: 30, from 19 to 43) | REC/REL 18 events for condition cued by keywords | before 12 years old; from 12 to 18 years; from 18 until now | happiness > rest | 10 |
| 2003 | Piefke [37] | fMRI | Talairach | n = 20 (10 F, 10 M, mean age: 26, SD: 3) | REC/REL 10 events for condition, cued by written sentences | childhood (up to 10 years); recent past (last 5 years) | happiness > negative | 4 |
| 2007 | Marci [71] | PET | MNI | n = 10 (5 F, 5 M, mean age: 33.9, SD: 11.9) | LIST.SCRIPT 2 events for condition | not defined | happiness > neutral | 4 |
| 2008 | Cerqueira [72] | fMRI | Talairach | n = 11 (5 F, 6 M, mean age: 32.4, SD: 7.2) | LIST.SCRIPT 3 events for condition | last 12 months | happiness > neutral happiness > irritability | 10 |
| 2010 | Cerqueira [73] | fMRI | Talairach | n = 11 (5 F, 6 M, mean age: 32.4, SD: 7.2) | LIST.SCRIPT 3 events for condition | last 6 months | happiness > neutral happiness > irritability | 5 |
| 2011 | Sitaram [74] | fMRI | MNI | n = 12 (mean age: 25 years, range: 22–26) | REC/REL 1 event for condition cued by emotional pictures | not defined | happiness > disgust | 112 |
| 2011 | Zotev [70] | fMRI | Talairach | n = 14 (M, mean age: 27.5, SD: 11.1) | REC/REL 3 happy events cued by the word “happy”; counting task as control condition | not defined | happiness > control | 22 |
| 2014 | Speer [79] | fMRI | Talairach | n = 19 (10 F, 9 M, mean age: 26.1, SD: 7.78) | REC/REL 21 episodes for condition cued by keywords | not defined | happiness > neutral | 27 |
| 2014 | Gong [66] | fMRI | MNI | n = 12 (F, mean age: 66.3, from 60 to 70) | REC/REL 10 events for condition cued by written sentences | before 12 years old; last 5 years (except the last month) | happiness > negative | 3 |
| 2014 | Ge [67] | fMRI | MNI | n = 27 (13 younger F, age from 18 to 22; 14 older F, age from 60 to 74) | REC/REL 5 events for condition cued by written sentences | last 5 years | happiness > negative | 3 |
| 2017 | Lempert [69] | fMRI | MNI | n = 35 (F; mean age: 20.86, SD: 2.9) | REC/REL 10 events for condition cued by written sentences | not defined | happiness > rest | 12 |
| 2018 | Xu [77] | fMRI | MNI | n = 25 (17 F, 8 M, mean age: 21.36, SD: 3.34) | REC/REL 9 events for condition cued by written sentences | before 18 years old | happiness > baseline happiness > negative | 21 |
| 2019 | Schie [68] | fMRI | MNI | n = 47 (F, mean age: 29.36; SD: 9.61) | REC/REL 4 events for condition cued by written sentences | not defined | happiness > neutral | 12 |
Notes: SD = standard deviations; n = sample size; F = females; M = males; REC/REL = recalling and reliving past emotional experiences; LIST.SCRIPT = listening autobiographical scripts; MNI = Montreal Neurological Institute.
3.3. Clusters of Neural Activity Changes
The brain regions identified in the meta-analysis are presented in Table 2. ALE maps were computed using GingerALE 3.0.2, at an FWE-corrected threshold of p < 0.05, with a minimum cluster size of K >150 mm3, and visualized using Mango (Figure 2). Eight activation clusters were found. One cluster included the left medial frontal gyrus and the left anterior cingulate (BA 10, 32). Other clusters were identified, including the left posterior cingulate (BA 23, 31), the left superior frontal gyrus (BA 9), and the anterior cingulate (BA 32). Another cluster was found in the left hypothalamus and the left medial globus pallidus. One cluster included the left parahippocampus (BA 35, 36) and parahippocampal structures, and another cluster was found in the left hippocampus and the lateral globus pallidus. Finally, the right thalamus, including the subthalamic nucleus, constituted another cluster of activation.

Table 2.
Areas of functional changes in brain activity associated with autobiographical recall of happy events.
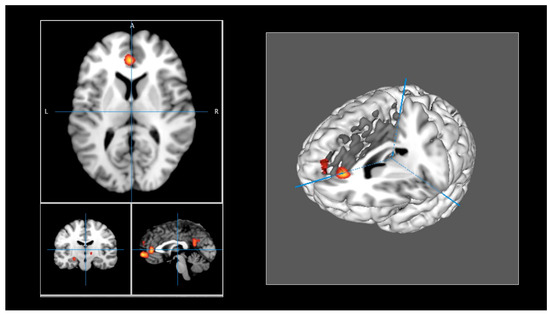
Figure 2.
(Left panel): ALE maps were computed using GingerALE 3.0.2, at an FWE-corrected threshold of p < 0.05, with a minimum cluster size of K > 150 mm3, and visualized using Mango. (Right panel): Activations were projected onto a 3D rendering model of the brain.
3.4. Characterization of the Clusters
To characterize in terms of behavior of the various clusters found with the ALE methods, we used a plug-in of the Mango software (v.4.1) [62] that automatically associates the different clusters with the different behavioral domains using the BrainMap database. BrainMap categorizes functional imaging experiments using five major behavioral domains (action, cognition, emotion, interoception, and perception) with 51 sub-domains. Each experiment in BrainMap is assigned one or more behavioral classifications along with the set of coordinates for reported activations, and these data provide the basic structure for forming behavioral probability distributions as 3D images. Region of interest (ROI) analysis is applied to these spatial probability images to assess behaviors.
The behavioral analysis (BD) was performed with a minimum threshold of activation of 38 foci (labeled as “row counts” in the output of the behavioral analysis plug-in in the Multi-image Analysis GUI). Several BD were identified within the domains of cognition (explicit memory, semantic language, social cognition) and emotion (happiness, fear) (See Figure 3).
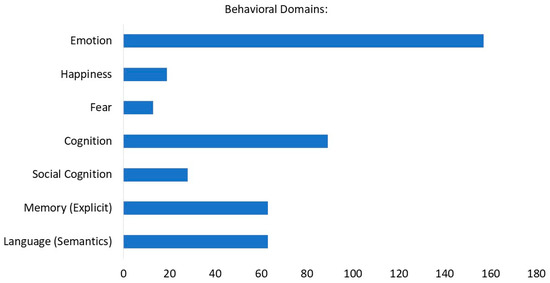
Figure 3.
Functional characterization by behavioral domain. The blue bars denote the number of foci for the particular behavioral domain within the selected ROI.
4. Discussion
The novel aim of this coordinate-based ALE meta-analysis was to quantitatively analyze the results of neuroimaging studies investigating cerebral activation changes during autobiographical recall of happy life events. This is the first attempt to consider autobiographical recall paradigms assessing happy AMs in a single analysis, to give a more objective perspective of the cerebral network involved when recalling AMs for happy events.
This review included 17 studies (12 fMRI, 5 PET), with an overall number of subjects of 340 for a total of 282 activation foci identified, which is considered a sufficient number to proceed with ALE analysis (as previously stated by Laird in her “Users’ Manual for BrainMap GingerALE 2.0”).
The outcome of the ALE was characterized by brain regions consistently activated when recalling AMs for happy events. The activation clusters encompassed the frontal gyrus and the cingulate cortex, the basal ganglia, the parahippocampal structures, the hypothalamus, and the thalamus.
Concerning frontal regions, the prefrontal cortex (PFC) is known to be part of the brain network involved in autobiographical recall processes [18]. Specifically, the medial PFC network, including the anterior and posterior midline regions, has been linked to self-referential processes implicated when recalling personal life events [22,23,80]. Here, we found a consistent activation of the superior and medial frontal gyrus during recall of happy AMs. Previous studies showed that the activation of brain areas within the medial PFC is more frequently associated with happy AMs, whereas activation of the lateral PFC is more frequently associated with sad AMs [30,63,64,81]. In the present meta-analysis, consistent activation of the medial (but not the lateral PFC) seems to support this lateral/medial differentiation in the processing of sad/happy AMs.
Additional frontal areas were represented by activation in the left anterior cingulate cortex (ACC) and the poster cingulate cortex (PCC). The cingulate cortex activity has been associated with happiness [45,82], although it seems likely that the cingulate cortex plays a key role in both positive and negative emotions [83]. In addition, the cingulate cortex is known to be involved in AMs [10,84,85] and the PCC has particularly strong reciprocal connections with medial temporal lobe memory structures such as the entorhinal and the parahippocampal cortices [86]. Furthermore, the PCC and the medial PFC together with other memory-related areas are part of the “brain default network” [87,88] which is crucial for self-representation and self-consciousness implicated in the recall of AMs. The ACC, closely interconnected to the medial PFC, is known to regulate both cognitive and emotional processing [89,90,91], and consistent activation of the ACC has been found during recall of emotional AMs [40].
Concerning the basal ganglia, activation foci were found in the lateral and medial globus pallidus and the subthalamic nucleus. The role of these structures in the reward circuit has been recognized in non-human primates [92,93,94] and more recently in humans [95]. Therefore, the activation of these reward-related areas could be explained by the hedonic features of happy AMs, reflecting hedonic happiness [42,43,47].
Finally, the activation found in the parahippocampal regions is consistent with the role of the medial temporal lobe and the hippocampus in AMs retrieval [18], whereas the activation of the hypothalamus and the thalamus may be related to the recall and reliving of sensory and bodily signals associated with happy personal memories [72,73].
The majority of clusters were identified in the left hemisphere, aligning with the consistent left-lateralized activation tendency reported in previous neuroimaging literature on autobiographical memory [20,84,96]. This inclination is likely attributed to the verbal modality of autobiographical recall tasks, often involving written keywords or short sentences, thereby implicating the left hemisphere.
Functional characterization of these activations was given by the automated regional behavioral analysis (BD) [62], suggesting that a high portion of the activation foci (n = 157) corresponds to the behavioral domain of emotion, with happiness being the most representative one. Other BD included cognitive domains, mainly represented by episodic memory as expected. A second cognitive domain shown is semantic language, possibly indicating an increase in semantic processing during recall of happy AMs. This may reflect the activation of the PFC and its role in the processing of semantic and conceptual information [35,97,98].
Taken together, the results of the present meta-analysis enable the identification of brain areas consistently activated during the recall of happy AMs. Frontal regions, such as the frontal gyrus and the cingulate cortex, might account for the cognitive processes involved in the subjective appraisal of recalled happy AMs, whereas the subthalamic nucleus and the globus pallidus may be responsible for the pleasure reactions associated with recalling happy AMs. Some of these areas, such as the basal ganglia, the PFC, and the cingulate cortex, align with those previously identified for happiness as a broad emotion [47]. In contrast, other areas, like the amygdala and the insula, did not consistently activate during the recall of happy AMs. This is in line with evidence indicating that the amygdala and the insula are more likely to exhibit increased activation during negative affect than positive affect, despite their responsiveness to both positive and negative affect more than neutral affect [99]. Additionally, early findings from a meta-analysis indicate consistent activation of the amygdala for AMs of fear and the insula for AMs of disgust [40].
The present work should be interpreted in light of some limitations. Firstly, only a small number of studies (9 out of 17) included the contrast between positive and negative events, and some studies focused on different types of negative emotional AM (e.g., sadness, irritability). Hence, it was not possible to run a separate analysis for the contrast of positive vs. negative AMs, because the results could be strongly driven by only a few experiments [60,100]. However, a direct comparison between negative and positive AMs would help to investigate and discuss possible overlap and discrepancies in brain activity during the recall of positive vs. negative AMs. Thus, this should be considered as an important limitation and results should be interpreted with caution.
Secondly, a factor potentially contributing to differential brain activations is related to the remoteness of the evoked AM. So far, some studies have shown greater activation of the medial temporal lobe during retrieval of recent compared to remote episodic memories [37,101,102], although other studies did not confirm this differential activation associated with remoteness [29,31,103,104,105,106,107]. In the current meta-analysis, remoteness was not included as a parameter, given that only eight studies adopted specific temporal information of the AMs (see Table 1), also showing discrepancies in the remoteness of the evoked AMs (e.g., recent vs. remote). Specifically, some studies focused on recent memories from 6–12 months to 5 years, whereas others included remote AMs from adulthood (e.g., from 18 years) or adolescence/childhood (e.g., before 12 years).
Another limitation is that only activation foci of happy AMs were analyzed, while deactivations were not considered in this study. The decision to exclude deactivations was influenced by the prevalence of articles primarily featuring activations, leaving an inadequate number of articles for a comprehensive meta-analysis of deactivations.
Finally, the demographic variables of the current sample such as the higher proportion of females should be considered when interpreting the results. Previous studies have demonstrated gender differences in AM, which are also reflected in different neural activations during AM recall [108,109,110]. Age is another crucial variable in the context of AM, as healthy aging tends to reduce the episodic richness of AM retrieval [111]. Thus, the wide age range (from 18 to 75) across studies in the meta-analysis may potentially mask age-related differences in the neural correlates of happy AMs that are worth investigating in specific age groups (for example, younger and older people).
Despite these limitations, the results obtained offer new insights into the neural correlates of positive AMs, identifying for the first time consistent brain activations across the existing studies using autobiographical recall methods with happy life events. Identifying the neural correlates of positive AMs in healthy individuals is the first step to extending the basic understanding of dysfunctional processes in affective disorders [112,113]. Individuals with depression have shown impairment in autobiographical memory retrieval, particularly in relation to positive material [48,49,114]. Future studies could investigate functional alterations during recall of positive AMs as possible biomarkers of individuals at risk of developing mood disorders. Furthermore, changes induced by pharmacological or psychological treatments have been described in depression [115]. Similarly, changes in the processing of positive AMs following pharmacological or psychological therapies for depression could be investigated to identify biomarkers of clinical response to treatment.
5. Conclusions
The present coordinate-based ALE meta-analysis identified a set of brain regions that are consistently activated during the recall of happy AMs. This included frontal regions (i.e., prefrontal gyrus and cingulate cortex) exerting cognitive control, basal ganglia structures (i.e., globus pallidus and subthalamic nucleus) related to reward, as well as the memory-related hippocampus and thalamic structures for sensory integration during the recall of AMs. Such a highly distributed brain network encompasses areas that mainly involve the emotion domain and cognitive domains of memory and semantic processing.
Author Contributions
Conceptualization, G.T., I.S. and M.L.R.; formal analysis, T.C.; methodology, F.C. and T.C.; writing—original draft, G.T. and T.C.; writing—review and editing, I.S., M.L.R. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sotgiu, I. The Psychology of Autobiographical Memory: History, Theory, Research; Palgrave Macmillan: Cham, Switzerland, 2021. [Google Scholar]
- Conway, M.A. Memory and the self. J. Mem. Lang. 2005, 53, 594–628. [Google Scholar] [CrossRef]
- Conway, M.A.; Singer, J.A.; Tagini, A. The self and autobiographical memory: Correspondence and coherence. Soc. Cogn. 2004, 22, 491–529. [Google Scholar] [CrossRef]
- Berntsen, D.; Rubin, D.C. Introduction. In Understanding Autobiographical Memory: Theories and Approaches; Cambridge University Press: Cambridge, UK, 2012; pp. 1–8. [Google Scholar]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Rubin, D.C. A Basic-Systems Approach to Autobiographical Memory. Curr. Dir. Psychol. Sci. 2005, 14, 79–83. [Google Scholar] [CrossRef]
- Rubin, D.C. The Basic-Systems Model of Episodic Memory. Perspect. Psychol. Sci. 2006, 1, 277–311. [Google Scholar] [CrossRef]
- Rubin, D.C. The basic systems model of autobiographical memory. In Understanding Autobiographical Memory: Theories and Approaches; Cambridge University Press: Cambridge, UK, 2012; pp. 11–32. [Google Scholar]
- Holland, A.C.; Kensinger, E.A. Emotion and autobiographical memory. Phys. Life Rev. 2010, 7, 88–131. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.J.; Pathman, T.; Inman, C.; Campanella, C.; Hamann, S. Neural correlates of autobiographical memory retrieval in children and adults. Memory 2017, 25, 450–466. [Google Scholar] [CrossRef]
- Sotgiu, I. How Do We Remember Happy Life Events? A Comparison Between Eudaimonic and Hedonic Autobiographical Memories. J. Psychol. Interdiscip. Appl. 2016, 150, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, I. Gender Differences and Similarities in Autobiographical Memory for Eudaimonic Happy Events. J. Happiness Stud. 2019, 20, 1457–1479. [Google Scholar] [CrossRef]
- Huta, V.; Waterman, A.S. Eudaimonia and Its Distinction from Hedonia: Developing a Classification and Terminology for Understanding Conceptual and Operational Definitions. J. Happiness Stud. 2013, 15, 1425–1456. [Google Scholar] [CrossRef]
- Delle Fave, A.; Brdar, I.; Freire, T.; Vella-Brodrick, D.; Wissing, M.P. The Eudaimonic and Hedonic Components of Happiness: Qualitative and Quantitative Findings. Soc. Indic. Res. 2011, 100, 185–207. [Google Scholar] [CrossRef]
- Vittersø, J.; Søholt, Y. Life satisfaction goes with pleasure and personal growth goes with interest: Further arguments for separating hedonic and eudaimonic well-being. J. Posit. Psychol. 2011, 6, 326–335. [Google Scholar] [CrossRef]
- Berntsen, D.; Rubin, D.C.; Siegler, I.C. Two versions of life: Emotionally negative and positive life events have different roles in the organization of life story and identity. Emotion 2011, 11, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, D.; Rubin, D.C. Cultural life scripts structure recall from autobiographical memory. Mem. Cogn. 2004, 32, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; St Jacques, P. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 2007, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A. Neuroimaging studies of autobiographical event memory. Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.W.M.; Macrae, C.N.; Wyland, C.L.; Caglar, S.; Inati, S.; Heatherton, T.F. Finding the self? An event-related fMRI study. J. Cogn. Neurosci. 2002, 14, 785–794. [Google Scholar] [CrossRef]
- Macrae, C.N. Medial Prefrontal Activity Predicts Memory for Self. Cereb. Cortex 2004, 14, 647–654. [Google Scholar] [CrossRef]
- Cabeza, R.; Ciaramelli, E.; Olson, I.R.; Moscovitch, M. The parietal cortex and episodic memory: An attentional account. Nat. Rev. Neurosci. 2008, 9, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Shannon, B.J.; Kahn, I.; Buckner, R.L. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 2005, 9, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Prince, S.E.; Daselaar, S.M.; Greenberg, D.L.; Budde, M.; Dolcos, F.; LaBar, K.S.; Rubin, D.C. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J. Cogn. Neurosci. 2004, 16, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.L.; Rubin, D.C. The neuropsychology of autobiographical memory. Cortex 2003, 39, 687–728. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.R.; Markowitsch, H.J.; Reinkemeier, M.; Bruckbauer, T.; Kassler, J.; Heiss, W.D. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. J. Neurosci. 1996, 16, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Frith, C.D. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J. Neurosci. 2003, 23, 5302–5307. [Google Scholar] [CrossRef]
- Markowitsch, H.J.; Thiel, A.; Reinkemeier, M.; Kessler, J.; Koyuncu, A.; Heiss, W.-D. Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behav. Neurol. 2000, 12, 181–190. [Google Scholar] [CrossRef]
- Addis, D.R.; Moscovitch, M.; Crawley, A.P.; McAndrews, M.P. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus 2004, 14, 752–762. [Google Scholar] [CrossRef]
- Greenberg, D.L.; Rice, H.J.; Cooper, J.J.; Cabeza, R.; Rubin, D.C.; LaBar, K.S. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 2005, 43, 659–674. [Google Scholar] [CrossRef]
- Talarico, J.M.; Labar, K.S.; Rubin, D.C. Emotional intensity predicts autobiographical memory experience. Mem. Cogn. 2004, 32, 1118–1132. [Google Scholar] [CrossRef]
- Berntsen, D.; Rubin, D.C. Emotionally charged autobiographical memories across the life span: The recall of happy, sad, traumatic and involuntary memories. Psychol. Aging 2002, 17, 636–652. [Google Scholar] [CrossRef]
- Kensinger, E.A. What factors need to be considered to understand emotional memories? Emot. Rev. 2009, 1, 120–121. [Google Scholar] [CrossRef]
- Markowitsch, H.J.; Vandekerckhove, M.M.P.; Lanfermann, H.; Russ, M.O. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 2003, 39, 643–665. [Google Scholar] [CrossRef]
- Piefke, M. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 2003, 126, 650–668. [Google Scholar] [CrossRef]
- Jallais, C.; Gilet, A.L. Inducing changes in arousal and valence: Comparison of two mood induction procedures. Behav. Res. Methods 2010, 42, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Westermann, R.; Spies, K.; Stahl, G.; Hesse, F.W. Relative effectiveness and validity of mood induction procedures: A meta-analysis. Eur. J. Soc. Psychol. 1996, 26, 557–580. [Google Scholar] [CrossRef]
- Phan, K.L.; Wager, T.; Taylor, S.F.; Liberzon, I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002, 16, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Reiman, E.M.; Axelrod, B.; Yun, L.S.; Holmes, A.; Schwartz, G.E. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J. Cogn. Neurosci. 1998, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Towards a Neuroscience of Well-Being: Implications of Insights from Pleasure Research; Springer: Dordrecht, The Netherlands, 2013; pp. 81–100. [Google Scholar]
- Kringelbach, M.L.; Berridge, K.C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 2009, 13, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Panksepp, J. The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 2006, 30, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Chemali, Z.N.; Chahine, L.M.; Naassan, G. On happiness: A minimalist perspective on a complex neural circuitry and its psychosocial constructs. J. Happiness Stud. 2008, 9, 489–501. [Google Scholar] [CrossRef]
- Machado, L.; Cantilino, A. A systematic review of the neural correlates of positive emotions. Rev. Bras. Psiquiatr. 2017, 39, 172–179. [Google Scholar] [CrossRef]
- Tanzer, J.R.; Weyandt, L. Imaging Happiness: Meta Analysis and Review. J. Happiness Stud. 2020, 21, 2693–2734. [Google Scholar] [CrossRef]
- Dillon, D.G. The neuroscience of positive memory deficits in depression. Front. Psychol. 2015, 6, 1295. [Google Scholar] [CrossRef]
- Werner-Seidler, A.; Moulds, M.L. Autobiographical memory characteristics in depression vulnerability: Formerly depressed individuals recall less vivid positive memories. Cogn. Emot. 2010, 25, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Suardi, A.; Sotgiu, I.; Costa, T.; Cauda, F.; Rusconi, M. The neural correlates of happiness: A review of PET and fMRI studies using autobiographical recall methods. Cogn. Affect. Behav. Neurosci. 2016, 16, 383–392. [Google Scholar] [CrossRef]
- Raemaekers, M.; Vink, M.; Zandbelt, B.; van Wezel, R.J.A.; Kahn, R.S.; Ramsey, N.F. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage 2007, 36, 532–542. [Google Scholar] [CrossRef]
- Wager, T.D.; Lindquist, M.A.; Nichols, T.E.; Kober, H.; Van Snellenberg, J.X. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage 2009, 45, S210–S221. [Google Scholar] [CrossRef]
- Wager, T.D.; Lindquist, M.; Kaplan, L. Meta-analysis of functional neuroimaging data: Current and future directions. Soc. Cogn. Affect. Neurosci. 2007, 2, 150–158. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Price, C.J.; Glahn, D.C.; Uecker, A.M.; Lancaster, J.L.; Turkeltaub, P.E.; Kochunov, P.; Fox, P.T. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005, 25, 155–164. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Nichols, T.E.; Laird, A.R.; Hoffstaedter, F.; Amunts, K.; Fox, P.T.; Bzdok, D.; Eickhoff, C.R. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016, 137, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Laird, A.R.; Fox, P.M.; Lancaster, J.L.; Fox, P.T. Implementation errors in the GingerALE Software: Description and recommendations. Hum. Brain Mapp. 2017, 38, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Laird, A.R.; Eickhoff, S.B.; Martinez, M.J.; Fox, P.M.; Fox, P.T. Automated regional behavioral analysis for human brain images. Front. Neuroinform. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Horwitz, B.; Herscovitch, P.; Post, R.M. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 1995, 152, 341–351. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Herscovitch, P.; Post, R.M. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol. Psychiatry 1996, 40, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Reiman, E.M.; Ahern, G.L.; Schwartz, G.; Davidson, R.E. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry 1997, 154, 926. [Google Scholar]
- Gong, X.; Fu, Y.; Wang, D.; Franz, E.; Long, Z. Remoteness Modulates the Effects of Emotional Valence on the Neural Network of Autobiographical Memory in Older Females. Int. J. Aging Hum. Dev. 2014, 79, 23–54. [Google Scholar] [CrossRef]
- Ge, R.; Fu, Y.; Wang, D.; Yao, L.; Long, Z. Age-related alterations of brain network underlying the retrieval of emotional autobiographical memories: An fMRI study using independent component analysis. Front. Hum. Neurosci. 2014, 8, 629. [Google Scholar] [CrossRef]
- Schie, C.C.; Chiu, C.; Rombouts, S.A.R.B.; Heiser, W.J.; Elzinga, B.M. When I relive a positive me: Vivid autobiographical memories facilitate autonoetic brain activation and enhance mood. Hum. Brain Mapp. 2019, 40, 4859–4871. [Google Scholar] [CrossRef]
- Lempert, K.M.; Speer, M.E.; Delgado, M.R.; Phelps, E.A. Positive autobiographical memory retrieval reduces temporal discounting. Soc. Cogn. Affect. Neurosci. 2017, 12, 1584–1593. [Google Scholar] [CrossRef]
- Zotev, V.; Krueger, F.; Phillips, R.; Alvarez, R.P.; Simmons, W.K.; Bellgowan, P.; Drevets, W.C.; Bodurka, J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS ONE 2011, 6, e24522. [Google Scholar] [CrossRef] [PubMed]
- Marci, C.D.; Glick, D.M.; Loh, R.; Dougherty, D.D. Autonomic and prefrontal cortex responses to autobiographical recall of emotions. Cogn. Affect. Behav. Neurosci. 2007, 7, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, C.T.; Almeida, J.R.C.; Gorenstein, C.; Gentil, V.; Leite, C.C.; Sato, J.R.; Amaro, E.; Busatto, G.F. Engagement of multifocal neural circuits during recall of autobiographical happy events. Braz. J. Med. Biol. Res. 2008, 41, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, C.T.; Almeida, J.R.C.; Sato, J.R.; Gorenstein, C.; Gentil, V.; Leite, C.C.; Amaro, E.; Busatto, G.F. Cognitive control associated with irritability induction: An autobiographical recall fMRI study. Rev. Bras. Psiquiatr. 2010, 32, 109–118. [Google Scholar] [CrossRef][Green Version]
- Sitaram, R.; Lee, S.; Ruiz, S.; Rana, M.; Veit, R.; Birbaumer, N. Real-time support vector classification and feedback of multiple emotional brain states. Neuroimage 2011, 56, 753–765. [Google Scholar] [CrossRef]
- Lang, P.J. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report; University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Ford, J.H.; Kensinger, E.A. The role of the amygdala in emotional experience during retrieval of personal memories. Memory 2019, 27, 1362–1370. [Google Scholar] [CrossRef]
- Xu, R.; Yang, J.; Feng, C.; Wu, H.; Huang, R.; Yang, Q.; Li, Z.; Xu, P.; Gu, R.; Luo, Y. jia Time is nothing: Emotional consistency of autobiographical memory and its neural basis. Brain Imaging Behav. 2018, 12, 1053–1066. [Google Scholar] [CrossRef]
- Damasio, A.R.; Grabowski, T.J.; Bechara, A.; Damasio, H.; Ponto, L.L.B.; Parvizi, J.; Hichwa, R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000, 3, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.E.; Bhanji, J.P.; Delgado, M.R. Savoring the Past: Positive Memories Evoke Value Representations in the Striatum. Neuron 2014, 84, 847–856. [Google Scholar] [CrossRef]
- Craik, F.I.M.; Moroz, T.M.; Moscovitch, M.; Stuss, D.T.; Winocur, G.; Tulving, E.; Kapur, S. In Search of the Self: A Positron Emission Tomography Study. Psychol. Sci. 1999, 10, 26–34. [Google Scholar] [CrossRef]
- Pelletier, M.; Bouthillier, A.; Lévesque, J.; Carrier, S.; Breault, C.; Paquette, V.; Mensour, B.; Leroux, J.M.; Beaudoin, G.; Bourgouin, P.; et al. Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport 2003, 14, 1111–1116. [Google Scholar] [CrossRef]
- Funahashi, S. Brain mechanisms of happiness. Psychologia 2011, 54, 222–233. [Google Scholar] [CrossRef]
- Davidson, R.J. Well-being and affective style: Neural substrates and biobehavioural correlates. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Turner, G.R.; Tisserand, D.; Hevenor, S.J.; Graham, S.J.; McIntosh, A.R. The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. J. Cogn. Neurosci. 2004, 16, 1633–1646. [Google Scholar] [CrossRef]
- Maddock, R.J.; Garrett, A.S.; Buonocore, M.H. Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 2001, 104, 667–676. [Google Scholar] [CrossRef]
- Suzuki, W.L.; Amaral, D.G. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. J. Comp. Neurol. 1994, 350, 497–533. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Whalen, P.J.; Rauch, S.L.; Etcoff, N.L.; McInerney, S.C.; Lee Michael, B.; Jenike, M.A. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 1998, 18, 411–418. [Google Scholar] [CrossRef]
- Hikosaka, O.; Bromberg-Martin, E.; Hong, S.; Matsumoto, M. New insights on the subcortical representation of reward. Curr. Opin. Neurobiol. 2008, 18, 203–208. [Google Scholar] [CrossRef]
- Espinosa-Parrilla, J.F.; Baunez, C.; Apicella, P. Modulation of neuronal activity by reward identity in the monkey subthalamic nucleus. Eur. J. Neurosci. 2015, 42, 1705–1717. [Google Scholar] [CrossRef]
- Espinosa-Parrilla, J.F.; Baunez, C.; Apicella, P. Linking reward processing to behavioral output: Motor and motivational integration in the primate subthalamic nucleus. Front. Comput. Neurosci. 2013, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Howell, N.A.; Prescott, I.A.; Lozano, A.M.; Hodaie, M.; Voon, V.; Hutchison, W.D. Preliminary evidence for human globus pallidus pars interna neurons signaling reward and sensory stimuli. Neuroscience 2016, 328, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, G.; Parker, A.; Head, K.; Gobet, F. LEFT LATERALIZATION IN AUTOBIOGRAPHICAL MEMORY: AN fMRI STUDY USING THE EXPERT ARCHIVAL PARADIGM. Int. J. Neurosci. 2008, 118, 191–209. [Google Scholar] [CrossRef]
- Dobbins, I.G.; Wagner, A.D. Domain-general and Domain-sensitive Prefrontal Mechanisms for Recollecting Events and Detecting Novelty. Cereb. Cortex 2005, 15, 1768–1778. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Selco, S.L.; Field, J.E.; Cohen, N.J. The Relationship between Skill Learning and Repetition Priming: Experimental and Computational Analyses. J. Exp. Psychol. Learn. Mem. Cogn. 1999, 25, 208–235. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Satpute, A.B.; Wager, T.D.; Weber, J.; Barrett, L.F. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cereb. Cortex 2016, 26, 1910–1922. [Google Scholar] [CrossRef]
- Müller, V.I.; Cieslik, E.C.; Laird, A.R.; Fox, P.T.; Radua, J.; Mataix-Cols, D.; Tench, C.R.; Yarkoni, T.; Nichols, T.E.; Turkeltaub, P.E.; et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 151–161. [Google Scholar] [CrossRef]
- Haist, F.; Gore, J.B.; Mao, H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat. Neurosci. 2001, 4, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Niki, K.; Luo, J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J. Cogn. Neurosci. 2002, 14, 500–507. [Google Scholar] [CrossRef]
- Conway, M.A.; Turk, D.J.; Miller, S.L.; Logan, J.; Nebes, R.D.; Meltzer, C.C.; Becker, J.T.; Conway, M.A. A Positron Emission Tomography (PET) Study of Autobiographical Memory Retrieval. Memory 1999, 7, 679–703. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, A.; Winocur, G.; Grady, C.L.; Hevenor, S.J.; Moscovitch, M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex 2004, 14, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Mayes, A.R.; Mackay, C.E.; Montaldi, D.; Downes, J.J.; Singh, K.D.; Roberts, N. Does retrieving decades-old spatial memories activate the medial temporal lobes less than retrieving recently acquired spatial memories? Neuroimage 2000, 11, S421. [Google Scholar] [CrossRef]
- Ryan, L.; Nadel, L.; Keil, K.; Putnam, K.; Schnyer, D.; Trouard, T.; Moscovitch, M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: Evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus 2001, 11, 707–714. [Google Scholar] [CrossRef]
- Steinvorth, S.; Corkin, S.; Halgren, E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuroimage 2006, 30, 285–298. [Google Scholar] [CrossRef]
- Compère, L.; Sperduti, M.; Gallarda, T.; Anssens, A.; Lion, S.; Delhommeau, M.; Martinelli, P.; Devauchelle, A.-D.; Oppenheim, C.; Piolino, P. Sex Differences in the Neural Correlates of Specific and General Autobiographical Memory. Front. Hum. Neurosci. 2016, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Bodurka, J.; Drevets, W.C. Functional neuroimaging of sex differences in autobiographical memory recall in depression. Psychol. Med. 2017, 47, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Grysman, A.; Hudson, J.A. Gender differences in autobiographical memory: Developmental and methodological considerations. Dev. Rev. 2013, 33, 239–272. [Google Scholar] [CrossRef]
- St. Jacques, P.L.; Rubin, D.C.; Cabeza, R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol. Aging 2012, 33, 1298–1310. [Google Scholar] [CrossRef]
- Young, K.D.; Erickson, K.; Nugent, A.C.; Fromm, S.J.; Mallinger, A.G.; Furey, M.L.; Drevets, W.C. Functional anatomy of autobiographical memory recall deficits in depression. Psychol. Med. 2012, 42, 345–357. [Google Scholar] [CrossRef]
- Young, K.D.; Bellgowan, P.; Bodurka, J.; Drevets, W. Functional Neuroimaging Correlates of Autobiographical Memory Deficits in Subjects at Risk for Depression. Brain Sci. 2015, 5, 144–164. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef]
- Atkinson, L.; Sankar, A.; Adams, T.M.; Fu, C.H.Y. Recent Advances in Neuroimaging of Mood Disorders: Structural and Functional Neural Correlates of Depression, Changes with Therapy, and Potential for Clinical Biomarkers. Curr. Treat. Options Psychiatry 2014, 1, 278–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).