Associations of Sleep Quality and Physical Activity with Diabetes Quality of Life in Korean Americans with Type 2 Diabetes: A Cross-Sectional Study
Abstract
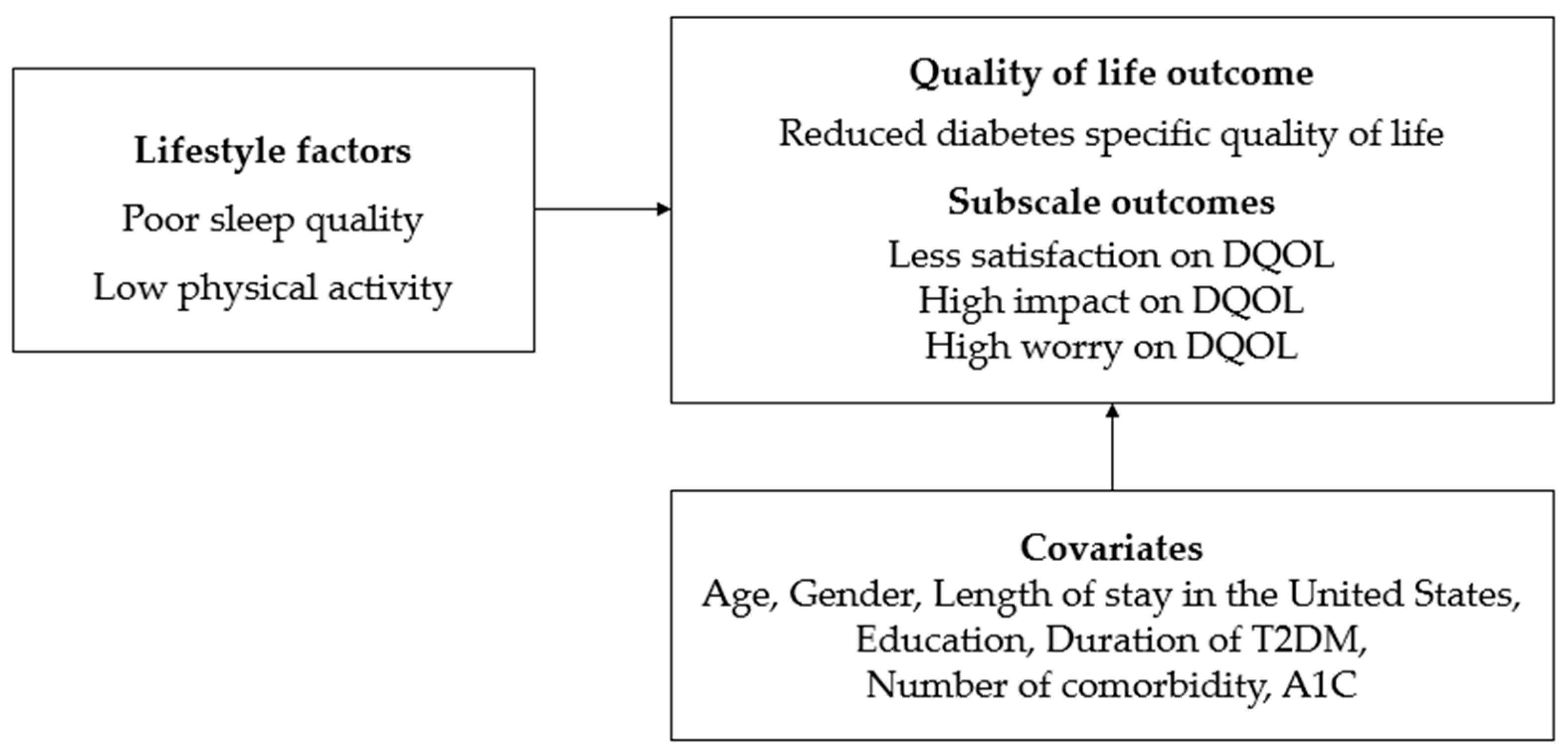
1. Introduction
- Lower satisfaction with diabetes management was anticipated for participants experiencing poor sleep quality or low physical activity.
- The perceived negative impact of diabetes on daily life was expected to be greater for those with poor sleep quality or low physical activity.
- Increased worry about diabetes was anticipated for those with poor sleep quality or low physical activity.
2. Methods
2.1. Participants
2.2. Data Collection
2.3. Measures
2.4. Analysis
3. Results
3.1. Descriptive Analyses of the Participants
3.2. Correlations of Sleep Quality and Physical Activity on Diabetes Quality of Life and Subscales
3.3. Relationships of Sleep Quality and Physical Activity with Diabetes Quality of Life and Subscales
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| DQOL | Diabetes Quality of Life |
| Kas | Korean Americans |
| PSQI | Pittsburgh Sleep Quality Index |
| IPAQ | International Physical Activity Questionnaire |
| SD | Standard Deviation |
| A1C | Glycosylated Hemoglobin |
| IQR | InterQuartile Range |
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Hilliard, M.E.; Isaacs, D. 10. Cardiovascular disease and risk management: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S158–S190. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L. 3. Prevention or delay of type 2 diabetes and associated comorbidities: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S41. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S. Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. IDF Diabetes Atlas. Diabetes Res. Clin. Pract. 2022, 183, 109–119. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau: QuickFacts: United States. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045219 (accessed on 1 November 2023).
- Choi, S.E.; Liu, M.; Palaniappan, L.P.; Wang, E.J.; Wong, N.D. Gender and ethnic differences in the prevalence of type 2 diabetes among Asian subgroups in California. J. Diabetes Complicat. 2013, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Echeverria, S.; Mayer, V.; Janevic, T. Diabetes risk and control in multi-ethnic US immigrant populations. Curr. Diabetes Rep. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Yang, K.; Lee, J.; Min, J.; Kim, K.H.; Dunbar, S.B.; Jennings, B.M. Understanding cultural issues in the diabetes self-management behaviors of Korean immigrants. Diabetes Educ. 2012, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Rush, E.; Henry, S. Health literacy in Korean immigrants at risk for type 2 diabetes. J. Immigr. Minor. Health 2013, 15, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.E. The “A to Z” of managing type 2 diabetes in culturally diverse populations. Front. Endocrinol. 2018, 9, 479. [Google Scholar] [CrossRef]
- Watkins, K.W.; Connell, C.M.; Fitzgerald, J.T.; Klem, L.; Hickey, T.; Ingersoll-Dayton, B. Effect of adults’ self-regulation of diabetes on quality-of-life outcomes. Diabetes Care 2000, 23, 1511–1515. [Google Scholar] [CrossRef]
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120. [Google Scholar] [CrossRef]
- Kumari, N.; Prakash, V.; Roy, S.S.; Kumar, M.; Mishra, H.; Dikshit, H. Impact of SARS-CoV-2 pandemic on Glycaemic control, metabolic status, treatment adherence, quality of life in diabetes mellitus patients in tertiary Care Hospital of Eastern India. Maedica 2022, 17, 88. [Google Scholar] [PubMed]
- John, R.; Pise, S.; Chaudhari, L.; Deshpande, P.R. Evaluation of quality of life in type 2 diabetes mellitus patients using quality of life instrument for indian diabetic patients: A cross-sectional study. J. Mid-Life Health 2019, 10, 81. [Google Scholar]
- Alshayban, D.; Joseph, R. Health-related quality of life among patients with type 2 diabetes mellitus in Eastern Province, Saudi Arabia: A cross-sectional study. PLoS ONE 2020, 15, e0227573. [Google Scholar] [CrossRef] [PubMed]
- Laverty, B.; Puthezhath Jayanandan, S.; Smyth, S. Understanding the relationship between sleep and quality of life in type 2 diabetes: A systematic review of the literature. J. Heal. Psychol. 2023, 28, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Qin, Y.; Zhang, P.; Chen, P.; Zhang, L.; Chang, G.; Li, T.; Qiao, C.; Zhang, N. Association of sleep quality and quality of life in type 2 diabetes mellitus: A cross-sectional study in China. Diabetes Res. Clin. Pract. 2015, 107, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Luyster, F.S.; Dunbar-Jacob, J. Sleep quality and quality of life in adults with type 2 diabetes. Diabetes Educ. 2011, 37, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Eckert, K. Impact of physical activity and bodyweight on health-related quality of life in people with type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.M.; Al Sayah, F.; Vallance, J.K.; Johnson, S.T.; Johnson, J.A. Association between physical activity and health-related quality of life in adults with type 2 diabetes. Can. J. Diabetes 2017, 41, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, G.; Zhang, P.; Xu, D.; Chen, L. Effect of exercise on the quality of life in type 2 diabetes mellitus: A systematic review. Qual. Life Res. 2017, 26, 515–530. [Google Scholar] [CrossRef]
- St John, A.; Davis, T.M.; Goodall, I.; Townsend, M.A.; Price, C.P. Nurse-based evaluation of point-of-care assays for glycated haemoglobin. Clin. Chim. Acta 2006, 365, 257–263. [Google Scholar] [CrossRef]
- Backhaus, J.; Niemann, T.; Hohagen, F.; Riemann, D.; Junghanns, K. Testretest reliability of the Pittsburgh Sleep Quality Index (PSQI) in patients with primary insomnia. World J. Biol. Psychia 2001, 2, 374–377. [Google Scholar]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- IPAQ Research Committee Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005. Available online: https://biobank.ndph.ox.ac.uk/showcase/refer.cgi?id=540 (accessed on 1 November 2023).
- DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988, 11, 725–732. [CrossRef] [PubMed]
- Choi, S.E.; Reed, P.L.; Sarkisian, C.A. Gender differences in the relationship between diabetes-specific quality of life and depressive symptoms in middle-aged and older Korean immigrants. Res. Gerontol. Nurs. 2013, 6, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.M.; Groot, M.D.; Samson, J.A. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994, 17, 267–274. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.K.; Bakomitrou, F.; Trikallinou, A.; Ganotopoulou, A.; Verras, C.; Christofilidis, G.; Bousboulas, S.; Μelidonis, A. Diabetes-dependent quality of life (ADDQOL) and affecting factors in patients with diabetes mellitus type 2 in Greece. BMC Res. Notes 2015, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Shibraumalisi, N.A.; Mat Nasir, N.; Md Yasin, M.; Isa, M.R. The Association Between Health Literacy and Quality of Life and Its Associated Factors Among Adults with Type 2 Diabetes Mellitus in Public Primary Care Clinic. J. Clin. Health Sci. 2020, 5, 60–74. [Google Scholar] [CrossRef]
- Jackson, I.L.; Onung, S.I.; Oiwoh, E.P. Self-care activities, glycaemic control and health-related quality of life of patients with type 2 diabetes in a tertiary hospital in Nigeria. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 137–143. [Google Scholar] [CrossRef]
- Gebremariam, G.T.; Biratu, S.; Alemayehu, M.; Welie, A.G.; Beyene, K.; Sander, B.; Gebretekle, G.B. Health-related quality of life of patients with type 2 diabetes mellitus at a tertiary care hospital in Ethiopia. PLoS ONE 2022, 17, e0264199. [Google Scholar] [CrossRef]
- Redekop, W.K.; Koopmanschap, M.A.; Stolk, R.P.; Rutten, G.E.; Wolffenbuttel, B.H.; Niessen, L.W. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care 2002, 25, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, J.; Jing, Z.; Yu, C.; Zhao, D.; Hao, W.; Zhou, C. The role of mental health and physical activity in the association between sleep quality and quality of life among rural elderly in China: A moderated mediation model. J. Affect. Disord. 2020, 273, 462–467. [Google Scholar] [CrossRef]
- Hu, J.; Wallace, D.C.; Tesh, A.S. Physical activity, obesity, nutritional health and quality of life in low-income hispanic adults with diabetes. J. Community Health Nurs. 2010, 27, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.F.; Ogwumike, O.O.; Oguntola, D.A.; Adeleye, J.O. Interrelationship among physical activity, quality of life, clinical and sociodemographic characteristics in a sample of Nigerian patients with type 2 diabetes. Afr. J. Physiother. Rehabil. Sci. 2015, 7, 12–18. [Google Scholar] [CrossRef]
- Seligowski, A.V.; Pless Kaiser, A.P.; Niles, B.L.; Mori, D.L.; King, L.A.; King, D.W. Sleep quality as a potential mediator between psychological distress and diabetes quality of life in veterans with type 2 diabetes. J. Clin. Psychol. 2013, 69, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.P.; Ehlers, D.K.; Salerno, E.A.; Fanning, J.; Kramer, A.F.; McAuley, E. Physical activity, sleep and quality of life in older adults: Influence of physical, mental and social well-being. Behav. Sleep Med. 2020, 18, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.S.; Brown, M.B.; Funnell, M.M.; Anderson, R.M. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. Diabetes Educ. 2008, 34, 266–276. [Google Scholar] [CrossRef]
- Tang, J.; Yang, Q.; Li, X.; Wu, W.; Niu, D.; Ding, P.; Liu, Z.; Xu, W.; Xu, L. Factors influencing quality of life in elderly patients with type 2 diabetes in community. Chin. J. Gen. Pract. 2018, 17, 848–850. [Google Scholar]

| Variables | Frequency (%) | Mean ± SD | Actual Range |
|---|---|---|---|
| Gender | |||
| Men | 37 (31.1) | ||
| Women | 82 (68.9) | ||
| Age (years) | 67.00 ± 9.68 | 43–85 | |
| Length of stay in the US (years) | 33.72 ± 10.45 | 7–54 | |
| Education | |||
| ≤High school | 62 (52.1) | ||
| >High school | 57 (47.9) | ||
| Employment | |||
| Employed | 40 (33.6) | ||
| Unemployed | 79 (66.4) | ||
| Living | |||
| With partner | 84 (70.6) | ||
| Without partner | 35 (29.4) | ||
| Duration of T2DM (years) | 9.84 ± 9.65 | 1–42 | |
| Use of insulin | |||
| Yes | 10 (8.4) | ||
| No | 109 (91.6) | ||
| Number of comorbidities | |||
| 0 | 17 (14.3) | ||
| 1 | 28 (23.5) | ||
| 2 | 44 (37.0) | ||
| 3 or more | 30 (25.2) | ||
| A1C (%) | 7.00 ± 1.09 | 5.4–10.4 |
| Variables | Frequency (%) | Mean ± SD | Actual Range |
|---|---|---|---|
| Sleep quality (PSQI) | 7.55 ± 3.67 | 1–17 | |
| Poor sleep (PSQI > 5) | 79 (66.4) | ||
| Good sleep (PSQI ≤ 5) | 40 (33.6) | ||
| Physical activity (IPAQ: hour/week), median (IQR) | 15.00 (6.83–38.80) | 0–104.70 | |
| Low activity | 40 (33.6) | ||
| Moderate activity | 55 (46.2) | ||
| High activity | 24 (20.2) | ||
| Diabetes Quality of Life: DQOL | 1.85 ± 0.28 | 1.28–2.83 | |
| Satisfaction | 2.80 ± 0.47 | 1.33–3.80 | |
| Impact | 1.51 ± 0.39 | 1.00–3.30 | |
| Worry | 1.16 ± 0.22 | 1.00–2.36 |
| Variables | DQOL | Subscales of DQOL | ||
|---|---|---|---|---|
| Satisfaction | Impact | Worry | ||
| Sleep quality | 0.32 *** | 0.20 * | 0.34 *** | 0.01 |
| Low physical activity | 0.34 ** | 0.29 ** | 0.30 *** | 0.06 |
| High physical activity | −0.24 ** | −0.15 | −0.25 ** | −0.06 |
| DQOL | Subscales of DQOL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Satisfaction | Impact | Worry | ||||||||||
| β | S.E. | p | β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Model 1 | ||||||||||||
| Sleep quality | 0.26 | 0.006 | 0.002 | 0.15 | 0.011 | 0.097 | 0.29 | 0.001 | 0.000 | −0.01 | 0.001 | 0.943 |
| Physical activity | ||||||||||||
| Low activity | 0.25 | 0.053 | 0.007 | 0.25 | 0.094 | 0.011 | 0.19 | 0.012 | 0.034 | 0.05 | 0.009 | 0.612 |
| Moderate activity | 1 | 1 | 1 | 1 | ||||||||
| High activity | −0.12 | 0.062 | 0.170 | −0.05 | 0.110 | 0.589 | −0.15 | 0.014 | 0.090 | −0.04 | 0.010 | 0.691 |
| Model 2 | ||||||||||||
| Sleep quality | 0.24 | 0.006 | 0.004 | 0.14 | 0.011 | 0.112 | 0.25 | 0.001 | 0.004 | 0.05 | 0.001 | 0.562 |
| Physical activity | ||||||||||||
| Low activity | 0.18 | 0.052 | 0.043 | 0.22 | 0.092 | 0.023 | 0.13 | 0.012 | 0.174 | −0.01 | 0.008 | 0.913 |
| Moderate activity | 1 | 1 | 1 | 1 | ||||||||
| High activity | −0.10 | 0.057 | 0.242 | −0.04 | 0.100 | 0.670 | −0.13 | 0.013 | 0.157 | −0.02 | 0.009 | 0.801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, M. Associations of Sleep Quality and Physical Activity with Diabetes Quality of Life in Korean Americans with Type 2 Diabetes: A Cross-Sectional Study. Healthcare 2024, 12, 756. https://doi.org/10.3390/healthcare12070756
Jeong M. Associations of Sleep Quality and Physical Activity with Diabetes Quality of Life in Korean Americans with Type 2 Diabetes: A Cross-Sectional Study. Healthcare. 2024; 12(7):756. https://doi.org/10.3390/healthcare12070756
Chicago/Turabian StyleJeong, Mihyun. 2024. "Associations of Sleep Quality and Physical Activity with Diabetes Quality of Life in Korean Americans with Type 2 Diabetes: A Cross-Sectional Study" Healthcare 12, no. 7: 756. https://doi.org/10.3390/healthcare12070756
APA StyleJeong, M. (2024). Associations of Sleep Quality and Physical Activity with Diabetes Quality of Life in Korean Americans with Type 2 Diabetes: A Cross-Sectional Study. Healthcare, 12(7), 756. https://doi.org/10.3390/healthcare12070756






