Abstract
mHealth has been utilized in the care of patients with chronic kidney disease, allowing the collection of patient health-related data, offering disease-related information, enabling the tracking and recording of biochemical parameters, and enabling communication with healthcare providers in real time through applications. mHealth may improve the health outcomes in patients with peritoneal dialysis. This systematic review aimed to summarize evidence regarding the functionality and usability of mHealth apps in patients with peritoneal dialysis. We conducted a comprehensive literature review, searching in five databases, including CINAHL, Cochrane, PsycINFO, PubMed, and Web of Science, to retrieve titles and abstracts related to peritoneal dialysis and mHealth applications for PRISMA recommendations from January 2013 to December 2023. Overall, 11 studies met all the inclusion criteria. The functionality of mHealth apps included inform, instruct, record, display, guide, remind/alert, and communicate. Most of the apps have multifunctionality. The usability was categorized into three aspects: efficiency (self-efficacy and usability), satisfaction, and effectiveness (underwent kidney transplantation and switched to hemodialysis, rehospitalization, peritonitis rate, infection rates at exit sites, mortality, fluid overload, inadequate solute clearance, biochemical values, quality of life, consumer quality index, and technology readiness). Generally, outcomes in the intervention group had better effects compared to those in the control group. Multifunctional mHealth apps show a good potential in improving the efficiency, satisfaction, and effectiveness for patients compared to traditional care. Future research should include more studies and participants to explore and verify the long-term effectiveness of mHealth apps.
1. Introduction
Unlike hemodialysis (HD), peritoneal dialysis (PD) does not require patients to visit hospitals for dialysis two to three times a week, allowing them to maintain their regular work and social activities, preserving their independence and freedom []. Additionally, the cost of hemodialysis was 1.37 times compared to peritoneal dialysis in Taiwan, and opting for PD can reduce healthcare costs. Despite these advantages, most patients continue to prefer HD []. The reluctance toward PD may stem from the fact that it involves self-administration or caregiver assistance, requiring patients to manage the dialysis process, including attaching the dialysis bag and manually recording related data on paper [], which can be challenging for some patients.
While patients can communicate with healthcare providers (HCP) over the phone to address any issues during daytime dialysis [], seeking advice from them at night may pose problems due to their unavailability. Furthermore, for patients without a medical background, HCP must impart additional disease-related knowledge and provide opportunities for them to practice dialysis techniques. The lack of a medical background and sudden complications can cause patients to feel overwhelmed, resulting in them choosing HD over PD []. Mobile health (mHealth) applications (apps) have the potential to increase patients’ willingness to adopt PD. These apps can record PD conditions, enhance patients’ self-practice of PD techniques at home, alleviate concerns about unmanageable health conditions, help patients with chronic kidney diseases understand their condition, strengthen disease self-management [,,], facilitate two-way communication and data sharing between patients and HCP, assist patients in gaining necessary knowledge and skills, and support patients in problem solving and decision making [,,,,], ultimately increasing patients’ sense of security and satisfaction.
mHealth provides healthcare services through portable devices such as smartphones, tablets, computers, and personal digital assistants [,]. mHealth delivers pre-established health services and provides guidance regarding the PD procedure and disease-related information in real time through features such as browsing, text messaging, communication, and videos [,,]. mHealth apps enable the collection of patient health-related data, including access to personal medical records, obtaining nutritional or dietary information, receiving treatment-related details, offering disease-related information, understanding health status, tracking exercise activities, weight, and dietary habits, monitoring medication dosage, recording blood pressure, and providing convenience and efficiency for patients with PD [,,,,,,,,]. Additionally, some mHealth apps have interactive features that allow patients to communicate immediately with physicians or nurses when difficulties arise []. Upon identifying problems in patient data transmissions, HCP can proactively contact the patients. These features contribute to patients feeling reassured and maintaining their independence [].
However, concerns regarding issues such as unsuitable design, data reliability [,], inadequate user technological literacy, and privacy and security [,] may affect patients’ willingness to use mHealth services, and the efficacy of such services remains unclear. If mHealth apps lack a scientific basis, certification, or regulatory requirements, they may be unsuitable or potentially harmful for patients []. A systematic literature review of the effectiveness of eHealth for patients with PD showed inconsistent results regarding infection rates, hospitalization outcomes, knowledge, skills, satisfaction, and quality of life []. However, van Eck van der Sluijs et al. (2021) suggested that eHealth can reduce the incidence of peritonitis []. Therefore, to determine the overall impact of mHealth apps on patients with PD, a comprehensive analysis through systematic evaluation is necessary. Thus, this study aimed to conduct a systematic review to explore the features, functions, and usability of mHealth apps used by patients with PD.
In terms of usage, each mobile app emphasizes different things, making it challenging to establish a standard for systematic evaluation []. To clarify the scope of assistance provided by mHealth apps for patients with PD, this study focused on the usability and functionality of mobile apps. Regarding functionality, we referred to the seven functions proposed by the Institute for Healthcare Informatics (IMS) for mHealth apps: inform, instruct, record, display, guide, remind/alert, and communicate []. These functions have been widely evaluated in mHealth apps, such as those for asthma [], chronic kidney diseases [], and heart failure []. In terms of usability assessment, Harrison et al. (2013) [] and Weichbroth (2020) [] advocated that an ideal mHealth app should be simple and user-friendly. According to Weichbroth (2020), an app’s usability can be assessed by exploring three aspects: efficiency, satisfaction, and effectiveness []. Therefore, in this study, we explored the aforementioned aspects in order to evaluate and analyze the usability of mHealth apps.
mHealth apps have been widely used to enhance the care of patients with PD. However, whether they effectively improve health requires a comprehensive and concrete analysis. Therefore, this systematic review aimed to investigate studies on the use of mHealth apps in patients with PD regarding the following aspects: (1) characteristics, (2) functionalities, and (3) usability.
2. Materials and Methods
This systematic review was reported in accordance with PRISMA guidelines, and was conducted by two reviewers following the systematic review and meta-analysis expansion methodology [,]. This review aimed to describe literature retrieval and study selection using the PRISMA checklist for reporting systematic reviews. In cases of inconsistencies in the results, a third reviewer was consulted. This review involved mapping the entirety of the relevant literature to identify and map features, functionalities, and usability related to mHealth apps for PD. The findings contribute not only to identifying empirical patterns and resources for clinical practice but also to recognizing the existing limitations of apps for future enhancement.
2.1. Search Strategy
Between January 2013 and December 2023, five databases—CINAHL, Cochrane, PsycINFO, PubMed, and Web of Science—were searched to retrieve titles and abstracts related to PD and mHealth apps. The search strategy incorporated search strings such as “mHealth”, “mobile health”, “mobile app”, “mobile application”, “smartphone application”, “app”, “apps”, “telemedicine”, “peritoneal”, “dialysis”, and “peritoneal dialysis” by Boolean operators. The following criteria were applied for selecting research articles: (1) full text; (2) English language; (3) studies classified according to Joanna Briggs Institute (JBI) levels of evidence, including experimental designs, quasi-experimental designs, observational–analytic designs, observational–descriptive studies, expert opinion, and bench research []; and (4) the exclusion of study protocols, scoping literature, literature reviews, systematic reviews, integrative analysis reviews, conference abstracts, letters to the editor, and editorials and similar articles.
2.2. Inclusion and Exclusion Criteria
A review of the use of mHealth apps in PD was conducted. The inclusion criteria were as follows: (1) apps targeted at patients with PD; (2) mobile app interventions delivered through smartphones, tablets, or web portals accessible via mobile devices; (3) apps aimed at enhancing patient care and the management of the disease. The exclusion criteria were as follows: (1) eHealth remote healthcare, electronic health records, and electronic medical records; and (2) designed and developed without adoption in patients.
2.3. Screening and Data Extraction
Title, abstract, and full-text screening was independently conducted by two reviewers (CS and FY). All identified articles were uploaded to EndNote 20, and duplicate articles were removed. The two reviewers used three predesigned forms to extract data from the eligible articles. The first form gathered the following information: author/year/country, research objectives, app name/device/platform/objectives, design/sample size/duration, intervention content, and outcome variables/results. The second form assessed the functionality of mHealth apps by utilizing the seven functions proposed by IMS: inform, instruct, record, display, guide, remind/alert, and communicate. The third form focused on evaluating the apps’ usability by exploring the aspects of efficiency, satisfaction, and effectiveness. The extracted data were synthesized and analyzed using the three aforementioned forms.
2.4. Selection Process
A total of 184 articles were extracted from the five databases. After removing duplicates (n = 10) as well as abstracts and articles that did not meet the inclusion criteria, 152 articles were excluded, leaving 22 articles for consideration. Two independent reviewers conducted the screening based on the inclusion and exclusion criteria and resolved any discrepancies through discussions. Based on the inclusion and exclusion criteria, 11 articles were subsequently excluded, resulting in a final set of 11 articles for the systematic review. The search results and study inclusion process are presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart (Figure 1).
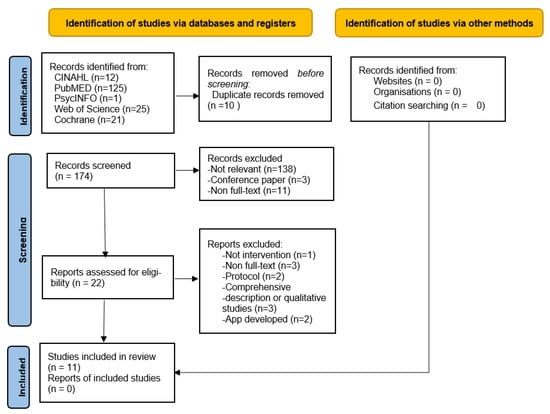
Figure 1.
Flow diagram of study selection.
2.5. Functionality Assessment
The app’s functionality was evaluated using the guidelines developed by the IMS [] as follows: (1) inform: provide information in the form of text, photos, videos, etc.; (2) instruct: offer instructional guidance; (3) record: gather physiological data and other relevant patient information; (4) display: show data entered by users and recipients in a graphical format; (5) guide: analyze user-inputted data and suggest relevant treatments; (6) remind/alert: send reminders or alerts to notify patients; (7) communicate: patients interact with HCP, peers, or community forums. These functionalities serve various purposes and enhance the overall capabilities of mHealth apps.
2.6. Usability Assessment
The usability of mHealth apps can be assessed by exploring their efficiency, satisfaction, and effectiveness [], as follows. (1) Efficiency refers to the user’s ability to achieve goals quickly and accurately as well as the error rate during use. (2) Satisfaction relates to the user’s comfort and happiness, as well as meeting expectations and fulfilling needs. (3) Effectiveness refers to the ability to accomplish tasks within a certain time frame. The two reviewers categorized the research results based on the aforementioned definitions of the components of usability. In cases of any inconsistencies in the classification, a third reviewer reassessed the cases until a consensus was reached. This systematic approach ensured a thorough and standardized evaluation of the usability of mHealth apps.
3. Results
3.1. Characteristics of the Included Studies
Eleven articles published between 2013 and 2023 were included in the final review. Table 1 presents the characteristics of each mHealth app. Three studies were conducted in Mexico [,,], two in Canada [,], two in China [,], and one each in Korea [], the United Kingdom [], Thailand [], and Italy [] (Table 1). The study designs varied, with three using experimental designs (randomized controlled trials (RCTs) [,,], six using observational–analytic designs [,,,,,], and two being observational–descriptive studies [,]. mHealth apps were used on various devices: 27.2% (3/11) on smartphones, 18.2% (2/11) on tablets, 9.1% (1/11) on computers, and 72.7% (8/11) on unspecified devices. In terms of operating systems, 45.5% (5/11) were Android-based, 36.4% (4/11) were iOS-based, 36.4% (4/11) were Internet-based, and 45.5% (5/11) were unspecified. The measurement durations of these studies ranged from 10 weeks to 285 months. The interrater agreement was analyzed to assess interrater reliability among reviewers, and values ranged from 0.95 to 1, indicating excellent interrater reliability.

Table 1.
Characteristics of the included studies on patients with peritoneal dialysis (PD).
3.2. Interventions Description
Regarding the functionality of mHealth apps, each app included “inform”, “instruct”, and “communicate” features with a 100% inclusion rate (11/11). Regarding other features, “display” was in 90.9% (10/11) of the apps, “guide” in 90.9% (10/11), “record” in 81.8% (9/11), and “remind/alert”—the least common feature—in 72.7% (8/11) of the apps (Table 2). The intervention content of these apps included disease-related information. Patients could record and transmit details such as the fluid volume during dialysis or the condition of dialysis wounds. Additionally, in the case of symptoms or problems during dialysis, patients could communicate and interact with HCP or peers. The interrater agreement was analyzed to assess interrater reliability among reviewers, whose values ranged from 0.95 to 1, indicating excellent interrater reliability.

Table 2.
IMS Functionality of mHealth apps for patients with peritoneal dialysis.
3.3. Outcomes
The outcomes were categorized into three main types: efficiency, satisfaction, and effectiveness (Table 3). In one study that investigated PD-related self-efficacy, no statistically significant differences were found []. Two studies that explored usability drew conflicting conclusions, with one study reporting positive [] and the other negative usability []. All five studies that assessed satisfaction reported positive outcomes [,,,,]. For acceptance, which was studied in one study, positive results were reported []. Regarding effectiveness, a lesser number of patients from the intervention group switched from PD to HD or kidney transplantation compared with those from the control group []; however, three studies reported no significant differences [,,]. In two studies, re-hospitalization rates showed no significant differences [,]. The peritonitis occurrence rates showed no significant differences in the two studies [,]. Infection rates at the exit site and mortality rates were examined in two studies, with one showing better outcomes in relation to fluid overload and inadequate solute clearance in the experimental group [], and the other reporting no significant differences []. Inconsistent results for biochemical parameters were found across studies. Serum albumin and hemoglobin levels were higher in the experimental group in two studies [,]. However, changes in serum calcium levels did not differ significantly in two studies [,]. Mixed results were obtained for serum phosphorus levels, with two studies reporting no significant differences [,] and one showing better outcomes in the experimental group []. While no significant differences were found in the changes in serum potassium levels in a study [], the calcium-phosphorus level was better in the experimental group in another study []. Taking calcium carbonate yielded no significant differences []. PD-related knowledge and health behaviors were better in the experimental group in one study []. Quality of life results varied, with one study showing better outcomes in the experimental group [] and two studies reporting no significant differences [,]. No significant differences were observed for the consumer quality index [] and technology readiness [].

Table 3.
Usability of mHealth apps for patients with peritoneal dialysis.
4. Discussion
In this study, a systematic review was conducted to identify and map the existing evidence regarding the features, functionalities, and usability of mHealth apps for patients with PD. The findings reveal that the apps primarily focused on self-efficacy in performing PD procedures, user satisfaction, and monitoring and improving health conditions. Regarding functionality, mHealth apps mostly encompassed features listed by the IMS. Regarding usability, the findings are similar to those found in Cartwright et al.’s (2021) systematic review on eHealth []; however, the results pertaining to the outcomes are inconsistent.
The most successful feature for mHealth apps should be multifunctionality []. In this systematic review, most apps were found to have multiple functions, such as record, display, guide, remind/alert, communicate [,], and facilitating remote monitoring []. They allow patients to upload data, such as fluid volumes during the PD process, enabling communication and interaction between HCP and patients to ensure treatment outcome. HCP can also track patient treatment results []. These results are consistent with those of Eberle et al. (2021) who found that apps improved laboratory data and facilitated health promotion []. Moreover, these apps provide disease-related information for patients with PD, resulting in increased user satisfaction.
The usability of an app can be assessed by exploring three aspects: efficiency, satisfaction, and effectiveness. Some results obtained in this review seem to differ from those in previous studies. In terms of efficiency, the apps did not seem to enhance patients’ self-efficacy. These results are inconsistent with the findings of Eberle et al. (2021), who indicated that patients with diabetes who used mHealth apps strengthened their self-efficacy []. This inconsistency may be attributed to the means of communicating or interacting with HCP, which are limited to phone calls, text messages, or monthly interviews, contributing to patients feeling less confident about dealing with disease-related situations. This could have influenced their lack of self-efficacy []. Furthermore, there was only one study on self-efficacy, and the intervention duration was only ten weeks; therefore, confirming whether apps can enhance self-efficacy is difficult. Similar findings were observed regarding usability. Lukkanalikitkul et al. (2022) believed that poor usability is due to unsuitable software development and limited Internet speeds, which do not meet user needs []. However, Olivares-Gandy et al. (2019), who focused on the app-development process, including finding shortcomings for improvement and allowing for timely adjustments to the app based on patient needs, found the usability to be effective [].
All study findings reported patient satisfaction with app usage [,,,,]. The primary advantage of mHealth apps is that they are not bound by time or space constraints, allowing users to access disease-related information, record personal data (e.g., blood pressure, weight, and PD fluid), and monitor their health status at any time. Patients instantly communicated with HCP on encountering any problems or difficulties. Patient satisfaction significantly improved if the app features were comprehensive, practical, user-friendly, and suitable for patients [,].
The effectiveness of apps in improving biochemical parameters revealed inconsistencies; while Eberle et al. (2021) showed some improvements in patients with diabetes, no statistically significant differences were found in others []. Continuous monitoring of nutritional management can help maintain normal hemoglobin and albumin levels in patients []. Learning fatigue, resulting in a low willingness to use apps, may occur when patients need to learn how to use apps correctly, which could be a contributing factor to the lack of improvement in physiological health indicators. In addition, the mHealth apps’ design not aligning with the needs of patients and being difficult to operate may contribute to increased frustration and discouragement among patients. It could lead to no ideally effective outcomes []. However, in a four-year study about the usage of a PD app, Xu et al. (2022) found improvements in all physiological indicators, for example cause-specific mortality and all-cause and cause-specific permanent transfer to hemodialysis, suggesting that the consistent and long-term use of apps to record daily life activities may yield effective outcomes [].
App usage can enhance patients’ quality of life, particularly in areas where the symptoms and disease impact daily living [,]. However, no significant differences were observed in users’ physiological and psychological domains of quality of life. Kiberd et al. (2018) indicated that the lower use of apps might be due to a high rate of patient dropout []. Farfan-Ruiz et al. (2021) indicated that improvements in users’ technological readiness might be associated with gender, educational level, and race [].
5. Conclusions
Although the functional features of mHealth apps for patients with PD are mostly complete, their effectiveness varies. This inconsistency may be due to the fact that only three RCT studies were included in this review, of which two study samples, both in the experimental and control groups, comprised fewer than 30 participants, which might have led to poor-quality evidence for intervention outcomes. Comparing the usability of apps between the experimental and control groups, some studies showed no significant differences in effectiveness, whereas others did. Health outcomes with mobile apps may be better than or equivalent to those of traditional healthcare. Future research should include more studies with larger sample sizes to explore and verify the long-term effectiveness of mHealth apps. In this systematic review, user satisfaction with apps was found to be generally high. Thus, using these apps in the care of patients with PD to promote the self-management of diseases and enhance health outcomes is recommended.
This study adhered to a systematic literature review approach by conducting a thorough and comprehensive search of the most common academic literature databases. Although the study findings suggest potential progress in the health of patients with PD as a result of using mHealth apps, this study has some limitations. First, out of the eleven studies, only three studies were RCTs. This may have resulted in weak evidence for the study outcomes. However, owing to the limited research on mHealth apps, researchers have considered various study designs to broaden the scope of the systematic review. Nevertheless, this approach may introduce selection bias, and the overall quality of the evidence may be low. Second, grey literature, non-full text articles, non-English publications, and conference reports in the field were excluded from this review. This omission may have led to valuable research in this field being excluded; therefore, the results should be interpreted cautiously. Third, this review only included studies published within the last 10 years, potentially overlooking valuable research published earlier. Finally, studies from different countries and regions were within the scope of the review, making it challenging to generalize the findings.
The ideal mHealth apps should have the functionality to record and monitor patients’ physiological conditions and provide reminders or alerts regarding abnormal situations to patients and HCP. More importantly, they must feature interactive functions with HCP to increase patients’ willingness to use them. Additionally, designing user-friendly software from the perspective of the patients is crucial. Only through user-friendly design can the mHealth apps fully function as an auxiliary tool for promoting healthcare.
Author Contributions
S.-M.C. and Y.-W.F.—Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Original draft preparation, Visualization, Supervision, and Project administration. S.-M.C. and S.-F.C.—Visualization, Supervision, Project administration. Validation, Investigation, and Data Curation. M.-L.W.—Software, Validation, and Formal analysis. S.-F.C.—Investigation. C.-K.P.—Data curation, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Science and Technology Council, Taiwan: NSTC 111-2314-B227-001 and Tzu Chi University of Science and Technology, Taiwan: TCCT-1111A26.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the first author.
Acknowledgments
The authors wish to acknowledge the National Science and Technology Council, Taiwan and Tzu Chi University of Science and Technology, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baumgart, A.; Manera, K.; Johnson, D.W.; Craig, J.C.; Shen, J.; Ruiz, L.; Wang, A.Y.-M.; Yip, T.; Fung, S.K.S.; Tong, M.; et al. Meaning of empowerment in peritoneal dialysis: Focus groups with patients and caregivers. Nephrol. Dial. Transplant. 2020, 35, 1949–1958. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, H.H.; Wu, M.J.; Hsu, B.G.; Tsai, J.C.; Kuo, C.C.; Lin, S.P.; Chen, T.H.; Sue, Y.M. Out-of-pocket costs and productivity losses in hemodialysis and peritoneal dialysis from a patient interview survey in Taiwan. BMJ Open 2019, 9, e023062. [Google Scholar] [CrossRef]
- Talbot, B.; Farnbach, S.; Tong, A.; Chadban, S.; Sen, S.; Garvey, V.; Gallagher, M.; Knight, J. Patient and Clinician Perspectives on the use of Remote Patient Monitoring in Peritoneal Dialysis. Can. J. Kidney Health Dis. 2022, 9, 20543581221084499. [Google Scholar] [CrossRef]
- Biebuyck, G.K.M.; Neradova, A.; de Fijter, C.W.H.; Jakulj, L. Impact of telehealth interventions added to peritoneal dialysis-care: A systematic review. BMC Nephrol. 2022, 23, 292. [Google Scholar] [CrossRef]
- He, T.; Liu, X.; Li, Y.; Wu, Q.; Liu, M.; Yuan, H. Remote home management for chronic kidney disease: A systematic review. J. Telemed. Telecare 2016, 23, 3–13. [Google Scholar] [CrossRef]
- Markossian, T.W.; Boyda, J.; Taylor, J.; Etingen, B.; Modave, F.; Price, R.; Kramer, H.J. A Mobile app to support self-management of chronic kidney disease: Development study. JMIR Hum. Factors 2021, 8, e29197. [Google Scholar] [CrossRef]
- Lukkanalikitkul, E.; Kongpetch, S.; Chotmongkol, W.; Morley, M.G.; Anutrakulchai, S.; Srichan, C.; Thinkhamrop, B.; Chunghom, T.; Wiangnon, P.; Thinkhamrop, W.; et al. Optimization of the Chronic Kidney Disease–Peritoneal Dialysis App to Improve Care for Patients on Peritoneal Dialysis in Northeast Thailand: User-Centered Design Study. JMIR Form. Res. 2022, 6, e37291. [Google Scholar] [CrossRef]
- Cartwright, E.J.; Goh, Z.Z.; Foo, M.; Chan, C.M.; Htay, H.; Griva, K. eHealth interventions to support patients in delivering and managing peritoneal dialysis at home: A systematic review. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2020, 41, 32–41. [Google Scholar] [CrossRef]
- Schrauben, S.J.; Appel, L.; Rivera, E.; Lora, C.M.; Lash, J.P.; Chen, J.; Hamm, L.L.; Fink, J.C.; Go, A.S.; Townsend, R.R.; et al. Mobile health (mHealth) technology: Assessment of availability, acceptability, and use in CKD. Am. J. Kidney Dis. 2021, 77, 941–950.e1. [Google Scholar] [CrossRef]
- Cao, F.; Li, L.; Lin, M.; Lin, Q.; Ruan, Y.; Hong, F. Application of instant messaging software in the follow-up of patients using peritoneal dialysis, a randomized controlled trial. J. Clin. Nurs. 2018, 27, 3001–3007. [Google Scholar] [CrossRef]
- Dey, V.; Jones, A.; Spalding, E.M. Telehealth: Acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med. 2016, 4, 2050312116670188. [Google Scholar] [CrossRef]
- Moss, R.J.; Süle, A.; Kohl, S. eHealth and mHealth. Eur. J. Hosp. Pharm. 2019, 26, 57–58. [Google Scholar] [CrossRef]
- Lewis, R.A.; Lunney, M.; Chong, C.; Tonelli, M. Identifying Mobile Applications Aimed at Self-Management in People With Chronic Kidney Disease. Can. J. Kidney Health Dis. 2019, 6, 2054358119834283. [Google Scholar] [CrossRef]
- Quach, S.; Benoit, A.; Oliveira, A.; Packham, T.L.; Goldstein, R.; Brooks, D. Features and characteristics of publicly available mHealth apps for self-management in chronic obstructive pulmonary disease. Digit. Health 2023, 9, 20552076231167007. [Google Scholar] [CrossRef]
- World Health Organization. M-Health Use of Appropriate Digital Technologies for Public Health; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Sluijs, A.V.E.V.d.; Vonk, S.; van Jaarsveld, B.C.; Bonenkamp, A.A.; Abrahams, A.C. Good practices for dialysis education, treatment, and eHealth: A scoping review. PLoS ONE 2021, 16, e0255734. [Google Scholar]
- Shen, H.; van der Kleij, R.M.J.J.; van der Boog, P.J.M.; Chang, X.; Chavannes, N.H. Electronic Health Self-Management Interventions for Patients with Chronic Kidney Disease: Systematic Review of Quantitative and Qualitative Evidence. J. Med. Internet Res. 2019, 21, e12384. [Google Scholar] [CrossRef]
- Ryan, S.; Chasaide, N.N.; Hanrahan, S.O.; Corcoran, D.; Caulfield, B.; Argent, R. mHealth apps for musculoskeletal rehabilitation: Systematic search in APP stores and content analysis. JMIR Rehabil. Assist. Technol. 2022, 9, e34355. [Google Scholar] [CrossRef]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and facilitators to the use of e-health by older adults: A scoping review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef]
- Wang, C.-S.; Ku, E. eHealth in kidney care. Nat. Rev. Nephrol. 2020, 16, 368–370. [Google Scholar] [CrossRef]
- IMS Institute for Healthcare Informatics. Patient Apps for Improved Healthcare; IMS Institute for Healthcare Informatics: Traverse City, MI, USA, 2013. [Google Scholar]
- Farzandipour, M.; Nabovati, E.; Sharif, R.; Arani, M.H.; Anvari, S. Patient self-management of asthma using mobile health applications: A systematic re-view of the functionalities and effects. Appl. Clin. Inform. 2017, 8, 1068–1081. [Google Scholar] [CrossRef]
- Creber, R.M.M.; Maurer, M.S.; Reading, M.; Hiraldo, G.; Hickey, K.T.; Iribarren, S. Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the Mobile Application Rating Scale (MARS). JMIR Mhealth Uhealth 2016, 4, e74. [Google Scholar] [CrossRef]
- Harrison, R.; Flood, D.; Duce, D. Usability of mobile applications: Literature review and rationale for a new usability model. J. Interact. Sci. 2013, 1, 1. [Google Scholar] [CrossRef]
- Weichbroth, P. Usability of mobile applications: A systematic literature study. IEEE Access 2020, 8, 55563–55577. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. JBI Levels of Evidence; Joanna Briggs Institute: Adelaide, Australia, 2014. [Google Scholar]
- Martínez García, M.A.; Fernández Rosales, M.S.; López Domínguez, E.; Hernández Velázquez, Y.; Domínguez Isidro, S. Telemonitoring system for patients with chronic kidney disease undergoing peritoneal dialysis: Usability assessment based on a case study. PLoS ONE 2018, 13, e0206600. [Google Scholar] [CrossRef]
- Olivares-Gandy, H.J.; Domínguez-Isidro, S.; López-Domínguez, E.; Hernández-Velázquez, Y.; Tapia-McClung, H.; de-la-Calleja, J. A telemonitoring system for nutritional intake in patients with chronic kidney dis-ease receiving peritoneal dialysis therapy. Comput. Biol. Med. 2019, 109, 1–13. [Google Scholar] [CrossRef]
- Polanco, E.; Aquey, M.; Collado, J.; Campos, E.; Guzman, J.; Cuevas-Budhart, M.A.; Divino-Filho, J.C.; Ramos-Sanchez, A. A COVID-19 pandemic-specific, structured care process for peritoneal dialysis patients facili-tated by tele-medicine: Therapy continuity, prevention, and complications management. Ther. Apher. Dial. 2021, 25, 970–978. [Google Scholar] [CrossRef]
- Farfan-Ruiz, A.C.; Czikk, D.; Leidecker, J.; Ramsay, T.; McCormick, B.; Wilson, K.; Zimmerman, D. Multidisciplinary team versus a “Phosphate-Counting” app for serum phosphate control: A ran-domized controlled trial. Kidney360 2021, 2, 290. [Google Scholar] [CrossRef]
- Kiberd, J.; Khan, U.; Stockman, C.; Radhakrishnan, A.; Phillips, M.; Kiberd, B.A.; West, K.A.; Soroka, S.; Chan, C.; Tennankore, K.K. Effectiveness of a web-based ehealth portal for delivery of care to home dialysis patients: A single-arm pilot study. Can. J. Kidney Health Dis. 2018, 5, 2054358118794415. [Google Scholar] [CrossRef]
- Xu, X.; Ma, T.; Tian, X.; Li, S.; Pei, H.; Zhao, J.; Xiong, Z.; Liao, Y.; Li, Y.; Lin, Q.; et al. Telemedicine and Clinical Outcomes in Peritoneal Dialysis: A Propensity-Matched Study. Am. J. Nephrol. 2022, 53, 663–674. [Google Scholar] [CrossRef]
- Chae, Y.J.; Kim, H.S. Effects of a mobile application on improving self-management of adult patients receiving peritoneal dialysis: A randomized controlled trial. Jpn. J. Nurs. Sci. 2023, 21, e12555. [Google Scholar] [CrossRef]
- Viglino, G.; Neri, L.; Barbieri, S.; Tortone, C. Videodialysis: A pilot experience of telecare for assisted peritoneal dialysis. J. Nephrol. 2020, 33, 177–182. [Google Scholar] [CrossRef]
- Eberle, C.; Löhnert, M.; Stichling, S. Effectiveness of disease-specific mHealth apps in patients with diabetes mellitus: Scoping review. JMIR Mhealth Uhealth 2021, 9, e23477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).