Innovative Strategies for Fertility Preservation in Female Cancer Survivors: New Hope from Artificial Ovary Construction and Stem Cell-Derived Neo-Folliculogenesis
Abstract
1. Oncofertility
2. Ovarian Tissue Cryostorage
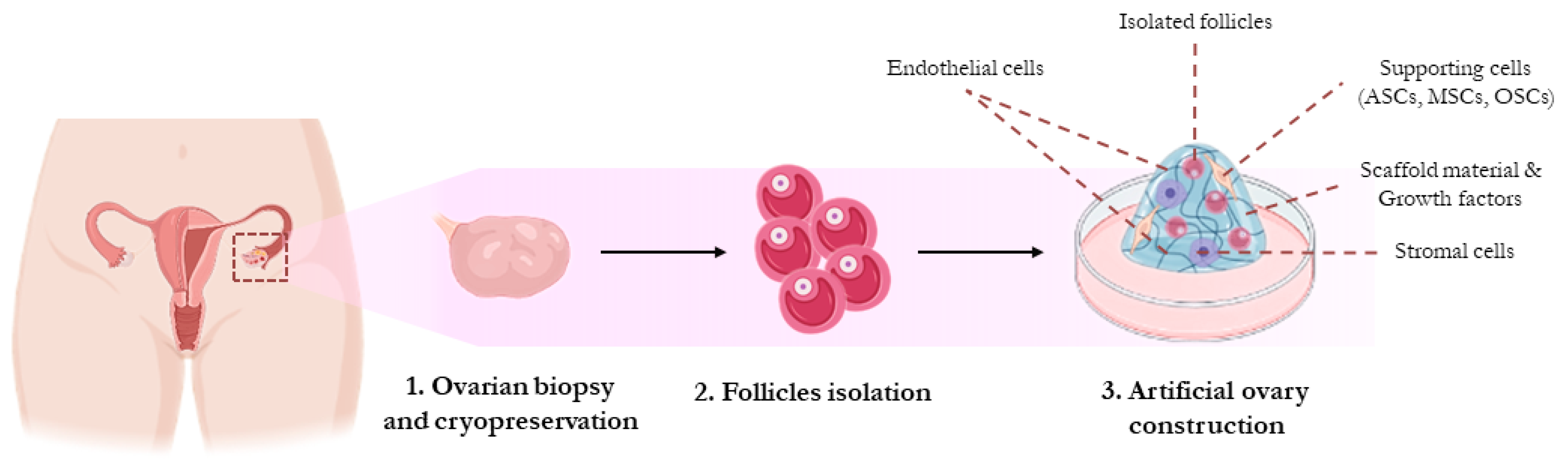
3. Artificial Ovary
4. In Vitro Maturation of Isolated Ovarian Stem Cells (OSCs)
5. Future Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yi, M.; Li, T.; Niu, M.; Luo, S.; Chu, Q.; Wu, K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: A population-based study. Biomark. Res. 2021, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Woodruff, T.K. Anticancer treatments and female fertility: Clinical concerns and role of oncologists in oncofertility practice. Expert Rev. Anticancer Ther. 2017, 17, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Gazouli, I.; Zarkavelis, G.; Papadaki, A.; Mavroeidis, L.; Gkoura, S.; Ntellas, P.; Amylidi, A.-L.; Tsali, L.; Kampletsas, E. Chemotherapy Associated Ovarian Failure. Front. Endocrinol. 2020, 11, 572388. [Google Scholar] [CrossRef]
- Brydøy, M.; Fosså, S.D.; Dahl, O.; Bjøro, T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. 2007, 46, 480–489. [Google Scholar] [CrossRef]
- Zavattaro, M.; Lanfranco, F.; Salvagno, F.; Motta, G.; Sestero, M.; Marinelli, L.; Canosa, S.; Revelli, A. Gonadal Failure and Infertility in Cancer Survivors: Clinical Management and Strategies for Prevention. In Endocrine and Metabolic Late Effects in Cancer Survivors; Frontiers of Hormone Research; S.Karger AG: Basel, Switzerland, 2021; Volume 54, pp. 58–68. [Google Scholar]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility Preservation for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef]
- Cui, W.; Stern, C.; Hickey, M.; Goldblatt, F.; Anazodo, A.; Stevenson, W.S.; Phillips, K.-A. Preventing ovarian failure associated with chemotherapy. Med. J. Aust. 2018, 209, 412–416. [Google Scholar] [CrossRef]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H.B. Toxicity of Chemotherapy and Radiation on Female Reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi160–vi170. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; De Palma, G.; Canosa, S.; Palini, S.; Dellino, M.; Revelli, A.; Paradiso, A.V. Human Ovarian Cortex biobanking: A Fascinating Resource for Fertility Preservation in Cancer. Int. J. Mol. Sci. 2020, 21, 3245. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar]
- Fabbri, R.; Pasquinelli, G.; Keane, D.; Magnani, V.; Paradisi, R.; Venturoli, S. Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod. Biomed. Online 2010, 21, 819–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salehnia, M.; Moghadam, E.A.; Velojerdi, M.R. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil. Steril. 2002, 78, 644–645. [Google Scholar] [CrossRef]
- Migishima, F.; Suzuki-Migishima, R.; Song, S.-Y.; Kuramochi, T.; Azuma, S.; Nishijima, M.; Yokoyama, M. Successful cryopreservation of mouse ovaries by vitrification. Biol. Reprod. 2003, 68, 881–887. [Google Scholar] [CrossRef][Green Version]
- Fabbri, R.; Vicenti, R.; Macciocca, M.; Pasquinelli, G.; Paradisi, R.; Battaglia, C.; Martino, N.A.; Venturoli, S. Good Preservation of Stromal Cells and No Apoptosis in Human Ovarian Tissue after Vitrification. BioMed Res. Int. 2014, 2014, 673537. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: A prospective cohort study. Fertil. Steril. 2018, 109, 478–485.e2. [Google Scholar] [CrossRef]
- Behl, S.; Joshi, V.B.; Larson, N.B.; Young, M.C.; Bilal, M.; Walker, D.L.; Khan, Z.; Granberg, C.F.; Chattha, A.; Zhao, Y. Vitrification versus slow freezing of human ovarian tissue: A systematic review and meta-analysis of histological outcomes. J. Assist. Reprod. Genet. 2023, 40, 455–464. [Google Scholar] [CrossRef]
- Wang, T.R.; Yan, J.; Lu, C.L.; Xia, X.; Yin, T.L.; Zhi, X.; Zhu, X.H.; Ding, T.; Hu, W.H.; Guo, H.Y.; et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum. Reprod. 2016, 31, 763–773. [Google Scholar] [CrossRef]
- Gu, R.; Ge, N.; Huang, B.; Fu, J.; Zhang, Y.; Wang, N.; Xu, Y.; Li, L.; Peng, X.; Zou, Y.; et al. Impacts of vitrification on the transcriptome of human ovarian tissue in patients with gynecological cancer. Front. Genet. 2023, 14, 1114650. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; De Stefano, C.; Ferraro, R.; Sudhakaran, S.; Gualtieri, R. Successful slush nitrogen vitrification of human ovarian tissue. Fertil. Steril. 2016, 105, 1523–1531.e1. [Google Scholar] [CrossRef] [PubMed]
- Barbato, V.; Gualtieri, R.; Capriglione, T.; Pallotta, M.M.; Braun, S.; Di Nardo, M.; Costanzo, V.; Ferraro, R.; Catapano, G.; Talevi, R. Slush nitrogen vitrification of human ovarian tissue does not alter gene expression and improves follicle health and progression in long-term in vitro culture. Fertil. Steril. 2018, 110, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Simon, P.; Emiliani, S.; Delbaere, A.; Englert, Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum. Reprod. Update 2009, 15, 649–665. [Google Scholar] [CrossRef]
- Jensen, A.K.; Macklon, K.T.; Fedder, J.; Ernst, E.; Humaidan, P.; Andersen, C.Y. Erratum to: 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: Focus on birth and perinatal outcome in 40 of these children. J. Assist. Reprod. Genet. 2017, 34, 337. [Google Scholar] [CrossRef]
- Van der Ven, H.; Liebenthron, J.; Beckmann, M.; Toth, B.; Korell, M.; Krüssel, J.; Frambach, T.; Kupka, M.; Hohl, M.K.; Winkler-Crepaz, K.; et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: Tissue activity, pregnancy and delivery rates. Hum. Reprod. 2016, 31, 2031–2041. [Google Scholar] [CrossRef]
- Jadoul, P.; Guilmain, A.; Squifflet, J.; Luyckx, M.; Votino, R.; Wyns, C.; Dolmans, M.M. Efficacy of ovarian tissue cryopreservation for fertility preservation: Lessons learned from 545 cases. Hum. Reprod. 2017, 32, 1046–1054. [Google Scholar] [CrossRef]
- Meirow, D.; Ra’Anani, H.; Shapira, M.; Brenghausen, M.; Chaim, S.D.; Aviel-Ronen, S.; Amariglio, N.; Schiff, E.; Orvieto, R.; Dor, J. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril. 2016, 106, 467–474. [Google Scholar] [CrossRef]
- Van Eyck, A.-S.; Bouzin, C.; Feron, O.; Romeu, L.; Van Langendonckt, A.; Donnez, J.; Dolmans, M.-M. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 2010, 93, 1676–1685. [Google Scholar] [CrossRef]
- Picardo, E.; Mitidieri, M.; Danese, S. Obstetrics and Ginecology Advances. In Cancer and Pregnacy. A Practical Approach for the Gynecologist; Nova Science Publishers: Hauppauge, NY, USA, 2021. [Google Scholar]
- Oktay, K.; Karlikaya, G. Ovarian Function after Transplantation of Frozen, Banked Autologous Ovarian Tissue. N. Engl. J. Med. 2000, 342, 1919. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Dolmans, M.-M.; Silber, S.; Meirow, D. Evaluation of Ovarian Tissue Transplantation: Results from Three Clinical Centers. Fertil. Steril. 2020, 114, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Corkum, K.S.; Rhee, D.S.; Wafford, Q.E.; Demeestere, I.; Dasgupta, R.; Baertschiger, R.; Malek, M.M.; Aldrink, J.H.; Heaton, T.E.; Weil, B.R.; et al. Fertility and Hormone Preservation and Restoration for Female Children and Adolescents Receiving Gonadotoxic Cancer Treatments: A Systematic Review. J. Pediatr. Surg. 2019, 54, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Roness, H.; Kristensen, S.G.; Andersen, C.Y. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum. Reprod. 2015, 30, 2453–2456. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.-H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kawamura, K.; Cheng, Y.; Liu, S.; Klein, C.; Liu, S.; Duan, E.-K.; Hsueh, A.J.W. Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. USA 2010, 107, 10280–10284. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- Eijkenboom, L.; Saedt, E.; Zietse, C.; Braat, D.; Beerendonk, C.; Peek, R. Strategies to safely use cryopreserved ovarian tissue to restore fertility after cancer: A systematic review. Reprod. Biomed. Online 2022, 45, 763–778. [Google Scholar] [CrossRef]
- Bastings, L.; Beerendonk, C.C.; Westphal, J.R.; Massuger, L.F.; Kaal, S.E.; van Leeuwen, F.E.; Braat, D.D.; Peek, R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: A systematic review. Hum. Reprod. Update 2013, 19, 483–506. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Hardan, I.; Dor, J.; Fridman, E.; Elizur, S.; Ra’Anani, H.; Slyusarevsky, E.; Amariglio, N.; Schiff, E.; Rechavi, G.; et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum. Reprod. 2008, 23, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Kourta, D.; Kanbar, M.; A Amorim, C.; Wyns, C. Cancer cell contamination and decontamination methods for ovaries and testes: Special focus on prepubertal gonads with a view to safe fertility restoration. Hum. Reprod. 2023, 38, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Shikanov, A. The artificial ovary: Current status and future perspectives. Future Oncol. 2016, 12, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kim, Y.Y.; Noh, K.; Ku, S.-Y. A new possibility in fertility preservation: The artificial ovary. J. Tissue Eng. Regen. Med. 2019, 13, 1294–1315. [Google Scholar] [CrossRef] [PubMed]
- Paulini, F.; Vilela, J.M.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.-M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef]
- Chiti, M.C.; Donnez, J.; Amorim, C.A.; Dolmans, M.-M. From isolation of human ovarian follicles to the artificial ovary: Tips and tricks. Minerva Ginecol. 2018, 70, 444–455. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Isachenko, E.; Rappl, G.; Rahimi, G.; Hanstein, B.; Morgenstern, B.; Mallmann, P.; Isachenko, V. Construction of human artificial ovary from cryopreserved ovarian tissue: Appearance of apoptosis and necrosis after enzymatic isolation of follicles. Cryobiology 2018, 84, 10–14. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Isachenko, V.; Rappl, G.; Rahimi, G.; Hanstein, B.; Morgenstern, B.; Mallmann, P.; Isachenko, E. Comparison of the enzymatic efficiency of Liberase TM and tumor dissociation enzyme: Effect on the viability of cells digested from fresh and cryopreserved human ovarian cortex. Reprod. Biol. Endocrinol. 2018, 16, 57. [Google Scholar] [CrossRef]
- Soares, M.; Sahrari, K.; Amorim, C.A.; Saussoy, P.; Donnez, J.; Dolmans, M.-M. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil. Steril. 2015, 104, 672–680.e2. [Google Scholar] [CrossRef] [PubMed]
- Revel, A.; Laufer, N.; Ben Meir, A.; Lebovich, M.; Mitrani, E. Micro-organ ovarian transplantation enables pregnancy: A case report. Hum. Reprod. 2011, 26, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Dath, C.; Dethy, A.; Van Langendonckt, A.; Van Eyck, A.S.; Amorim, C.A.; Luyckx, V.; Donnez, J.; Dolmans, M.M. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum. Reprod. 2011, 26, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Magoffin, D.A. Ovarian theca cell. Int. J. Biochem. Cell Biol. 2005, 37, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Sahrari, K.; Chiti, M.C.; Amorim, C.A.; Ambroise, J.; Donnez, J.; Dolmans, M.-M. The best source of isolated stromal cells for the artificial ovary: Medulla or cortex, cryopreserved or fresh? Hum. Reprod. 2015, 30, 1589–1598. [Google Scholar] [CrossRef]
- Kizuka-Shibuya, F.; Tokuda, N.; Takagi, K.; Adachi, Y.; Lee, L.; Tamura, I.; Maekawa, R.; Tamura, H.; Suzuki, T.; Owada, Y.; et al. Locally existing endothelial cells and pericytes in ovarian stroma, but not bone marrow-derived vascular progenitor cells, play a central role in neovascularization during follicular development in mice. J. Ovarian Res. 2014, 7, 10. [Google Scholar] [CrossRef]
- Vanacker, J.; Luyckx, V.; Dolmans, M.-M.; Des Rieux, A.; Jaeger, J.; Van Langendonckt, A.; Donnez, J.; Amorim, C.A. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012, 33, 6079–6085. [Google Scholar] [CrossRef]
- Chen, J.; Todorov, P.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Isachenko, V. Construction and cryopreservation of an artificial ovary in cancer patients as an element of cancer therapy and a promising approach to fertility restoration. Hum. Fertil. 2022, 25, 651–661. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, C.; Zhang, X.; He, X.; Liu, Y. Design and Application Strategies of Natural Polymer Biomaterials in Artificial Ovaries. Ann. Biomed. Eng. 2023, 51, 461–478. [Google Scholar] [CrossRef]
- Kim, J.; Perez, A.S.; Claflin, J.; David, A.; Zhou, H.; Shikanov, A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. Npj Regen. Med. 2016, 1, 16010. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.; Torrance, C.; Gosden, R.G. Morphological study of cultured preantral ovarian follicles of mice after transplantation under the kidney capsule. Reproduction 1990, 89, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.M.; Orellana, R.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C.A. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 2016, 31, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.-M.; Vanacker, J.; Legat, C.; Fortuño Moya, C.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef]
- Vanacker, J.; Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Amorim, C.A. First transplantation of isolated murine follicles in alginate. Regen. Med. 2014, 9, 609–619. [Google Scholar] [CrossRef]
- Rios, P.D.; Kniazeva, E.; Lee, H.C.; Xiao, S.; Oakes, R.S.; Saito, E.; Jeruss, J.S.; Shikanov, A.; Woodruff, T.K.; Shea, L.D. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol. Bioeng. 2018, 115, 2075–2086. [Google Scholar] [CrossRef]
- Kniazeva, E.; Hardy, A.N.; Boukaidi, S.A.; Woodruff, T.K.; Jeruss, J.S.; Shea, L.D. Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model. Sci. Rep. 2015, 5, 17709. [Google Scholar] [CrossRef]
- Rajabzadeh, A.R.; Eimani, H.; Mohseni Koochesfahani, H.; Shahvardi, A.-H.; Fathi, R. Morphological study of isolated ovarian preantral follicles using fibrin gel plus platelet lysate after subcutaneous transplantation. Cell J. 2015, 17, 145–152. [Google Scholar]
- Laronda, M.M.; Jakus, A.E.; Whelan, K.A.; Wertheim, J.A.; Shah, R.N.; Woodruff, T.K. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015, 50, 20–29. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Hassanpour, A.; Talaei-Khozani, T.; Kargar-Abarghouei, E.; Razban, V.; Vojdani, Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther. 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Pors, S.E.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S.G. Initial steps in reconstruction of the human ovary: Survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, K.-C.; Yan, J.-F.; Zhang, J.-J.; Wang, S.-X. Extracellular matrix-derived scaffolds in constructing artificial ovaries for ovarian failure: A systematic methodological review. Hum. Reprod. Open 2023, 2023, hoad014. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Isachenko, E.; Sanchez, R.; Dattena, M.; Mallmann, P.; Rahimi, G. Cryopreservation of Whole Ovine Ovaries with Pedicles as a Model for Human: Parameters of Perfusion with Simultaneous Saturations by Cryoprotectants. Clin. Lab. 2015, 61, 415–420. [Google Scholar] [CrossRef]
- Alaee, S.; Asadollahpour, R.; Hosseinzadeh Colagar, A.; Talaei-Khozani, T. The decellularized ovary as a potential scaffold for maturation of preantral ovarian follicles of prepubertal mice. Syst. Biol. Reprod. Med. 2021, 67, 413–427. [Google Scholar] [CrossRef]
- Sarabadani, M.; Tavana, S.; Mirzaeian, L.; Fathi, R. Co-culture with peritoneum mesothelial stem cells supports the in vitro growth of mouse ovarian follicles. J. Biomed. Mater. Res. Part A 2021, 109, 2685–2694. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Y.; Hou, C.; Li, Z.; Yang, S.; Liang, X.; Zhou, L.; Guo, J.; Zhang, J.; Huang, X. Ovary-derived Decellularized Extracellular Matrix-based Bioink for Fabricating 3D Primary Ovarian Cells-laden Structures for Mouse Ovarian Failure Correction. Int. J. Bioprint. 2022, 8, 597. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M.; Pellicer, A.; Diaz-Garcia, C.; Sanchez Serrano, M.; Schmidt, K.T.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef]
- Oktay, K.; Bedoschi, G.; Pacheco, F.; Turan, V.; Emirdar, V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am. J. Obstet. Gynecol. 2016, 214, 94.e1–94.e9. [Google Scholar] [CrossRef]
- Abir, R.; Fisch, B.; Jessel, S.; Felz, C.; Ben-Haroush, A.; Orvieto, R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil. Steril. 2011, 95, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Park, L.; Bodine, R.; Ginsberg, M.; Zaninovic, N.; Man, O.A.; Schattman, G.; Rosenwaks, Z.; James, D. Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci. Rep. 2017, 7, 8203. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Masasa, H.; Orenbuch, A.; Levaot, N.; Shachar Goldenberg, M.; Cohen, S. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials 2019, 205, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J. Ovary cryopreservation and transplantation for fertility preservation. Mol. Hum. Reprod. 2012, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J. Assist. Reprod. Genet. 2012, 29, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Kelsey, T.W.; Anderson, R.A. Ovarian cryopreservation: Experimental or established and a cure for the menopause? Reprod. Biomed. Online 2012, 25, 93–95. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Torres-de la Roche, L.A.; Kahlert, U.D.; Isachenko, V.; Huang, H.; Hennefründ, J.; Yan, X.; Chen, Q.; Shi, W.; Li, Y. Artificial Ovary for Young Female Breast Cancer Patients. Front. Med. 2022, 9, 837022. [Google Scholar] [CrossRef]
- Wang, W.; Pei, C.; Isachenko, E.; Zhou, Y.; Wang, M.; Rahimi, G.; Liu, W.; Mallmann, P.; Isachenko, V. Automatic Evaluation for Bioengineering of Human Artificial Ovary: A Model for Fertility Preservation for Prepubertal Female Patients with a Malignant Tumor. Int. J. Mol. Sci. 2022, 23, 12419. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Manavella, D.D.; Cacciottola, L.; Pommé, S.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen–thawed human ovarian tissue. Hum. Reprod. 2018, 33, 1107–1116. [Google Scholar] [CrossRef]
- Manavella, D.D.; Cacciottola, L.; Desmet, C.M.; Jordan, B.F.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: A potential way to improve ovarian tissue transplantation. Hum. Reprod. 2018, 33, 270–279. [Google Scholar] [CrossRef]
- Manavella, D.D.; Cacciottola, L.; Payen, V.L.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol. Hum. Reprod. 2019, 25, 184–193. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.; Lee, S. Role of Stem Cells in the Ovarian Tissue Cryopreservation and Transplantation for Fertility Preservation. Int. J. Mol. Sci. 2021, 22, 12482. [Google Scholar] [CrossRef] [PubMed]
- Damous, L.L.; Nakamuta, J.S.; Carvalho, A.E.; Carvalho, K.C.; Soares, J.M., Jr.; Simões Mde, J.; Krieger, J.E.; Baracat, E.C. Does adipose tissue-derived stem cell therapy improve graft quality in freshly grafted ovaries? Reprod. Biol. Endocrinol. 2015, 13, 108. [Google Scholar] [CrossRef]
- Xia, X.; Yin, T.; Yan, J.; Yan, L.; Jin, C.; Lu, C.; Wang, T.; Zhu, X.; Zhi, X.; Wang, J.; et al. Mesenchymal Stem Cells Enhance Angiogenesis and Follicle Survival in Human Cryopreserved Ovarian Cortex Transplantation. Cell Transplant. 2015, 24, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-M.; Yan, J.; Li, R.; Li, M.; Yan, L.-Y.; Wang, T.-R.; Zhao, H.-C.; Zhao, Y.; Yu, Y.; Qiao, J. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum. Reprod. 2013, 28, 2784–2793. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.; Yan, J.; Yan, L.; Lu, C.; Zhu, X.; Wang, T.; Yin, T.; Li, R.; Chang, H.-M.; et al. Mesenchymal stem cell-derived angiogenin promotes primodial follicle survival and angiogenesis in transplanted human ovarian tissue. Reprod. Biol. Endocrinol. 2017, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, L.; Wang, S.; Sheng, X.; Yan, G.; Xu, L.; Liu, J.; Liu, M.; Zhen, X.; Ding, L.; et al. Transplantation of umbilical cord–derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. Vitr. Cell. Dev. Biol.—Anim. 2019, 55, 302–311. [Google Scholar] [CrossRef]
- Park, B.-W.; Pan, B.; Toms, D.; Huynh, E.; Byun, J.-H.; Lee, Y.-M.; Shen, W.; Rho, G.-J.; Li, J.; Dyce, P.W.; et al. Ovarian-cell-like cells from skin stem cells restored estradiol production and estrus cycling in ovariectomized mice. Stem Cells Dev. 2014, 23, 1647–1658. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef]
- Green, S.H.; Zuckerman, S. Quantitative aspects of the growth of the human ovum and follicle. J. Anat. 1951, 85, 373–375. [Google Scholar] [PubMed]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Telfer, E.E. Purification of germline stem cells from adult mammalian ovaries: A step closer towards control of the female biological clock? Mol. Hum. Reprod. 2009, 15, 393–398. [Google Scholar] [CrossRef]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef] [PubMed]
- White, Y.A.R.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; Zelinski, M.B. Ovarian follicle culture: Advances and challenges for human and nonhuman primates. Fertil. Steril. 2013, 99, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Stimpfel, M.; Skutella, T.; Cvjeticanin, B.; Meznaric, M.; Dovc, P.; Novakovic, S.; Cerkovnik, P.; Vrtacnik-Bokal, E.; Virant-Klun, I. Isolation, characterization and differentiation of cells expressing pluripotent/multipotent markers from adult human ovaries. Cell Tissue Res. 2013, 354, 593–607. [Google Scholar] [CrossRef]
- Parte, S.; Bhartiya, D.; Patel, H.; Daithankar, V.; Chauhan, A.; Zaveri, K.; Hinduja, I. Dynamics associated with spontaneous differentiation of ovarian stem cells in vitro. J. Ovarian Res. 2014, 7, 25. [Google Scholar] [CrossRef]
- Hernandez, S.F.; Vahidi, N.A.; Park, S.; Weitzel, R.P.; Tisdale, J.; Rueda, B.R.; Wolff, E.F. Characterization of extracellular DDX4- or Ddx4-positive ovarian cells. Nat. Med. 2015, 21, 1114–1116. [Google Scholar] [CrossRef]
- Wang, N.; Tilly, J.L. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle 2010, 9, 339–349. [Google Scholar] [CrossRef]
- Park, E.S.; Woods, D.C.; Tilly, J.L. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil. Steril. 2013, 100, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, X.; Zheng, T.; Hu, C.; Pan, Z.; Huang, J.; Li, J.; Li, W.; Zheng, Y. The hippo signaling pathway regulates ovarian function via the proliferation of ovarian germline stem cells. Cell. Physiol. Biochem. 2017, 41, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Tilly, J.L. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat. Protoc. 2013, 8, 966–988. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Takai, Y.; Ishihara, O.; Seki, H.; Woods, D.C.; Tilly, J.L. Extracellular matrix signaling activates differentiation of adult ovary-derived oogonial stem cells in a species-specific manner. Fertil. Steril. 2019, 111, 794–805. [Google Scholar] [CrossRef]
- Silvestris, E.; Cafforio, P.; D’oronzo, S.; Felici, C.; Silvestris, F.; Loverro, G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Hum. Reprod. 2018, 33, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; Cafforio, P.; Felici, C.; Cormio, G.; D’Oronzo, S. Ddx4+ Oogonial Stem Cells in Postmenopausal Women’s Ovaries: A Controversial, Undefined Role. Cells 2019, 8, 650. [Google Scholar] [CrossRef]
- Silvestris, E.; Minoia, C.; Guarini, A.; Opinto, G.; Negri, A.; Dellino, M.; Tinelli, R.; Cormio, G.; Paradiso, A.V.; De Palma, G. Ovarian Stem Cells (OSCs) from the Cryopreserved Ovarian Cortex: A Potential for Neo-Oogenesis in Women with Cancer-Treatment Related Infertility: A Case Report and a Review of Literature. Curr. Issues Mol. Biol. 2022, 44, 2309–2320. [Google Scholar] [CrossRef]
- Parte, S.; Bhartiya, D.; Manjramkar, D.D.; Chauhan, A.; Joshi, A. Stimulation of ovarian stem cells by follicle stimulating hormone and basic fibroblast growth factor during cortical tissue culture. J. Ovarian Res. 2013, 6, 20. [Google Scholar] [CrossRef]
- Mirzaeian, L.; Eivazkhani, F.; Saber, M.; Moini, A.; Esfandiari, F.; Valojerdi, M.R.; Fathi, R. In-vivo oogenesis of oogonial and mesenchymal stem cells seeded in transplanted ovarian extracellular matrix. J. Ovarian Res. 2023, 16, 56. [Google Scholar] [CrossRef]
- Woods, D.C.; Tilly, J.L. The next (re)generation of ovarian biology and fertility in women: Is current science tomorrow’s practice? Fertil. Steril. 2012, 98, 3–10. [Google Scholar] [CrossRef]
- Horan, C.J.; Williams, S.A. Oocyte stem cells: Fact or fantasy? Reproduction 2017, 154, R23–R35. [Google Scholar] [CrossRef]
- De Roo, C.; Tilleman, K. In Vitro Maturation of Oocytes Retrieved from Ovarian Tissue: Outcomes from Current Approaches and Future Perspectives. J. Clin. Med. 2021, 10, 4680. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; D’oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In Vitro Generation of Oocytes from Ovarian Stem Cells (OSCs): In Search of Major Evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ozkavukcu, S.; Ku, S.-Y. Current and Future Perspectives for Improving Ovarian Tissue Cryopreservation and Transplantation Outcomes for Cancer Patients. Reprod. Sci. 2021, 28, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Anckaert, E.; De Rycke, M.; Smitz, J. Culture of oocytes and risk of imprinting defects. Hum. Reprod. Update 2013, 19, 52–66. [Google Scholar] [CrossRef]
- Ozakpinar, O.B.; Maurer, A.M.; Ozsavci, D. Ovarian stem cells: From basic to clinical applications. World J. Stem Cells 2015, 7, 757–768. [Google Scholar] [CrossRef]
- Akahori, T.; Woods, D.C.; Tilly, J.L. Female Fertility Preservation through Stem Cell-based Ovarian Tissue Reconstitution In Vitro and Ovarian Regeneration In Vivo. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119848007. [Google Scholar] [CrossRef]
- Telfer, E.E.; Fauser, B.C. Important steps towards materializing the dream of developing an artificial ovary. Reprod. Biomed. Online 2016, 33, 333–334. [Google Scholar] [CrossRef]
- Canosa, S.; Cimadomo, D.; Conforti, A.; Maggiulli, R.; Giancani, A.; Tallarita, A.; Golia, F.; Fabozzi, G.; Vaiarelli, A.; Gennarelli, G.; et al. The effect of extended cryo-storage following vitrification on embryo competence: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2022, 39, 873–882. [Google Scholar] [CrossRef]
- Canosa, S.; Maggiulli, R.; Cimadomo, D.; Innocenti, F.; Fabozzi, G.; Gennarelli, G.; Revelli, A.; Bongioanni, F.; Vaiarelli, A.; Ubaldi, F.M.; et al. Cryostorage management of reproductive cells and tissues in ART: Status, needs, opportunities and potential new challenges. Reprod. Biomed. Online 2023, 47, 103252. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Amorim, C.A. FERTILITY PRESERVATION: Construction and use of artificial ovaries. Reproduction 2019, 158, F15–F25. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Manavella, D.D. Recent advances in fertility preservation. J. Obstet. Gynaecol. Res. 2019, 45, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Woodruff, T.K. From bench to bedside: Current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet. Gynecol. Scand. 2019, 98, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Sfakianoudis, K.; Tsioulou, P.; Rapani, A.; Giannelou, P.; Kiriakopoulos, N.; Pantou, A.; Vlahos, N.; Anifandis, G.; Bolaris, S.; et al. What will the future hold for artificial organs in the service of assisted reproduction: Prospects and considerations. Front. Med. 2019, 13, 627–638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canosa, S.; Revelli, A.; Gennarelli, G.; Cormio, G.; Loizzi, V.; Arezzo, F.; Petracca, E.A.; Carosso, A.R.; Cimadomo, D.; Rienzi, L.; et al. Innovative Strategies for Fertility Preservation in Female Cancer Survivors: New Hope from Artificial Ovary Construction and Stem Cell-Derived Neo-Folliculogenesis. Healthcare 2023, 11, 2748. https://doi.org/10.3390/healthcare11202748
Canosa S, Revelli A, Gennarelli G, Cormio G, Loizzi V, Arezzo F, Petracca EA, Carosso AR, Cimadomo D, Rienzi L, et al. Innovative Strategies for Fertility Preservation in Female Cancer Survivors: New Hope from Artificial Ovary Construction and Stem Cell-Derived Neo-Folliculogenesis. Healthcare. 2023; 11(20):2748. https://doi.org/10.3390/healthcare11202748
Chicago/Turabian StyleCanosa, Stefano, Alberto Revelli, Gianluca Gennarelli, Gennaro Cormio, Vera Loizzi, Francesca Arezzo, Easter Anna Petracca, Andrea Roberto Carosso, Danilo Cimadomo, Laura Rienzi, and et al. 2023. "Innovative Strategies for Fertility Preservation in Female Cancer Survivors: New Hope from Artificial Ovary Construction and Stem Cell-Derived Neo-Folliculogenesis" Healthcare 11, no. 20: 2748. https://doi.org/10.3390/healthcare11202748
APA StyleCanosa, S., Revelli, A., Gennarelli, G., Cormio, G., Loizzi, V., Arezzo, F., Petracca, E. A., Carosso, A. R., Cimadomo, D., Rienzi, L., Vaiarelli, A., Ubaldi, F. M., & Silvestris, E. (2023). Innovative Strategies for Fertility Preservation in Female Cancer Survivors: New Hope from Artificial Ovary Construction and Stem Cell-Derived Neo-Folliculogenesis. Healthcare, 11(20), 2748. https://doi.org/10.3390/healthcare11202748










