Optimizing the Ovarian Tissue Cryopreservation in the ‘Oncofertility’ Institutional Program at an Italian National Cancer Institute
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implementing a Working Team for Counselling Patients and Ovarian Cortex Sampling
2.2. Patients’ Characteristics and Enrollment Criteria
2.3. Ovarian Cortex Recruitment and Sampling
2.4. Ovarian Cortex Cryopreservation Procedure: Slow Freezing vs. Ultra-Rapid Freezing
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
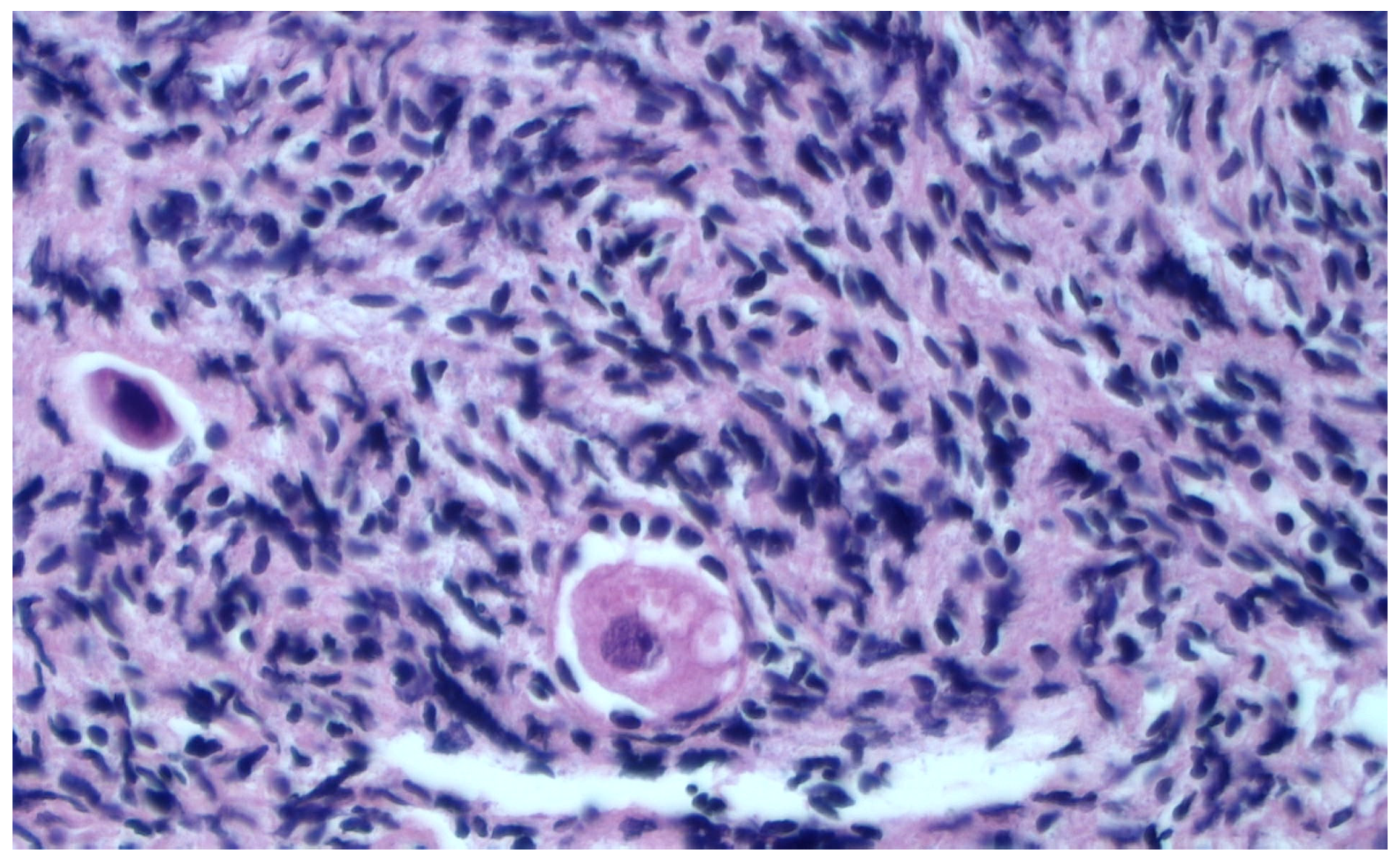
Histological Evaluation of Thawed Strips
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, B. The basic benefits of ultraviolet technology. Water Cond. Purif. 1995, 26–27. [Google Scholar]
- Silvestris, E.; De Palma, G.; Canosa, S.; Palini, S.; Dellino, M.; Revelli, A.; Paradiso, A.V. Human Ovarian Cortex biobanking: A Fascinating Resource for Fertility Preservation in Cancer. Int. J. Mol. Sci. 2020, 21, 3245. [Google Scholar] [CrossRef] [PubMed]
- Parmegiani, L.; Minasi, M.G.; Arnone, A.; Casciani, V.; Cognigni, G.E.; Viñoles, R.; Varricchio, M.T.; Quintero, L.A.; Greco, E.; Filicori, M. “Universal Warming” protocol for vitrified oocytes to streamline cell exchange for transnational donation programs: A multi-center study. J. Assist. Reprod. Genet. 2020, 37, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, E.J.; Louwe, L.A.; Rooijers, M.; van der Westerlaken, L.A.J.; Klijn, N.F.; Pilgram, G.S.K.; de Kroon, C.D.; Hilders, C.G.J.M. Ovarian tissue cryopreservation: Low usage rates and high live-birth rate after transplantation. Acta Obstet. Gynecol. Scand. 2020, 99, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Karlikaya, G. Ovarian Function after Transplantation of Frozen, Banked Autologous Ovarian Tissue. N. Engl. J. Med. 2000, 342, 1919. [Google Scholar] [CrossRef] [PubMed]
- Kometas, M.; Christman, G.M.; Kramer, J.; Rhoton-Vlasak, A. Methods of Ovarian Tissue Cryopreservation: Is Vitrification Superior to Slow Freezing?—Ovarian Tissue Freezing Methods. Reprod. Sci. 2021, 28, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.G.; Kimler, B.F.; Smith, B.M.; Woodruff, T.K.; Pavone, M.E.; Duncan, F.E. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium’s National Physicians Cooperative. Future Oncol. 2018, 14, 363–378. [Google Scholar] [CrossRef]
- Arapaki, A.; Christopoulos, P.; Kalampokas, E.; Triantafyllidou, O.; Matsas, A.; Vlahos, N.F. Ovarian Tissue Cryopreservation in Children and Adolescents. Children 2022, 9, 1256. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Stoop, D.; Cobo, A.; Silber, S. Fertility preservation for age-related fertility decline. Lancet 2014, 384, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Consiglio Superiore di Sanità. Tutela Della Fertilità nei Pazienti Oncologici. Available online: https://www.statoregioni.it/media/1413/p-1-csr-atto-rep-n-27-21-feb2019.pdf (accessed on 12 April 2023).
- Himabindu, Y.; Sriharibabu, M.; Gopinathan, K.; Satish, U.; Louis, F.; Gopinath, P. Anti-mullerian hormone and antral follicle count as predictors of ovarian response in assisted reproduction. J. Hum. Reprod. Sci. 2013, 6, 27–31. [Google Scholar] [CrossRef] [PubMed]
- AbdelHafez, F.F.; Desai, N.; Abou-Setta, A.M.; Falcone, T.; Goldfarb, J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: A systematic review and meta-analysis. Reprod. Biomed. Online 2010, 20, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K.; Shea, L.D. The Role of the Extracellular Matrix in Ovarian Follicle Development. Reprod. Sci. 2007, 14, 6–10. [Google Scholar] [CrossRef]
- Sanfilippo, S.; Canis, M.; Romero, S.; Sion, B.; Déchelotte, P.; Pouly, J.-L.; Janny, L.; Smitz, J.; Brugnon, F. Quality and functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J. Assist. Reprod. Genet. 2011, 28, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Keros, V.; Xella, S.; Hultenby, K.; Pettersson, K.; Sheikhi, M.; Volpe, A.; Hreinsson, J.; Hovatta, O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum. Reprod. 2009, 24, 1670–1683. [Google Scholar] [CrossRef]
- Courbiere, B.; Massardier, J.; Salle, B.; Mazoyer, C.; Guerin, J.-F.; Lornage, J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil. Steril. 2005, 84, 1065–1071. [Google Scholar] [CrossRef]
- Santos, M.L.; Pais, A.S.; Santos, T.A. Fertility preservation in ovarian cancer patients. Gynecol. Endocrinol. 2021, 37, 483–489. [Google Scholar] [CrossRef]
- Medrano, J.V.; Andrés, M.d.M.; García, S.; Herraiz, S.; Vilanova-Pérez, T.; Goossens, E.; Pellicer, A. Basic and Clinical Approaches for Fertility Preservation and Restoration in Cancer Patients. Trends Biotechnol. 2018, 36, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Osuga, Y. Where are oncofertility and fertility preservation treatments heading in 2016? Future Oncol. 2016, 12, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Behrman, S.; Sawada, Y. Heterologous and Homologous Inseminations with Human Semen Frozen and Stored in a Liquid-Nitrogen Refrigerator. Fertil. Steril. 1966, 17, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Donnez, J. Fertility preservation in women for medical and social reasons: Oocytes vs. ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Mukaida, T. Cryopreservation of animal and human embryos by vitrification. Reprod. Biomed. Online 2004, 9, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Falcone, T.; Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil. Steril. 2020, 114, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J.; DeRosa, M.; Goldsmith, S.; Fan, Y.; Castleman, L.; Melnick, J. Cryopreservation and transplantation of ovarian tissue: Results from one center in the USA. J. Assist. Reprod. Genet. 2018, 35, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef]
- Bahroudi, Z.; Zarnaghi, M.R.; Izadpanah, M.; Abedelahi, A.; Niknafs, B.; Nasrabadi, H.T.; Seghinsara, A.M. Review of ovarian tissue cryopreservation techniques for fertility preservation. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102290. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef]
- Minoia, C.; Viviani, S.; Silvestris, E.; Palini, S.; Parissone, F.; De Palma, G.; Fedina, A.; Cormio, G.; Guarini, A.; Gini, G.; et al. Fertility preservation and monitoring in adult patients diagnosed with lymphoma: Consensus-based practical recommendations by the Fondazione Italiana Linfomi (FIL) & Società Italiana della Riproduzione Umana (SIRU). Front. Oncol. 2023, 13, 1252433. [Google Scholar] [CrossRef] [PubMed]
- Sonmezer, M.; Shamonki, M.I.; Oktay, K. Ovarian tissue cryopreservation: Benefits and risks. Cell Tissue Res. 2005, 322, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sonmezer, M.; Oktay, K. Fertility preservation in female patients. Hum. Reprod. Update 2004, 10, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Hossay, C.; Nguyen, T.Y.T.; Poirot, C. Fertility Preservation: How to Preserve Ovarian Function in Children, Adolescents and Adults. J. Clin. Med. 2021, 10, 5247. [Google Scholar] [CrossRef] [PubMed]
- Viviani, S.; Caccavari, V.; Gerardi, C.; Ramadan, S.; Allocati, E.; Minoia, C.; Guarini, A.; Di Russo, A. Male and Female Fertility: Prevention and Monitoring Hodgkin’ Lymphoma and Diffuse Large B-Cell Lymphoma Adult Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Viviani, S.; Dellino, M.; Ramadan, S.; Peracchio, C.; Marcheselli, L.; Minoia, C.; Guarini, A. Fertility preservation strategies for patients with lymphoma: A real-world practice survey among Fondazione Italiana Linfomi centers. Tumori 2022, 108, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; Minoia, C.; Guarini, A.; Opinto, G.; Negri, A.; Dellino, M.; Tinelli, R.; Cormio, G.; Paradiso, A.V.; De Palma, G. Ovarian Stem Cells (OSCs) from the Cryopreserved Ovarian Cortex: A Potential for Neo-Oogenesis in Women with Cancer-Treatment Related Infertility: A Case Report and a Review of Literature. Curr. Issues Mol. Biol. 2022, 44, 2309–2320. [Google Scholar] [CrossRef]


| N | Age | AMH (ng/mL) | AFC | Diagnosis |
|---|---|---|---|---|
| 1 | 34 | 2.70 | 8 | Uterine fibromatosis |
| 2 | 28 | 4.89 | 18 | Left ovarian cyst |
| 3 | 30 | 3.56 | 15 | Uterine fibromatosis |
| 4 | 29 | 5.45 | 20 | Endometriosis |
| 5 | 30 | 3.75 | 16 | Cervical cancer |
| 6 | 33 | 2.89 | 10 | Uterine fibromatosis |
| 7 | 31 | 3.56 | 10 | Left ovarian cyst |
| 8 | 32 | 3.01 | 14 | Cervical cancer |
| 9 | 33 | 2.42 | 9 | Endometriosis |
| 10 | 24 | 3.98 | 14 | Right ovarian cyst |
| 11 | 34 | 3.98 | 11 | Cervical cancer |
| Techniques | No. of Follicles after Thawing | Morphological Quality Classification after Thawing |
|---|---|---|
| Slow freezing | 149 | 47 intact |
| 102 damaged | ||
| Ultra-rapid freezing | 37 | 27 intact |
| 10 damaged |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestris, E.; Minoia, C.; De Palma, G.; Popescu, O.; Altavilla, A.; Guarini, A.; Pavone, F.; Loizzi, V.; Cormio, G.; Depalo, R. Optimizing the Ovarian Tissue Cryopreservation in the ‘Oncofertility’ Institutional Program at an Italian National Cancer Institute. Healthcare 2023, 11, 2727. https://doi.org/10.3390/healthcare11202727
Silvestris E, Minoia C, De Palma G, Popescu O, Altavilla A, Guarini A, Pavone F, Loizzi V, Cormio G, Depalo R. Optimizing the Ovarian Tissue Cryopreservation in the ‘Oncofertility’ Institutional Program at an Italian National Cancer Institute. Healthcare. 2023; 11(20):2727. https://doi.org/10.3390/healthcare11202727
Chicago/Turabian StyleSilvestris, Erica, Carla Minoia, Giuseppe De Palma, Ondina Popescu, Anna Altavilla, Attilio Guarini, Fabio Pavone, Vera Loizzi, Gennaro Cormio, and Raffaella Depalo. 2023. "Optimizing the Ovarian Tissue Cryopreservation in the ‘Oncofertility’ Institutional Program at an Italian National Cancer Institute" Healthcare 11, no. 20: 2727. https://doi.org/10.3390/healthcare11202727
APA StyleSilvestris, E., Minoia, C., De Palma, G., Popescu, O., Altavilla, A., Guarini, A., Pavone, F., Loizzi, V., Cormio, G., & Depalo, R. (2023). Optimizing the Ovarian Tissue Cryopreservation in the ‘Oncofertility’ Institutional Program at an Italian National Cancer Institute. Healthcare, 11(20), 2727. https://doi.org/10.3390/healthcare11202727







