Comparability of the Effectiveness of Different Types of Exercise in the Treatment of Achilles Tendinopathy: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion–Exclusion Criteria
2.3. Methodological Quality
2.4. Level of Research Evidence
3. Results
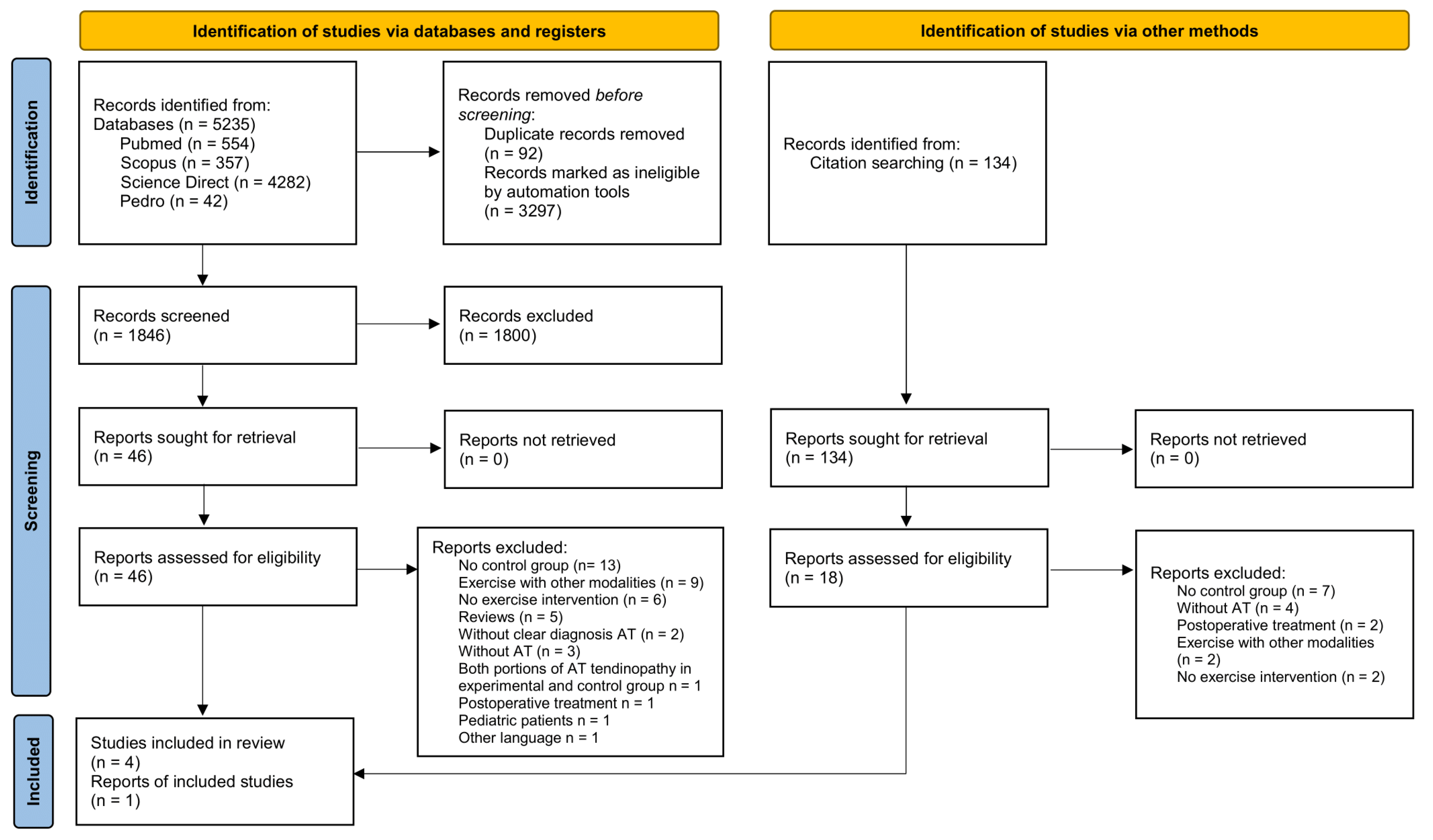
3.1. Description of Studies
3.2. Characteristics of Studies
3.3. Methodological Quality
3.4. Interventions
3.5. Compliance
3.6. Results of Outcome Measures in the Intervention and Control Groups
3.7. Groups Comparison
3.8. Exercise Effectiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kane, S.; Olewinski, L.H.; Tamminga, K.S. Management of Chronic Tendon Injuries. Am. Fam. Physician 2019, 100, 147–157. [Google Scholar] [PubMed]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef]
- van der Plas, A.; de Jonge, S.; de Vos, R.J.; van der Heide, H.J.L.; Verhaar, J.A.N.; Weir, A.; Tol, J.L. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2012, 46, 214–218. [Google Scholar] [CrossRef]
- Sobhani, S.; Dekker, R.; Postema, K.; Dijkstra, P.U. Epidemiology of ankle and foot overuse injuries in sports: A systematic review: Ankle and Foot Overuse Injuries. Scand. J. Med. Sci. Sports 2013, 23, 669–686. [Google Scholar] [CrossRef]
- Knapik, J.J.; Pope, R. Achilles Tendinopathy: Pathophysiology, Epidemiology, Diagnosis, Treatment, Prevention, and Screening. J. Spec. Oper. Med. 2020, 20, 125–140. [Google Scholar] [CrossRef]
- van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.J.; Caldwell, D.M.; Verhaar, J.A.N.; de Vos, R.-J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sports Med. 2021, 55, 249–256. [Google Scholar] [CrossRef]
- Malliaras, P. Physiotherapy management of Achilles tendinopathy. J. Physiother. 2022, 68, 221–237. [Google Scholar] [CrossRef]
- Feilmeier, M. Noninsertional Achilles Tendinopathy Pathologic Background and Clinical Examination. Clin. Podiatr. Med. Surg. 2017, 34, 129–136. [Google Scholar] [CrossRef]
- Maffulli, N.; Saxena, A.; Wagner, E.; Torre, G. Achilles insertional tendinopathy: State of the art. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2019, 4, 48–57. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Phisitkul, P.; Pearce, C.J. Achilles tendinopathy—Pathophysiology: State of the art. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2018, 3, 304–314. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef]
- Matthews, W.; Ellis, R.; Furness, J.; Hing, W.A. The clinical diagnosis of Achilles tendinopathy: A scoping review. PeerJ 2021, 9, e12166. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Alfredson, H.; Cucchiarini, M.; Madry, H.; Marmotti, A.; Mouton, C.; Oliveira, J.; Pereira, H.; Peretti, G.M.; Romero-Rodriguez, D.; et al. Current trends in tendinopathy: Consensus of the ESSKA basic science committee. Part I: Biology, biomechanics, anatomy and an exercise-based approach. J. Exp. Orthop. 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Visser, T.S.O.S.; van der Vlist, A.C.; van Oosterom, R.F.; van Veldhoven, P.; Verhaar, J.A.N.; de Vos, R.-J. Impact of chronic Achilles tendinopathy on health-related quality of life, work performance, healthcare utilisation and costs. BMJ Open Sport Exerc. Med. 2021, 7, e001023. [Google Scholar] [CrossRef] [PubMed]
- Wiegerinck, J.I.; Kerkhoffs, G.M.; van Sterkenburg, M.N.; Sierevelt, I.N.; van Dijk, C.N. Treatment for insertional Achilles tendinopathy: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2012, 21, 1345–1355. [Google Scholar] [CrossRef]
- Li, H.-Y.; Hua, Y.-H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Mobilia, G.; Dimartino, S.; Di Stefano, G.; Culmone, A.; Testa, G. Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review. J. Funct. Morphol. Kinesiol. 2019, 4, 46. [Google Scholar] [CrossRef]
- Aicale, R.; Oliviero, A.; Maffulli, N. Management of Achilles and patellar tendinopathy: What we know, what we can do. J. Foot Ankle Res. 2020, 13, 59. [Google Scholar] [CrossRef]
- Feeney, K.M. The Effectiveness of Extracorporeal Shockwave Therapy for Midportion Achilles Tendinopathy: A Systematic Review. Cureus 2022, 14, e26960. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Thomeé, R.; Eriksson, B.I.; Karlsson, J. Continued Sports Activity, Using a Pain-Monitoring Model, during Rehabilitation in Patients with Achilles Tendinopathy: A Randomized Controlled Study. Am. J. Sports Med. 2007, 35, 897–906. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Hanlon, S.; Sprague, A. Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. J. Athl. Train. 2020, 55, 438–447. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Dunn, W.R.; Thomson, A.B. Nonoperative Treatment of Midportion Achilles Tendinopathy: A Systematic Review. Clin. J. Sport Med. 2009, 19, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Chimenti, R.; Cuddeford, T.; Houck, J.; Matheson, J.W.; McDonough, C.M.; Paulseth, S.; Wukich, D.K.; Carcia, C.R. Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision 2018: Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability and Health From the Orthopaedic Section of the American Physical Therapy Association. J. Orthop. Sports Phys. Ther. 2018, 48, A1–A38. [Google Scholar] [CrossRef] [PubMed]
- Stanish, W.D.; Rubinovich, R.M.; Curwin, S. Eccentric exercise in chronic tendinitis. Clin. Orthop. Relat. Res. 1986, 208, 65–68. [Google Scholar] [CrossRef]
- Alfredson, H.; Pietilä, T.; Jonsson, P.; Lorentzon, R. Heavy-Load Eccentric Calf Muscle Training For the Treatment of Chronic Achilles Tendinosis. Am. J. Sports Med. 1998, 26, 360–366. [Google Scholar] [CrossRef]
- Arnal-Gómez, A.; Espí-López, G.V.; Cano-Heras, D.; Muñoz-Gómez, E.; Balbastre Tejedor, I.; Ramírez Iñiguez-de la Torrez, M.V.; Vicente-Herrero, M.T. Efficacy of eccentric exercise as a treatment for Achilles Tendinopathy: Literature review. Arch. Prev. Riesgos Labor. 2020, 23, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Anderson, K.M.; Goodell, C.K.; Sargeant, J.M. Conducting Systematic Reviews of Intervention Questions I: Writing the Review Protocol, Formulating the Question and Searching the Literature. Zoonoses Public Health 2014, 61, 28–38. [Google Scholar] [CrossRef]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; van Tulder, M. 2009 Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group. Spine 2009, 34, 1929–1941. [Google Scholar] [CrossRef]
- van Tulder, M.; Furlan, A.; Bombardier, C.; Bouter, L. Updated Method Guidelines for Systematic Reviews in the Cochrane Collaboration Back Review Group. Spine 2003, 28, 1290–1299. [Google Scholar] [CrossRef]
- Niesen-Vertommen, S.L.; Taunton, J.E.; Clement, D.B.; Mosher, R.E. The Effect of Eccentric Versus Concentric Exercise in the Management of Achilles Tendonitis. Clin. J. Sport Med. 1992, 2, 109–113. [Google Scholar] [CrossRef]
- Mafi, N.; Lorentzon, R.; Alfredson, H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, D.; Lee, G. Effect of Eccentric Strengthening on Pain, Muscle Strength, Endurance, and Functional Fitness Factors in Male Patients with Achilles Tendinopathy. Am. J. Phys. Med. Rehabil. 2013, 92, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.; Kongsgaard, M.; Kjær, B.H.; Øhlenschlæger, T.; Kjær, M.; Magnusson, S.P. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef]
- Habets, B.; van Cingel, R.E.; Backx, F.J.; van Elten, H.J.; Zuithoff, P.; Huisstede, B.M. No Difference in Clinical Effects When Comparing Alfredson Eccentric and Silbernagel Combined Concentric-Eccentric Loading in Achilles Tendinopathy: A Randomized Controlled Trial. Orthop. J. Sports Med. 2021, 9, 232596712110312. [Google Scholar] [CrossRef]
- Rees, J.D.; Lichtwark, G.A.; Wolman, R.L.; Wilson, A.M. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology 2008, 47, 1493–1497. [Google Scholar] [CrossRef]
- Couppé, C.; Svensson, R.B.; Silbernagel, K.G.; Langberg, H.; Magnusson, S.P. Eccentric or Concentric Exercises for the Treatment of Tendinopathies? J. Orthop. Sports Phys. Ther. 2015, 45, 853–863. [Google Scholar] [CrossRef]
- Roos, E.M.; Engstrom, M.; Lagerquist, A.; Soderberg, B. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy—A randomized trial with 1-year follow-up. Scand. J. Med. Sci. Sports 2004, 14, 286–295. [Google Scholar] [CrossRef]
- Stevens, M.; Tan, C.-W. Effectiveness of the Alfredson Protocol Compared with a Lower Repetition-Volume Protocol for Midportion Achilles Tendinopathy: A Randomized Controlled Trial. J. Orthop. Sports Phys. Ther. 2014, 44, 59–67. [Google Scholar] [CrossRef]
- Kedia, M.; Williams, M.; Jain, L.; Barron, M.; Bird, N.; Blackwell, B.; Richardson, D.R.; Ishikawa, S.; Murphy, G.A. The effects of conventional physical therapy and eccentric strengthening for insertional achilles tendinopathy. Int. J. Sports Phys. Ther. 2014, 9, 488–497. [Google Scholar] [PubMed]
- Stasinopoulos, D.; Manias, P. Comparing two eccentric exercise programmes for the management of Achilles tendinopathy. A pilot trial. J. Bodyw. Mov. Ther. 2013, 17, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dimitrios, S. Exercise for tendinopathy. World J. Methodol. 2015, 5, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Head, J.; Mallows, A.; Debenham, J.; Travers, M.J.; Allen, L. The efficacy of loading programmes for improving patient-reported outcomes in chronic midportion Achilles tendinopathy: A systematic review. Musculoskelet. Care 2019, 17, 283–299. [Google Scholar] [CrossRef]
- Murphy, M.C.; Travers, M.J.; Chivers, P.; Debenham, J.R.; Docking, S.I.; Rio, E.K.; Gibson, W. Efficacy of heavy eccentric calf training for treating mid-portion Achilles tendinopathy: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 1070–1077. [Google Scholar] [CrossRef]
- Rompe, J.D.; Furia, J.; Maffulli, N. Eccentric Loading Compared with Shock Wave Treatment for Chronic Insertional Achilles Tendinopathy: A Randomized, Controlled Trial. J. Bone Jt. Surg. 2008, 90, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, T.; Jud, H.M.; Fröhlich, V.; Mündermann, A.; Grau, S. Whole-Body Vibration Versus Eccentric Training or a Wait-and-See Approach for Chronic Achilles Tendinopathy: A Randomized Clinical Trial. J. Orthop. Sports Phys. Ther. 2013, 43, 794–803. [Google Scholar] [CrossRef]
- Avancini, C.; de Oliveira, L.F.; Menegaldo, L.L.; Vieira, T.M. Variations in the Spatial Distribution of the Amplitude of Surface Electromyograms Are Unlikely Explained by Changes in the Length of Medial Gastrocnemius Fibres with Knee Joint Angle. PLoS ONE 2015, 10, e0126888. [Google Scholar] [CrossRef]
- Roberts, T.J.; Azizi, E. Flexible mechanisms: The diverse roles of biological springs in vertebrate movement. J. Exp. Biol. 2011, 214, 353–361. [Google Scholar] [CrossRef]
- Seiberl, W.; Hahn, D.; Power, G.A.; Fletcher, J.R.; Siebert, T. Editorial: The Stretch-Shortening Cycle of Active Muscle and Muscle-Tendon Complex: What, Why and How It Increases Muscle Performance? Front. Physiol. 2021, 12, 693141. [Google Scholar] [CrossRef]
- Prudêncio, D.A.; Maffulli, N.; Migliorini, F.; Serafim, T.T.; Nunes, L.F.; Sanada, L.S.; Okubo, R. Eccentric exercise is more effective than other exercises in the treatment of mid-portion Achilles tendinopathy: Systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2023, 15, 9. [Google Scholar] [CrossRef]
- Komi, P.V. Measurement of the Force-Velocity Relationship in Human Muscle under Concentric and Eccentric Contractions. In Medicine and Sport Science; Cerquiglini, S., Venerando, A., Wartenweiler, J., Eds.; S. Karger AG: Basel, Switzerland, 1973; Volume 8, pp. 224–229. [Google Scholar] [CrossRef]
- Kramer, J.F.; MacDERMID, J. Isokinetic Measures During Concentric-Eccentric Cycles of the Knee Extensors. Aust. J. Physiother. 1989, 35, 9–14. [Google Scholar] [CrossRef]
- McCRORY, J.L.; Martin, D.F.; Lowery, R.B.; Cannon, D.W.; Curl, W.W.; Read, H.M.; Hunter, D.M.; Craven, T.; Messier, S.P. Etiologic factors associated with Achilles tendinitis in runners. Med. Sci. Sports Exerc. 1999, 31, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Malan, T.P. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain Model. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Baur, H.; Müller, S.; Hirschmüller, A.; Cassel, M.; Weber, J.; Mayer, F. Comparison in lower leg neuromuscular activity between runners with unilateral mid-portion Achilles tendinopathy and healthy individuals. J. Electromyogr. Kinesiol. 2011, 21, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.K. Cervical Outcome Measures: Testing for Postural Stability and Balance. J. Manip. Physiol. Ther. 2008, 31, 540–546. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Brorsson, A.; Lundberg, M. The Majority of Patients With Achilles Tendinopathy Recover Fully When Treated With Exercise Alone: A 5-Year Follow-Up. Am. J. Sports Med. 2011, 39, 607–613. [Google Scholar] [CrossRef]
- Byrnes, K.; Wu, P.-J.; Whillier, S. Is Pilates an effective rehabilitation tool? A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 192–202. [Google Scholar] [CrossRef]
- Cruz, J.C.; Liberali, R.; da Cruz, T.M.F.; Netto, M.I.A. The Pilates method in the rehabilitation of musculoskeletal disorders: A systematic review. Fisioter. Mov. 2016, 29, 609–622. [Google Scholar] [CrossRef]
- Anderson, B.D.; Spector, A. Introduction to Pilates-Based Rehabilitation. Orthop. Phys. Ther. 2000, 9, 395–410. [Google Scholar]
- Akbas, E.; Ulaş Erdem, E.U. Does Pilates-Based Approach Provide Additional Benefit over Traditional Physiotherapy in the Management of Rotator Cuff Tendinopathy? A Randomised Controlled Trial. Ann. Sports Med. Res. 2016, 3, 1083. [Google Scholar]
- Wajswelner, H.; Metcalf, B.; Bennell, K. Clinical Pilates versus General Exercise for Chronic Low Back Pain: Randomized Trial. Med. Sci. Sports Exerc. 2012, 44, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Bianco, A.; Paoli, A.; Messina, G.; Montalto, M.A.; Bellafiore, M.; Battaglia, G.; Iovane, A.; Palma, A. Pain Perception and Stabilometric Parameters in People With Chronic Low Back Pain after a Pilates Exercise Program: A Randomized Controlled Trial. Medicine 2016, 95, e2414. [Google Scholar] [CrossRef] [PubMed]
- Eliks, M.; Zgorzalewicz-Stachowiak, M.; Zeńczak-Praga, K. Application of Pilates-based exercises in the treatment of chronic non-specific low back pain: State of the art. Postgrad. Med. J. 2019, 95, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Çelik, D.; Turkel, N. The effectiveness of Pilates for partial anterior cruciate ligament injury. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2357–2364. [Google Scholar] [CrossRef]
- Schembri, L. How Does a Standing Exercise Programme Focusing on Hip-Abduction Strength Affect Anterior Knee Pain in Runners? Res. Health Sci. 2016, 2, 24. [Google Scholar] [CrossRef][Green Version]
- Denham-Jones, L.; Gaskell, L.; Spence, N.; Pigott, T. A systematic review of the effectiveness of Pilates on pain, disability, physical function, and quality of life in older adults with chronic musculoskeletal conditions. Musculoskelet. Care 2022, 20, 10–30. [Google Scholar] [CrossRef]
| Authors | Sample | Sex | Symptoms Duration | Age | Intervention | Control | Outcome Measures | Results | Score |
|---|---|---|---|---|---|---|---|---|---|
| Habets et al., 2021 [35] | 40 non-professional athletes Mid-portion AT AG: 18 SG: 22 | Men (55%) and women (45%) | >3 months | 18–65 | Alfredson Group (progressive loading eccentric exercises) | Silbernagel Group (Concentric-eccentric (progressive loading)-plyometrics) | VISA-A VAS-ADL VAS-sports EQ -5D GPE 0, 12, 26 weeks, 1 year | Positive for both teams | 9/12 |
| Beyer et al., 2015 [34] | 44 (out of 58 initially) non-professional athletes Mid-portion AT ECC: 25 HSR: 22 | Men (68.1%) and women (31.9%) | >3 months | 18–60 | Alfredson Group (eccentric exercises-progressive loading) | Heavy Slow Resistance (HSR) | VISA-A VAS (heel) VAS (running) Tendon swelling (A-P, ultrasound) Neovascularization (Doppler) 0, 12, 52 weeks | Positive for both teams | 8/12 |
| Yu et al., 2012 [33] | 32 EG: 16 CG: 16 | Men | 6 months at least | 20–30 | Curvin and Stanish&Alfredson et al. [24] (combination)-eccentric | Mafi et al., 2001 [32] concentric and stretches | VAS Knee and ankle muscle strength Endurance with an isokinetic dynamometer Dynamic balance (Biodex Balance Platform) Dexterity (side-step test) Agility (SargnetJump Test) 8 weeks | Pain improved more in the intervention group (IG) Knee extension, ankle dorsiflexion, plantar flexion strength (IG)-plantar flexion endurance ONLY in (IG) Dynamic balance- agility positive in both groups TBI, agility-large difference in IG | 9/12 |
| Mafi et al., 2001 [32] | 44 EG: 22 CG: 22 Mid-portion AT | Men (54.5%) and women (45.5%) | 3–120 months | 48–58 | Alfredson Group(progressive loading eccentric exercises) | Progressive loading concentric exercises | VAS Satisfaction with treatment 6, 12 weeks | Significant reduction in pain and satisfaction in IG | 8/12 |
| Niesen-Vertommen et al., 1992 [31] | 17 EG: 8 CG: 9 | Men (58.8%) and women (41.2%) | 1 month–2.6 years | 22–49 | Curvin and Stanish (progressive loading eccentric exercises) | Progressive loading concentric exercises | Pain 0–10 Return to activities 0–10 Maximum torque in concentric and eccentric Plantar flexion at speeds of 30 and 50 degrees/sec Isokinetic dynamometer 0, 4, 8, 12 weeks | Pain reduction, maximum torque- return to activities positive in both groups- emphasis in the IG | 7/12 |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Maximum Score | Score | Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Niesen-Vertommen et al. [31] | ? | ? | − | − | + | + | + | ? | + | + | + | + | 12 | 7/12 | 58% |
| Mafi et al. [32] | + | + | − | − | − | + | + | ? | + | + | + | + | 12 | 8/12 | 67% |
| Yu et al. [33] | + | + | − | − | − | + | + | + | + | + | + | + | 12 | 9/12 | 75% |
| Beyer et al. [34] | + | + | − | − | ? | + | + | ? | + | + | + | + | 12 | 8/12 | 67% |
| Habets et al. [35] | + | + | − | − | + | + | + | + | + | + | + | + | 12 | 10/12 | 83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivrika, A.P.; Papadamou, E.; Kypraios, G.; Lamnisos, D.; Georgoudis, G.; Stasinopoulos, D. Comparability of the Effectiveness of Different Types of Exercise in the Treatment of Achilles Tendinopathy: A Systematic Review. Healthcare 2023, 11, 2268. https://doi.org/10.3390/healthcare11162268
Sivrika AP, Papadamou E, Kypraios G, Lamnisos D, Georgoudis G, Stasinopoulos D. Comparability of the Effectiveness of Different Types of Exercise in the Treatment of Achilles Tendinopathy: A Systematic Review. Healthcare. 2023; 11(16):2268. https://doi.org/10.3390/healthcare11162268
Chicago/Turabian StyleSivrika, Aikaterini Pantelis, Eleni Papadamou, George Kypraios, Demetris Lamnisos, George Georgoudis, and Dimitrios Stasinopoulos. 2023. "Comparability of the Effectiveness of Different Types of Exercise in the Treatment of Achilles Tendinopathy: A Systematic Review" Healthcare 11, no. 16: 2268. https://doi.org/10.3390/healthcare11162268
APA StyleSivrika, A. P., Papadamou, E., Kypraios, G., Lamnisos, D., Georgoudis, G., & Stasinopoulos, D. (2023). Comparability of the Effectiveness of Different Types of Exercise in the Treatment of Achilles Tendinopathy: A Systematic Review. Healthcare, 11(16), 2268. https://doi.org/10.3390/healthcare11162268









