Pulmonary Fat Embolism Following Liposuction and Fat Grafting: A Review of Published Cases
Abstract
1. Introduction
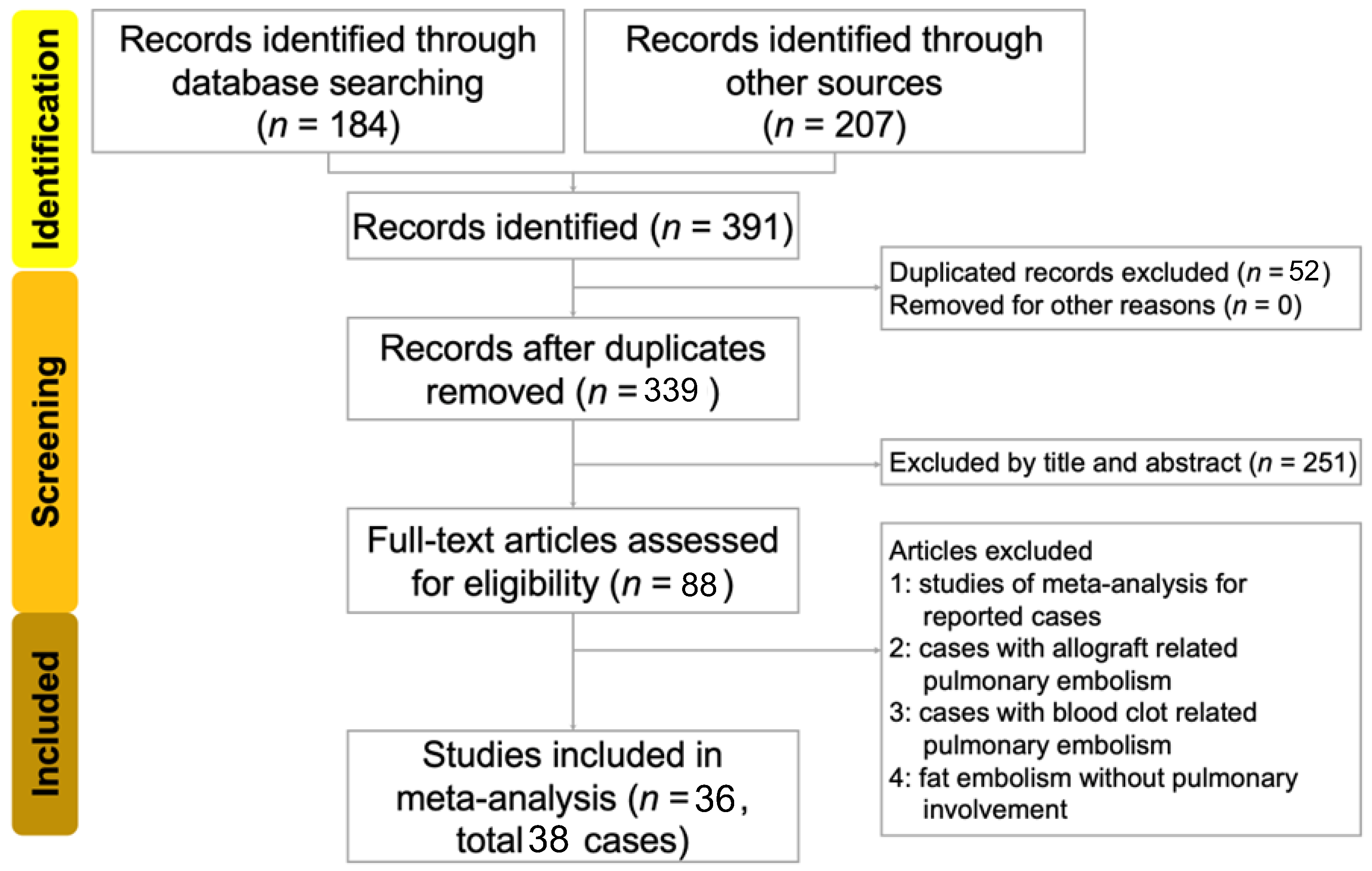
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
3. Results
3.1. Characteristics of Enrolled Studies
3.2. Symptoms, Laboratory Tests, and Diagnostic Measurements of Enrolled Patients
3.3. Treatment
3.4. Resolution Time for Pulmonary Embolism
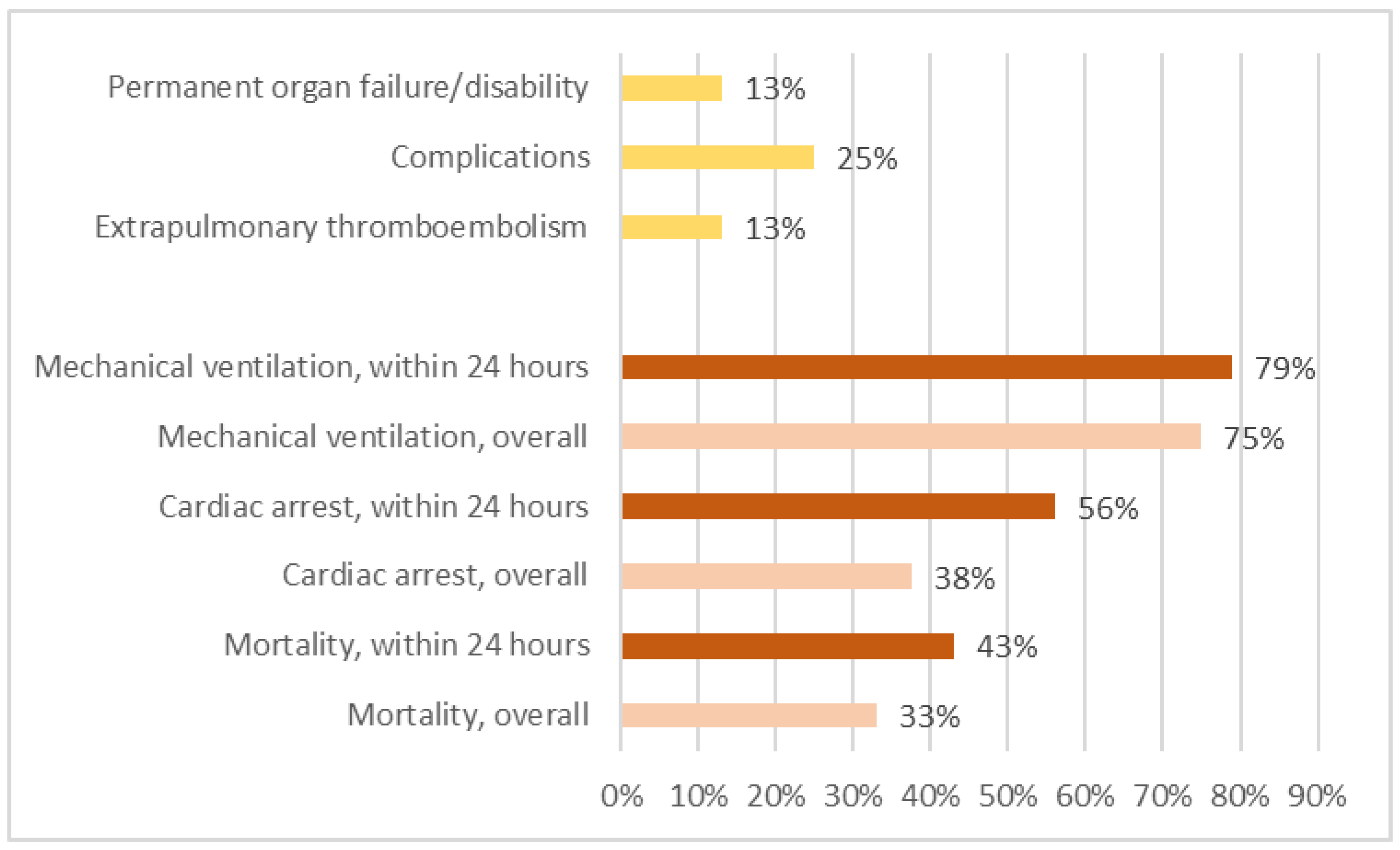
3.5. Outcome
4. Discussion
4.1. Demographics and Diagnosis
4.2. Management of Pulmonary Fat Embolism and Complications
4.3. Hospital Course and Outcome
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonell, S.; Barlow, F.K.; Griffiths, S. The cosmetic surgery paradox: Toward a contemporary understanding of cosmetic surgery popularisation and attitudes. Body Image 2021, 38, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.L.; Moore, E.E. The integral role of the plastic surgeon at a level I trauma center. Plast. Reconstr. Surg. 2003, 112, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Nejadsarvari, N.; Ebrahimi, A.; Ebrahimi, A.; Hashem-Zade, H. Medical Ethics in Plastic Surgery: A Mini Review. World J. Plast. Surg. 2016, 5, 207–212. [Google Scholar] [PubMed]
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report—2016 Cosmetic Plastic Surgery Statistics. Chicago. 2016. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics (accessed on 1 October 2022).
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Slevec, J.; Tiggemann, M. Attitudes toward cosmetic surgery in middle-aged women: Body image, aging anxiety, and the media. Psychol. Women Q. 2010, 34, 65–74. [Google Scholar] [CrossRef]
- Halk, A.B.; Habbema, L.; Genders, R.E.; Hanke, C.W. Safety Studies in the Field of Liposuction: A Systematic Review. Derm. Surg. 2019, 45, 171–182. [Google Scholar] [CrossRef]
- Doornaert, M.; Colle, J.; Maere, E.D.; Declercq, H.; Blondeel, P. Autologous fat grafting: Latest insights. Ann. Med. Surg. 2018, 37, 47–53. [Google Scholar] [CrossRef]
- Housman, T.S.; Lawrence, N.; Mellen, B.G.; George, M.N.; Filippo, J.S.; Cerveny, K.A.; De Marco, M.; Feldman, S.R.; Fleischer, A.B. The safety of liposuction: Results of a national survey. Derm. Surg. 2002, 28, 971–978. [Google Scholar] [CrossRef]
- Cantu, C.A.; Pavlisko, E.N. Liposuction-Induced Fat Embolism Syndrome: A Brief Review and Postmortem Diagnostic Approach. Arch. Pathol. Lab. Med. 2018, 142, 871–875. [Google Scholar] [CrossRef]
- He, Z.; Shi, Z.; Li, C.; Ni, L.; Sun, Y.; Arioli, F.; Wang, Y.; Ammirati, E.; Wang, D.W. Single-case metanalysis of fat embolism syndrome. Int. J. Cardiol. 2021, 345, 111–117. [Google Scholar] [CrossRef]
- Newbigin, K.; Souza, C.A.; Torres, C.; Marchiori, E.; Gupta, A.; Inacio, J.; Armstrong, M.; Peña, E. Fat embolism syndrome: State-of-the-art review focused on pulmonary imaging findings. Respir. Med. 2016, 113, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kadar, A.; Shah, V.S.; Mendoza, D.P.; Lai, P.S.; Aghajan, Y.; Piazza, G.; Camargo, E.C.; Viswanathan, K. Case 39-2021: A 26-Year-Old Woman with Respiratory Failure and Altered Mental Status. N. Engl. J. Med. 2021, 385, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.S.; Kohler, L.J.; Ruiz, R.; Cohle, S.D.; Ravichandran, P. Deaths associated with liposuction: Case reports and review of the literature. J. Forensic Sci. 2002, 47, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Crapo, R.O.; Broadbent, T.R.; Woolf, R.M. Pulmonary complications following abdominal lipectomy. Plast. Reconstr. Surg. 1983, 71, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Christman, K.D. Death following suction lipectomy and abdominoplasty. Plast. Reconstr. Surg. 1986, 78, 428. [Google Scholar] [CrossRef]
- Ross, R.M.; Johnson, G.W. Fat embolism after liposuction. Chest 1988, 93, 1294–1295. [Google Scholar] [CrossRef]
- Boezaart, A.P.; Clinton, C.W.; Braun, S.; Oettle, C.; Lee, N.P. Fulminant adult respiratory distress syndrome after suction lipectomy. A case report. S. Afr. Med. J. 1990, 78, 693–695. [Google Scholar]
- Laub, D.R., Jr.; Laub, D.R. Fat embolism syndrome after liposuction: A case report and review of the literature. Ann. Plast. Surg. 1990, 25, 48–52. [Google Scholar] [CrossRef]
- Currie, I.; Drutz, H.P.; Deck, J.; Oxorn, D. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: Case report and review of the literature. Int. Urogynecol. J. 1997, 8, 377–380. [Google Scholar] [CrossRef]
- Fourme, T.; Vieillard-Baron, A.; Loubières, Y.; Julié, C.; Page, B.; Jardin, F. Early fat embolism after liposuction. Anesthesiology 1998, 89, 782–784. [Google Scholar] [CrossRef]
- Folador, J.C.; Bier, G.E.; Camargo, R.F.; Sperandio, M. Fat embolism syndrome: Report of a case associated to liposuction. J. De Pneumol. 1999, 25, 114–117. [Google Scholar] [CrossRef]
- Scroggins, C.; Barson, P.K. Fat embolism syndrome in a case of abdominal lipectomy with liposuction. Md. Med. J. 1999, 48, 116–118. [Google Scholar] [PubMed]
- Rothmann, C.; Ruschel, N.; Streiff, R.; Pitti, R.; Bollaert, P.E. Fat pulmonary embolism after liposuction. Ann. Fr. D’anesthesie Reanim. 2006, 25, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Wessman, D.E.; Kim, T.T.; Parrish, J.S. Acute Respiratory Distress following Liposuction. Mil. Med. 2007, 172, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.N.; Mendes, D.M.; Toufen, C.; Arrunátegui, G.; Caruso, P.; de Carvalho, C.R. Adult respiratory distress syndrome due to fat embolism in the postoperative period following liposuction and fat grafting. J. Bras. Pneumol. 2008, 34, 622–625. [Google Scholar] [CrossRef]
- Erba, P.; Farhadi, J.; Schaefer, D.J.; Pierer, G. Fat embolism syndrome after combined aesthetic surgery. J. Plast. Surg. Hand Surg. 2011, 45, 51–53. [Google Scholar] [CrossRef]
- Gleeson, C.M.; Lucas, S.; Langrish, C.J.; Barlow, R.J. Acute fatal fat tissue embolism after autologous fat transfer in a patient with lupus profundus. Derm. Surg. 2011, 37, 111–115. [Google Scholar] [CrossRef]
- Shiffman, M.A. Fat Tissue Embolism following Fat Transfer. Am. J. Cosmet. Surg. 2012, 29, 145–149. [Google Scholar] [CrossRef]
- Zeidman, M.; Durand, P.; Kundu, N.; Doumit, G. Fat embolism after liposuction in Klippel-Trenaunay syndrome. J. Craniofac. Surg. 2013, 24, 1319–1321. [Google Scholar] [CrossRef]
- Cohen, L.; Engdahl, R.; Latrenta, G. Hypoxia after abdominal and thigh liposuction: Pulmonary embolism or fat embolism? Eplasty 2014, 14, ic19. [Google Scholar] [PubMed]
- Hostiuc, S.; Francisc, A.; Ceauşu, M.; Negoi, I.; Carantino, A. Lethal complications of laser assisted liposuction. Case report. Rom. J. Leg. Med. 2014, 22, 173–176. [Google Scholar] [CrossRef]
- Astarita, D.C.; Scheinin, L.A.; Sathyavagiswaran, L. Fat transfer and fatal macroembolization. J. Forensic Sci. 2015, 60, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.W.; Ban, T.H.; Rhee, C.K. A Case of Acute Fulminant Fat Embolism Syndrome after Liposuction Surgery. Tuberc. Respir. Dis. 2015, 78, 423–427. [Google Scholar] [CrossRef]
- Cárdenas-Camarena, L.; Bayter, J.E.; Aguirre-Serrano, H.; Cuenca-Pardo, J. Deaths Caused by Gluteal Lipoinjection: What Are We Doing Wrong? Plast. Reconstr. Surg. 2015, 136, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Gao, S.; Hu, Z.; Guo, Y.; Cai, J. Fat embolism as a rare complication of large-volume liposuction in a plastic patient. J. Forensic Sci. Med. 2015, 1, 68–71. [Google Scholar]
- Vidua, R.K.; Murty, O.P. Cosmetic Laser Liposuction Fatal Case. J. Forensic Med. Toxicol. 2015, 32, 45–47. [Google Scholar]
- Ali, A.; Theobald, G.; Arshad, M.A. Fat attacks!: A case of fat embolisation syndrome postliposuction. Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Sasaki, Y.; Uchi, H.; Furue, M. A case of fat embolism syndrome after liposuction. Nishinihon J. Dermatol. 2017, 79, 459–462. [Google Scholar] [CrossRef]
- Zilg, B.; Råsten-Almqvist, P. Fatal Fat Embolism after Penis Enlargement by Autologous Fat Transfer: A Case Report and Review of the Literature. J. Forensic Sci. 2017, 62, 1383–1385. [Google Scholar] [CrossRef]
- Peña, W.; Cárdenas-Camarena, L.; Bayter-Marin, J.E.; McCormick, M.; Durán, H.; Ramos-Gallardo, G.; Robles-Cervantes, J.A.; Macias, A.A. Macro fat embolism after gluteal augmentation with fat: First survival case report. Aesthetic Surg. J. 2019, 39, NP380–NP383. [Google Scholar] [CrossRef] [PubMed]
- Saon, M.; Walker, D.; Nair, G.B.; Al-Katib, S. Pulmonary fat embolism syndrome after liposuction surgery. Clin. Pulm. Med. 2019, 26, 32–35. [Google Scholar]
- Recinos, S.; Barillas, S.; Rodas, A.; Ardebol, J. A rare case of fat embolism syndrome secondary to abdominal liposuction and gluteal fat infiltration. J. Surg. Case Rep. 2020, 2020, rjaa441. [Google Scholar] [CrossRef] [PubMed]
- Kaiser Ururahy Nunes Fonseca, E.; Chate, R.C. Macroscopic Fat Embolism after Cosmetic Surgery. Radiol. Cardiothorac. Imaging 2022, 4, e210316. [Google Scholar] [CrossRef] [PubMed]
- Foula, A.S.; Ahmed, M.A.; Foula, M.S.; Nassar, M.W. Sudden Cardiac Arrest during a Prolonged Liposuction and Lipofilling Procedure: A Case Report. Cureus 2022, 14, e25985. [Google Scholar]
- Pham, M.Q. Fat Embolism after Plastic Surgery: A Case Report. Plast. Aesthetic Nurs. 2022, 42, 27–30. [Google Scholar] [CrossRef]
- Wolfe, E.M.; Weber, L.E.; Wo, L.M.; Samaha, M.J.; Mathew, P.; Garcia, O.; Singh, D. Two Cases Surviving Macro Fat Emboli Complications Following Gluteal Fat Grafting. Aesthet. Surg. J. 2022, 42, 902–906. [Google Scholar] [CrossRef]
- Scarpino, M.; Lanzo, G.; Lolli, F.; Grippo, A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int. J. Gen. Med. 2019, 12, 39–48. [Google Scholar] [CrossRef]
- Bayter-Marin, J.E.; Cárdenas-Camarena, L.; Aguirre-Serrano, H.; Durán, H.; Ramos-Gallardo, G.; Robles-Cervantes, J.A. Understanding Fatal Fat Embolism in Gluteal Lipoinjection: A Review of the Medical Records and Autopsy Reports of 16 Patients. Plast. Reconstr. Surg. 2018, 142, 1198–1208. [Google Scholar] [CrossRef]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
| Year, Author | Country | Sex | Age | Surgery | Cardiac Arrest | Mechanical Ventilation | Mortality |
|---|---|---|---|---|---|---|---|
| 1983, Hunter GR [15] | US | F | 37 | Liposuction | No | Unknown | Alive |
| 1986, Christman KD [16] | US | F | 56 | Liposuction | Yes | Yes | Dead |
| 1988, Ross RM [17] | US | F | 44 | Liposuction | No | Yes | Alive |
| 1990, Boezaart AP [18] | South Africa | F | 39 | Liposuction | No | Yes | Alive |
| 1990, Laub Jr DR [19] | US | F | 51 | Liposuction | No | No | Alive |
| 1997, Currie I [20] | Canada | F | 69 | Liposuction, fat grafting | Yes | Yes | Dead |
| 1998, Fourme T [21] | France | F | 29 | Liposuction | No | No | Alive |
| 1999, Folador JC [22] | Brazil | F | 40 | Liposuction | No | No | Alive |
| 1999, Scroggins C [23] | US | F | 54 | Liposuction | No | Yes | Alive |
| 2002, Platt MS-1 [14] | US | F | 82 | Liposuction | Yes | Yes | Dead |
| 2002, Platt MS-2 [14] | US | M | 50 | Liposuction | Yes | Yes | Dead |
| 2006, Rothmann C [24] | France | F | 24 | Liposuction | No | Yes | Alive |
| 2007, Wessman DE [25] | US | M | 31 | Liposuction | No | No | Alive |
| 2008, Costa AN [26] | Brazil | M | 53 | Liposuction, fat grafting | No | Yes | Alive |
| 2011, Erba P [27] | Switzerland | F | 46 | Liposuction | No | Yes | Alive |
| 2011, Gleeson CM [28] | UK | F | 37 | Liposuction, fat grafting | Yes | Yes | Dead |
| 2012, Shiffman MA [29] | US | F | 40 | Liposuction, fat grafting | Yes | Yes | Dead |
| 2013, Zeidman M [30] | US | F | 24 | Liposuction | No | Yes | Alive |
| 2014, Cohen L [31] | US | F | 58 | Liposuction | Unknown | Unknown | Unknown |
| 2014, Hostiuc S [32] | Romania | F | 56 | Liposuction | Yes | Yes | Dead |
| 2015, Astarita DC [33] | US | F | 42 | Liposuction, fat grafting | Yes | Yes | Dead |
| 2015, Byeon SW [34] | Korea | M | 21 | Liposuction | No | Yes | Alive |
| 2015, Cárdenas-Camarena L [35] | Colombia | F | 37 | Liposuction, fat grafting | Yes | Yes | Dead |
| 2015, Fu X [36] | China | F | 30 | Liposuction, fat grafting | No | No | Alive |
| 2015, Vidua RK [37] | India | F | 39 | Liposuction | Yes | No | Dead |
| 2016, Souza RL [12] | Brazil | F | 42 | Liposuction, fat grafting | No | Yes | Dead |
| 2017, Ali A [38] | UK | F | 45 | Liposuction | No | Yes | Alive |
| 2017, Sasaki Y [39] | Japan | F | 29 | Liposuction | No | Yes | Alive |
| 2017, Zilg B [40] | Sweden | M | 31 | Liposuction, fat grafting | Yes | Yes | Dead |
| 2019, Peña W [41] | Mexico | F | 41 | Liposuction, fat grafting | Yes | Yes | Alive |
| 2019, Saon MD [42] | US | F | 52 | Liposuction | No | No | Alive |
| 2020, Recinos S [43] | Guatemala | M | 37 | Liposuction, fat grafting | Yes | Yes | Alive |
| 2021, Kadar A [13] | US | F | 26 | Liposuction | No | Yes | Alive |
| 2022, Fonseca EKUN [44] | Brazil | F | 32 | Liposuction | Unknown | Unknown | Unknown |
| 2022, Foula AS [45] | Egypt | F | 29 | Liposuction | Yes | Yes | Alive |
| 2022, Pham MQ [46] | Vietnam | F | 37 | Liposuction | No | No | Alive |
| 2022, Wolfe EM-1 [47] | US | F | 28 | Liposuction, fat grafting | No | Yes | Alive |
| 2022, Wolfe EM-2 [47] | US | F | 26 | Liposuction, fat grafting | No | Yes | Alive |
| Demographic Data | Percentage | Body Parts | Percentage |
|---|---|---|---|
| Age (years) | 39.0 (30.3–49.0) * | Abdomen/flank | 21 (55%) |
| Sex (female) | 32 (84%) | Lower limbs | 14 (37%) |
| Comorbidity | 7 (18%) | Buttocks | 12 (32%) |
| Surgery | Breast/chest | 9 (24%) | |
| Liposuction | 38 (100%) | Upper limbs | 3 (8%) |
| Fat grafting | 13 (34%) | Head/neck | 2 (5%) |
| Others # | 3 (8%) | Penis | 2 (5%) |
| Symptoms | Percentage | Diagnostic Measurements | Percentage |
|---|---|---|---|
| Dyspnea | 21 (55%) | Examinations | |
| Hypotension | 16 (42%) | CT scan | 18/18 (100%) |
| Tachycardia | 14 (37%) | CXR | 16/19 (84%) |
| Hypoxia | 12 (32%) | Echocardiogram | 8/12 (67%) |
| Altered mental state | 9 (24%) | Bronchoalveolar lavage | 4/4 (100%) |
| Cardiac arrest | 8 (21%) | Autopsy | 10/10 (100%) |
| Fever | 7 (18%) | Pulmonary angiogram | 3/3 (100%) |
| Skin rash/petechiae | 5 (13%) | Laboratory tests | |
| Chest pain | 4 (11%) | PaO2/FiO2 ≤ 200 mmHg | 18/18 (100%) |
| Cyanosis | 4 (11%) | White cell count > 1000, < 4000/μL | 9/11 (82%) |
| Cough | 3 (8%) | Hemoglobin < 12 g/dL | 8/14 (57%) |
| Hemoptysis | 3 (8%) | Platelet < 150,000/μL | 5/11 (45%) |
| Syncope | 2 (5%) | Creatine > 1.2 g/L | 2/5 (40%) |
| Bradycardia | 2 (5%) | T bilirubin > 1.2 g/L | 2/3 (67%) |
| Neurologic deficit | 2 (5%) | D-dimer > 500 mg/L | 4/4 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-M.; Chen, K.-T.; Lee, K.-C.; Hsu, C.-C.; Chien, Y.-C. Pulmonary Fat Embolism Following Liposuction and Fat Grafting: A Review of Published Cases. Healthcare 2023, 11, 1391. https://doi.org/10.3390/healthcare11101391
Kao Y-M, Chen K-T, Lee K-C, Hsu C-C, Chien Y-C. Pulmonary Fat Embolism Following Liposuction and Fat Grafting: A Review of Published Cases. Healthcare. 2023; 11(10):1391. https://doi.org/10.3390/healthcare11101391
Chicago/Turabian StyleKao, Yu-Ming, Kuo-Tai Chen, Kuo-Chang Lee, Chien-Chin Hsu, and Yeh-Cheng Chien. 2023. "Pulmonary Fat Embolism Following Liposuction and Fat Grafting: A Review of Published Cases" Healthcare 11, no. 10: 1391. https://doi.org/10.3390/healthcare11101391
APA StyleKao, Y.-M., Chen, K.-T., Lee, K.-C., Hsu, C.-C., & Chien, Y.-C. (2023). Pulmonary Fat Embolism Following Liposuction and Fat Grafting: A Review of Published Cases. Healthcare, 11(10), 1391. https://doi.org/10.3390/healthcare11101391






