Strategies of Screening and Treating Post-Extubation Dysphagia: An Overview of the Situation in Greek-Cypriot ICUs
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Settings
2.2. Participants
2.3. Data Collection and Instrument
- Demographics
- Domain 1: Current practice
- (a)
- PED management with questions (7 multiple choice, 3 checkboxes and 1 matrix) about the existing protocols for screening, methods used to confirm the presence of PED and responsibilities of every ICU team member for assessing PED.
- (b)
- Prevention of aspiration pneumonia related to PED (2 matrix questions).
- (c)
- PED treatment interventions (1 matrix, 1 checkbox and 1 multiple choice question).
- Domain 2: Scope of the Problem
- Domain 3: Perceived Best Practice
Translation and Cultural Adaptation of the Instrument
2.4. Ethics Approval
2.5. Data Analysis
3. Results
3.1. Current Practices on PED
3.1.1. PED Management
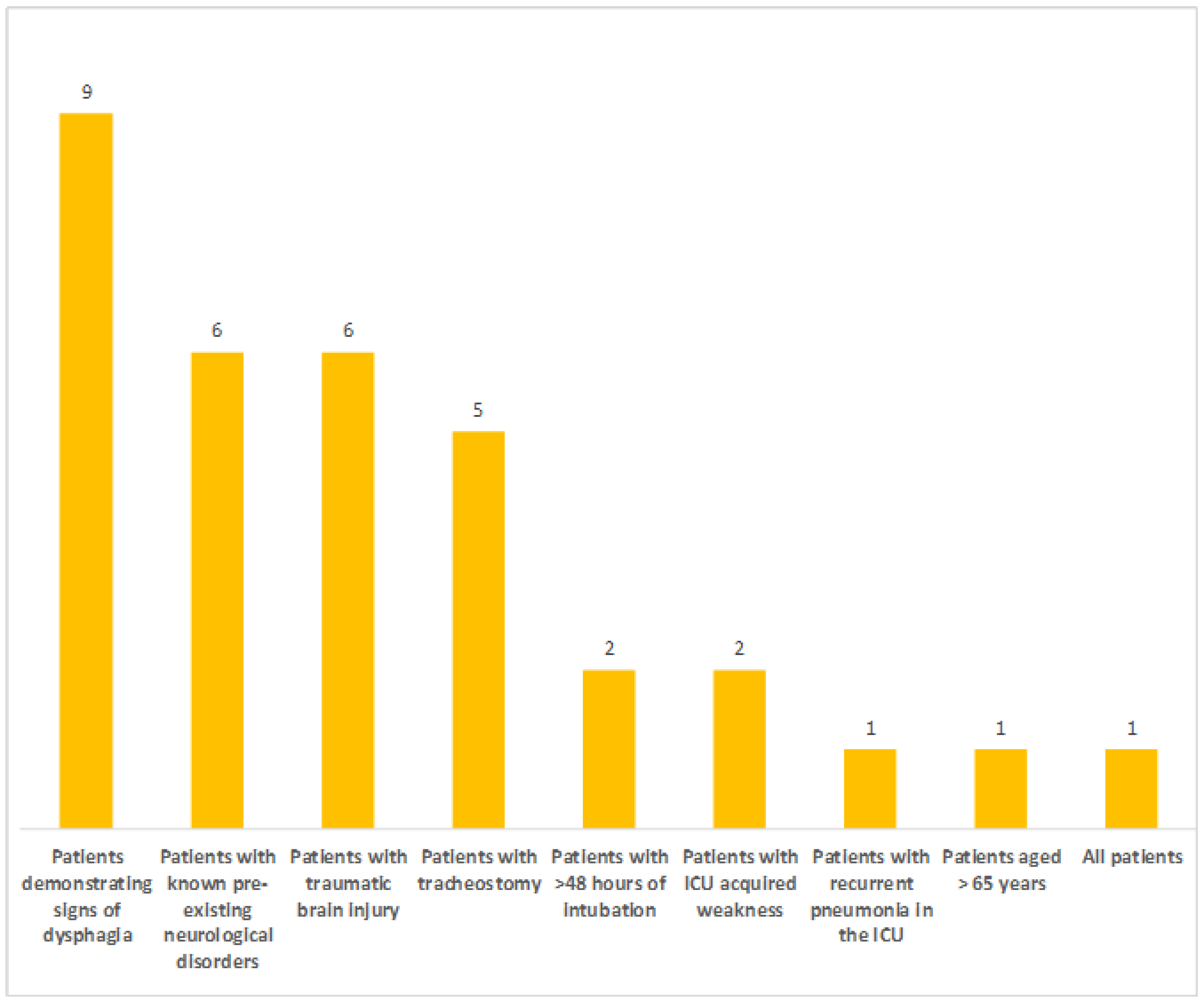
Existing Protocols and Subgroups of Patients Screened
Timing of Screening
- After ICU admission
- After extubation
Methods Used for PED Assessment
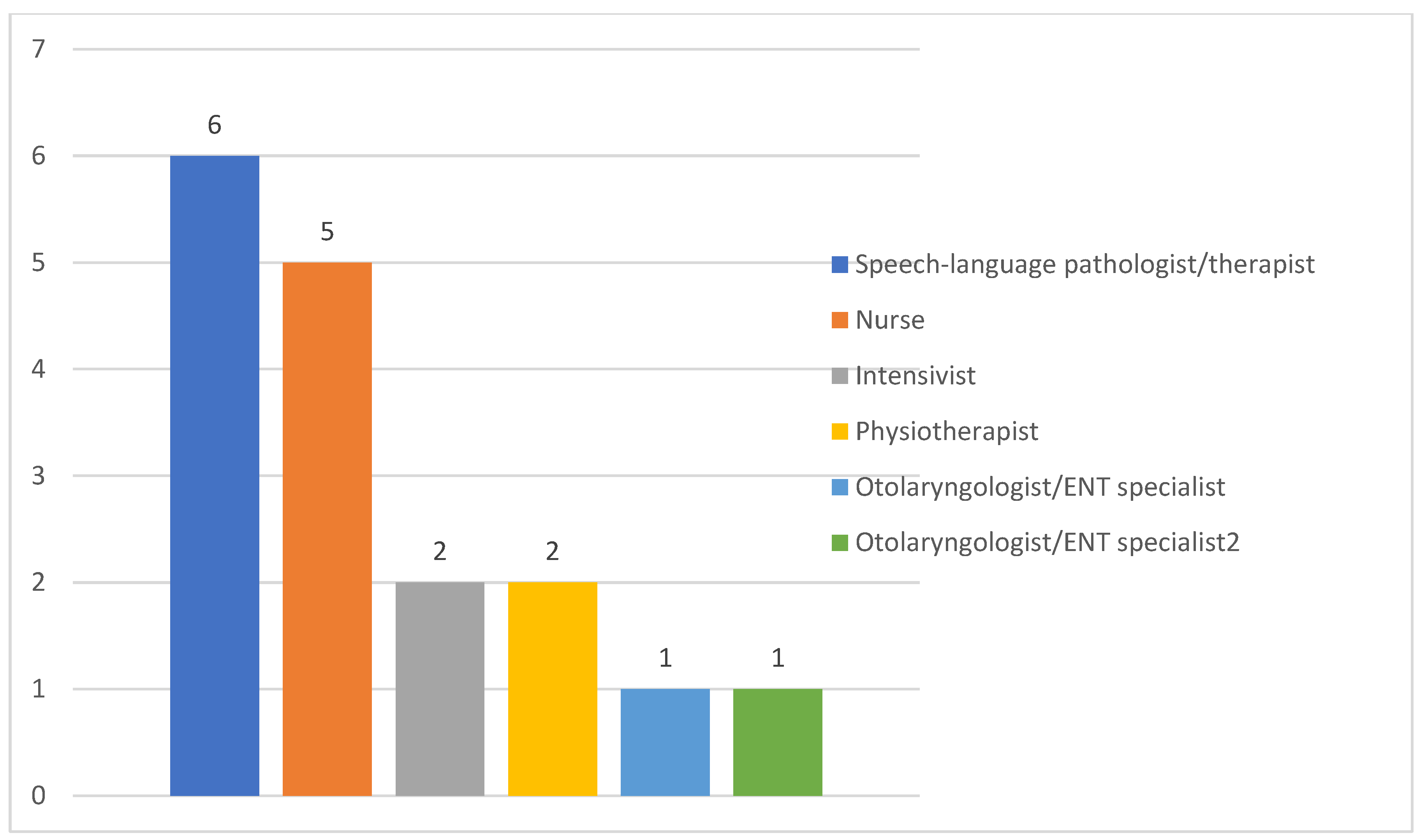
Responsibilities of ICU Team Members
3.1.2. Prevention of Aspiration Pneumonia Related to PED
Aspiration/Aspiration Pneumonia Resulting from Liquids/Solid Food
Aspiration Pneumonia Resulting from Saliva Production
3.1.3. Interventions to Treat PED
3.2. Scope of the Problem
3.2.1. Awareness of PED Incidence
3.2.2. Awareness of PED Consequences
3.3. Perceived Best Practices on PED
3.3.1. Protocols and Routine Screening
3.3.2. Availability of Screening and Treating Methods
3.3.3. Barriers to Standardized Screening and Treatment
3.3.4. Facilitators to Standardized Screening and Treatment
4. Discussion
4.1. Current Practices on PED
4.1.1. PED Management
Existing Protocols and Subgroups of Patients Screened
Timing of Screening
- After ICU admission
- After extubation
Methods Used for PED Assessment
Responsibilities of ICU Team Members
4.1.2. Prevention of Aspiration Pneumonia Related to PED
Aspiration/Aspiration Pneumonia Resulting from Liquids/Solid Food
Aspiration Pneumonia Resulting from Saliva Production
4.1.3. Interventions to Treat PED
4.2. Scope of the Problem
4.2.1. Awareness of PED Incidence
4.2.2. Awareness of PED Consequences
4.3. Perceived Best Practices on PED
4.3.1. Protocols and Routine Screening
4.3.2. Availability of Screening and Treating Methods
4.3.3. Barriers to Standardized Screening and Treatment
4.3.4. Facilitators to Standardized Screening and Treatment
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suntrup-Krueger, S.; Muhle, P.; Kampe, I.; Egidi, P.; Ruck, T.; Lenze, F.; Jungheim, M.; Gminski, R.; Labeit, B.; Claus, I.; et al. Effect of capsaicinoids on neurophysiological, biochemical, and mechanical parameters of swallowing function. Neurotherapeutics 2021, 18, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en (accessed on 4 January 2023).
- Rajati, F.; Ahmadi, N.; Naghibzadeh, Z.A.; Kazeminia, M. The global prevalence of oropharyngeal dysphagia in different populations: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Krishnaswami, S.; Steger, E.; Conover, E.; Vaezi, M.F.; Ciucci, M.R.; Francis, D.O. Economic and survival burden of dysphagia among inpatients in the United States. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2018, 31, dox131. [Google Scholar] [CrossRef] [PubMed]
- Kertscher, B.; Speyer, R.; Fong, E.; Georgiou, A.M.; Smith, M. Prevalence of oropharyngeal dysphagia in the Netherlands: A telephone survey. Dysphagia 2015, 30, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Colletti, C.M.; Ding, M.C. Post-stroke Dysphagia: Recent insights and unanswered questions. Curr. Neurol. Neurosci. Rep. 2020, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Uemura, H.; Kimura, T.; Nishimura, A.; Aoki, K.; Otsuka, S.; Ueda, K.; Kitahara, T. Evaluation of usefulness of tongue pressure measurement device for dysphagia associated with treatment of patients with head and neck cancer (ELEVATE). Medicine 2023, 102, e33954. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, S.J.; Jakobsen, D.; Riberholt, C.G.; Poulsen, I.; Curtis, D.J. Protocol for a scoping review study to identify and map treatments for dysphagia following moderate to severe acquired brain injury. BMJ Open 2019, 9, e029061. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Moret, C.S.; Dziewas, R.; Schefold, J.C. Dysphagia in the intensive care unit: Epidemiology, mechanisms, and clinical management. Crit. Care 2019, 23, 103. [Google Scholar] [CrossRef]
- Mc Intyre, M.; Doeltgen, S.; Dalton, N.; Koppa, M.; Chimunda, T. Post Extubation dysphagia incidence in critically ill patients: A systematic review and meta-analysis. Aust. Crit. Care 2020, 34, 67–75. [Google Scholar] [CrossRef]
- Schefold, J.C.; Berger, D.; Zürcher, P.; Lensch, M.; Perren, A.; Jakob, S.M.; Parviainen, I.; Takala, J. Dysphagia in mechanically ventilated ICU patients (Dynamics): A prospective observational trial. Crit. Care Med. 2017, 45, 2061–2069. [Google Scholar] [CrossRef]
- Brodsky, M.B.; Huang, M.; Shanholtz, C.; Mendez-Tellez, P.A.; Palmer, J.B.; Colantuoni, E.; Needham, D.M. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5-Year longitudinal study. Ann. Am. Thorac. Soc. 2017, 14, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Rofes, L.; Muriana, D.; Palomeras, E.; Vilardell, N.; Palomera, E.; Alvarez-Berdugo, D.; Casado, V.; Clavé, P. Prevalence, risk factors and complications of oropharyngeal dysphagia in stroke patients: A cohort study. Neurogastroenterol. Motil. 2018, 23, e13338. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Martín-Martínez, A.; Alarcón, C.; Riera, S.A.; Miró, J.; Amadó, C.; Clavé, P.; Ortega, O. A Comparative Study between the Three Waves of the Pandemic on the Prevalence of Oropharyngeal Dysphagia and Malnutrition among Hospitalized Patients with COVID-19. Nutrients 2022, 16, 3826. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Moser, M.; Waskowski, J.; Pfortmueller, C.A.; Schefold, J.C. Dysphagia post-extubation affects long-term mortality in mixed adult ICU patients-Data from a large prospective observational study with systematic dysphagia screening. Crit. Care Explor. 2022, 4, e0714. [Google Scholar] [CrossRef] [PubMed]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef]
- Zielske, J.; Bohne, S.; Brunkhorst, F.M.; Axer, H.; Guntinas-Lichius, O. Acute and long-term dysphagia in critically ill patients with severe sepsis: Results of a prospective controlled observational study. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 3085–3093. [Google Scholar] [CrossRef]
- Hongo, T.; Yumoto, T.; Naito, H.; Fujiwara, T.; Kondo, J.; Nozaki, S.; Nakao, A. Frequency, associated factors, and associated outcomes of dysphagia following sepsis. Aust. Crit. Care 2022, 36, 521–527. [Google Scholar] [CrossRef]
- Royals, W.J.; Gillis, R.J.; Campbell, J.L. A Decision Guide for Assessing the Recently Extubated Patient’s Readiness for Safe Oral Intake. Crit. Care Nurse 2023, 43, 42–51. [Google Scholar] [CrossRef]
- Christensen, M.; Trapl, M. Development of a modified swallowing screening tool to manage post-extubation dysphagia. Nurs. Crit. Care 2018, 23, 102–107. [Google Scholar] [CrossRef]
- Dallal York, J.; Miller, S.; Chapin, J.; Gore, S.; Jeng, E.I.; Plowman, E.K. Swallowing screening practice patterns for nurses in the cardiac surgery intensive care unit. J. Clin. Nurs. 2020, 29, 4573–4582. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Kaldan, G.; Nielsen, B.H.; Kristensen, G.J.; Shiv, L.; Egerod, I. Intensive care professionals’ perspectives on dysphagia management: A focus group study. Aust. Crit. Care 2023, 36, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Marian, T.; Dünser, M.; Citerio, G.; Koköfer, A.; Dziewas, R. Are intensive care physicians aware of dysphagia? The MADICU survey results. Intensive Care Med. 2018, 44, 973–975. [Google Scholar] [CrossRef] [PubMed]
- McCarty, E.B.; Chao, T.N. Dysphagia and swallowing disorders. Med. Clin. N. Am. 2021, 105, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Denk-Linnert, D.M.; Farneti, D.; Nawka, T.; Am Zehnhoff-Dinnesen, A.; Moerman, M.; Zorowka, P.; Farahat, M.; Schindler, A.; Geneid, A. Position statement of the Union of European Phoniatricians (UEP): Fees and phoniatricians’ role in multidisciplinary and multiprofessional dysphagia management team. Dysphagia 2023, 38, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Omura, K.; Komine, A.; Yanagigawa, M.; Chiba, N.; Osada, M. Frequency and outcome of post-extubation dysphagia using nurse-performed swallowing screening protocol. Nurs. Crit. Care 2019, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.; Spronk, L.E.J.; Egerod, I.; McGaughey, J.; McRae, J.; Rose, L.; Brodsky, M.B.; DICE Study Investigators. Dysphagia in Intensive Care Evaluation (DICE): An international cross-sectional survey. Dysphagia 2022, 37, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Darvall, J.N.; Bellomo, R.; Bailey, M.; Paul, E.; Young, P.J.; Reid, A.; Rockwood, K.; Pilcher, D. Routine frailty screening in critical illness: A population based cohort study in Australia and New Zealand. Chest 2021, 160, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Darvall, J.N.; Bellomo, R.; Bailey, M.; Paul, E.; Young, P.J.; Rockwood, K.; Pilcher, D. Frailty and outcomes from pneumonia in critical illness: A population-based cohort study. Br. J. Anaesth. 2020, 125, 730–738. [Google Scholar] [CrossRef]
- Kalaiselvan, M.S.; Yadav, A.; Kaur, R.; Menon, A.; Wasnik, S. Prevalence of frailty in ICU and its impact on patients’ outcomes. Indian J. Crit. Care Med. 2023, 27, 335–341. [Google Scholar] [CrossRef]
- Hollinghurst, J.; Smithard, D.G. Identifying dysphagia and demographic associations in older adults using electronic health records: A national longitudinal observational study in Wales (United Kingdom) 2008–2018. Dysphagia 2022, 37, 1612–1622. [Google Scholar] [CrossRef]
- Smithard, D.G. Dysphagia: A Geriatric Giant? Med. Clin. Rev. 2016, 2, 5. [Google Scholar] [CrossRef]
- Hu, A. June is national dysphagia awareness month. Ear Nose Throat J. 2017, 96, 194–199. [Google Scholar] [CrossRef] [PubMed]
- STROBE—Strengthening the Reporting of Observational Studies in Epidemiology. Available online: https://www.strobe-statement.org/ (accessed on 7 January 2023).
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P.; ISPOR Task Force for Translation and Cultural Adaptation. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef]
- Van Snippenburg, W.; Kröner, A.; Flim, M.; Hofhuis, J.; Buise, M.; Hemler, R.; Spronk, P. Awareness and management of dysphagia in Dutch intensive care units: A nationwide survey. Dysphagia 2019, 34, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Macht, M.; Wimbish, T.; Clark, B.J.; Benson, A.B.; Burnham, E.L.; Williams, A.; Moss, M. Diagnosis and treatment of post-extubation dysphagia: Results from a national survey. J. Crit. Care 2012, 27, 578–586. [Google Scholar] [CrossRef]
- Brodsky, M.B.; Nollet, J.L.; Spronk, P.E.; González-Fernández, M. Prevalence, pathophysiology, diagnostic modalities, and treatment options for dysphagia in critically ill patients. Am. J. Phys. Med. Rehabil. 2020, 99, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Altman, K.W.; Yu, G.P.; Schaefer, S.D. Consequence of dysphagia in the hospitalized patient: Impact on prognosis and hospital resources. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Zürcher, P.; Schefold, J.C. Clinical approaches to assess post-extubation dysphagia (PED) in the critically ill. Dysphagia 2019, 34, 475–486. [Google Scholar] [CrossRef]
- Hongo, T.; Yamamoto, R.; Liu, K.; Yaguchi, T.; Dote, H.; Saito, R.; Masuyama, T.; Nakatsuka, K.; Watanabe, S.; Kanaya, T.; et al. Association between timing of speech and language therapy initiation and outcomes among post-extubation dysphagia patients: A multicenter retrospective cohort study. Crit. Care 2022, 26, 98. [Google Scholar] [CrossRef]
- Brodsky, M.B.; Suiter, D.M.; González-Fernández, M.; Michtalik, H.J.; Frymark, T.B.; Venediktov, R.; Schooling, T. Screening accuracy for aspiration using bedside water swallow tests: A systematic review and meta-analysis. Chest 2016, 150, 148–163. [Google Scholar] [CrossRef]
- Zuercher, P.; Moret, C.; Schefold, J.C. Dysphagia in the intensive care unit in Switzerland (DICE)—Results of a national survey on the current standard of care. Swiss Med. Wkly. 2019, 149, w20111. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.; Montgomery, E.; Garstang, Z.; Cleary, E. The role of speech and language therapists in the intensive care unit. J. Intensive Care Soc. 2020, 21, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.; Mills, C.; Walshe, M. Perspectives on speech and language pathology practices and service provision in adult critical care settings in Ireland and international settings: A cross-sectional survey. Int. J. Speech Lang. Pathol. 2023, 25, 219–230. [Google Scholar] [CrossRef] [PubMed]
- ASHA Multiskilled Personnel, Position Statement. American Speech-Language-Hearing Association. Available online: https://www.asha.org/policy/ps1997-00225/ (accessed on 10 July 2023).
- Cardinal, L.A.; Freeman-Sanderson, A.; Togher, L. The speech pathology workforce in intensive care units: Results from a national survey. Aust. Crit. Care 2020, 33, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.; Heaton, S.; Bassett, L. Triaging dysphagia: Nurse screening for dysphagia in an acute hospital. J. Clin. Nurs. 2009, 18, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Peng, S.Y.; Phua, J.; Sum, C.L.; Concepcion, J. Nurse-performed screening for postextubation dysphagia: A retrospective cohort study in critically ill medical patients. Crit. Care 2016, 20, 326. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Zafar, H.; Al-Eisa, E.S.; Iqbal, Z.A. Effect of posture on swallowing. Afr. Health Sci. 2017, 17, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.Y.; Yi, C.H.; Park, I.W.; Yong, J.H. Effects of sitting posture and bolus volume on activation of swallowing-related muscles. J. Oral Rehabil. 2020, 47, 577–583. [Google Scholar] [CrossRef]
- Iordanou, S.; Papathanassoglou, E.; Middleton, N.; Palazis, L.; Timiliotou-Matsentidou, C.; Raftopoulos, V. Device-associated health care-associated infections: The effectiveness of a 3-year prevention and control program in the Republic of Cyprus. Nurs. Crit. Care 2020, 27, 602–611. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, X.; Zhang, Q.; Li, C.; Worthington, H.V.; Hua, F. Oral. hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2020, 12, CD008367. [Google Scholar] [CrossRef]
- Langmore, S.E.; Terpenning, M.S.; Schork, A.; Chen, Y.; Murray, J.T.; Lopatin, D.; Loesche, W.J. Predictors of aspiration pneumonia: How important is dysphagia? Dysphagia 1998, 13, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Seedat, J.; Penn, C. Implementing oral care to reduce aspiration pneumonia amongst patients with dysphagia in a South African setting. S. Afr. J. Commun. Disord. 2016, 63, 102. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Yoshida, M.; Ohrui, T.; Mukaiyama, H.; Okamoto, H.; Hoshiba, K.; Ihara, S.; Yanagisawa, S.; Ariumi, S.; Morita, T.; et al. Oral care reduces pneumonia in older patients in nursing homes. J. Am. Geriatr. Soc. 2002, 50, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Tablan, O.C.; Anderson, L.J.; Besser, R.; Bridges, C.; Hajjeh, R.; CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care–associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm. Rep. 2004, 26, 1–36. [Google Scholar]
- Watando, A.; Ebihara, S.; Ebihara, T.; Okazaki, T.; Takahashi, H.; Asada, M.; Sasaki, H. Daily oral care and cough reflex sensitivity in elderly nursing home patients. Chest 2004, 126, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Jost, W.; Bäumer, T.; Beutner, D.; Degenkolb-Weyers, S.; Groß, M.; Grosheva, M.; Hakim, S.; Kahl, K.G.; Laskawi, R.; et al. Hypersalivation: Update of the German S2k guideline (AWMF) in short form. J. Neural Transm. 2019, 126, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, H.J. Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur. J. Phys. Rehabil. Med. 2017, 53, 426–432. [Google Scholar] [CrossRef]
- Park, J.S.; An, D.H.; Oh, D.H.; Chang, M.Y. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: A randomized pilot study. NeuroRehabilitation 2018, 42, 191–197. [Google Scholar] [CrossRef]
- Park, J.S.; Hwang, N.K. Chin tuck against resistance exercise for dysphagia rehabilitation: A systematic review. J. Oral Rehabil. 2021, 48, 968–977. [Google Scholar] [CrossRef]
- El Gharib, A.Z.G.; Berretin-Felix, G.; Rossoni, D.F.; Seiji Yamada, S. Effectiveness of Therapy on Post-Extubation Dysphagia: Clinical and Electromyographic Findings. Clin. Med. Insights Ear Nose Throat 2019, 12, 1179550619873364. [Google Scholar] [CrossRef]
- Suntrup, S.; Marian, T.; Schröder, J.B.; Suttrup, I.; Muhle, P.; Oelenberg, S.; Hamacher, C.; Minnerup, J.; Warnecke, T.; Dziewas, R. Electrical pharyngeal stimulation for dysphagia treatment in tracheotomized stroke patients: A randomized controlled trial. Intensive Care Med. 2015, 41, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.; McCabe, D.; Wheeler-Hegland, K.; Frymark, T.; Mullen, R.; Musson, N.; Schooling, T.H.C. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III–Impact of dysphagia treatments on populations with neurological disorders. J. Rehabil. Res. Dev. 2009, 46, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.C.; Medeiros, G.C.; Zambon, L.S.; Zilberstein, B.; Andrade, C.R.F. Evaluation and classification of post-extubation dysphagia in critically ill patients. Rev. Col. Bras. Cir. 2018, 45, e1687. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.B.; Gellar, J.E.; Dinglas, V.D.; Colantuoni, E.; Mendez-Tellez, P.A.; Shanholtz, C.; Palmer, J.B.; Needham, D.M. Duration of oral endotracheal intubation is associated with dysphagia symptoms in acute lung injury patients. J. Crit. Care 2014, 29, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.B.; Levy, M.J.; Jedlanek, E.; Pandian, V.; Blackford, B.; Price, C.; Cole, G.; Hillel, A.T.; Best, S.R.; Akst, L.M. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: A systematic review. Crit. Care Med. 2018, 46, 2010–2017. [Google Scholar] [CrossRef]
- Rousou, J.A.; Tighe, D.A.; Garb, J.L.; Krasner, H.; Engelman, R.M.; Flack, J.E.; Deaton, D.W. Risk of dysphagia after transesophageal echocardiography during cardiac operations. Ann. Thorac. Surg. 2000, 69, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Macht, M.; Wimbish, T.; Bodine, C.; Moss, M. ICU-acquired swallowing disorders. Crit. Care Med. 2013, 41, 2396–2405. [Google Scholar] [CrossRef]
- Naved, S.A.; Siddiqui, S.; Khan, F.H. APACHE-II score correlation with mortality and length of stay in an intensive care unit. J. Coll. Phys. Surg. Pak. 2011, 21, 4–8. [Google Scholar]
- Zeng, L.; Song, Y.; Dong, Y.; Wu, Q.; Zhang, L.; Yu, L.; Gao, L.; Shi, Y. Risk score for predicting dysphagia in patients after neurosurgery: A prospective observational trial. Front. Neurol. 2021, 12, 605687. [Google Scholar] [CrossRef]
- Melgaard, D.; Rodrigo-Domingo, M.; Mørch, M.M. The prevalence of oropharyngeal dysphagia in acute geriatric patients. Geriatriacs 2018, 3, 15. [Google Scholar] [CrossRef]
- Matsuo, H.; Yoshimura, Y.; Ishizaki, N.; Ueno, T. Dysphagia is associated with functional decline during acute-care hospitalization of older patients. Geriatr. Gerontol. Int. 2017, 17, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.D.; Modlinski, R.M.; Poulsen, S.H.; Rosenvinge, P.M.; Rasmussen, H.H.; Holst, M. Prevalence of signs of dysphagia and associated risk factors in geriatric patients admitted to an acute medical unit. Clin. Nutr. ESPEN 2021, 41, 208–216. [Google Scholar] [CrossRef] [PubMed]
- NCVHS. National Committee on Vital and Health Statistics: Classifying and Reporting Functional Status Subcommittee on Populations National Committee on Vital and Health Statistics (NCVHS) Classifying and Reporting Functional Status. Available online: https://www.ncvhs.hhs.gov/wp-content/uploads/2017/08/010617rp.pdf (accessed on 17 January 2023).
- Regala, M.; Marvin, S.; Ehlenbach, W.J. Association between post-extubation dysphagia and long-term mortality among critically ill older adults. J. Am. Geriatr. Soc. 2019, 67, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Connor, L.; Dean, J.; McNett, M.; Tydings, D.M.; Shrout, A.; Gorsuch, P.F.; Hole, A.; Moore, L.; Brown, R.; Melnyk, B.M.; et al. Evidence-based practice improves patient outcomes and healthcare system return on investment: Findings from a scoping review. Worldviews Evid. Based Nurs. 2023, 20, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dinglas, V.D.; Faraone, L.N.; Needham, D.M. Understanding patient-important outcomes after critical illness: A synthesis of recent qualitative, empirical, and consensus-related studies. Curr. Opin. Crit. Care 2018, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Glotta, A.; Galli, A.; Biggiogero, M.; Bona, G.; Mauri, R.; Saporito, A.; Capdevil, X. Dysphagic disorder in a cohort of COVID-19 patients: Evaluation and evolution. Ann. Med. Surg. 2021, 69, 102837. [Google Scholar] [CrossRef] [PubMed]
- Clayton, N.A.; Walker, E.; Freeman-Sanderson, A. Clinical profile and recovery pattern of dysphagia in the COVID-19 patient: A prospective observational cohort within NSW. Aust. Crit. Care 2023, 36, 262. [Google Scholar] [CrossRef] [PubMed]
- Anesi, G.L.; Kerlin, M.P. The impact of resource limitations on care delivery and outcomes: Routine variation, the coronavirus disease 2019 pandemic, and persistent shortage. Curr. Opin. Crit. Care 2021, 27, 513–519. [Google Scholar] [CrossRef]
- Kozlow, J.H.; Berenholtz, S.M.; Garrett, E.; Dorman, T.; Pronovost, P.J. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit. Care Med. 2003, 31, 1930–1937. [Google Scholar] [CrossRef]
- Temsah, M.H.; Jamal, A.; Aljamaan, F.; Al-Tawfiq, J.A.; Al-Eyadhy, A. ChatGPT-4 and the Global Burden of Disease Study: Advancing Personalized Healthcare Through Artificial Intelligence in Clinical and Translational Medicine. Cureus 2023, 23, e39384. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, H.; Qi, S.; Cheng, K. Artificial Intelligence in Intensive Care Medicine: Toward a ChatGPT/GPT-4 Way? Ann. Biomed. Eng. 2023, 51, 1898–1903. [Google Scholar] [CrossRef]




| ICU Patient Capacity in Beds | ICU Type | Hospital Capacity | SLP/SLT Available | |
|---|---|---|---|---|
| 1 | 5–9 ** | Mixed medical/surgical | <200 | No |
| 2 | 5–9 * | Mixed medical/surgical/neurosurgical | <200 | No |
| 3 | 5–9 * | Mixed medical/surgical | 200–499 | No |
| 4 | 5–9 * | Mixed medical/surgical | <200 | No |
| 5 | 5–9 ** | Medical/surgical/Neurosurgical/cardiothoracic | <200 | Yes, not ICU-dedicated |
| 6 | 5–9 ** | Mixed medical/surgical | <200 | No |
| 7 | 5–9 ** | Medical/surgical/Neurosurgical/cardiothoracic | <200 | Yes, not ICU-dedicated |
| 8 | 10–14 ** | Mixed medical/surgical | <200 | Yes, not ICU-dedicated |
| 9 | 10–14 * | Mixed medical/surgical | 200–499 | No |
| 10 | 10–14 * | Mixed medical/surgical | <200 | Yes, not ICU-dedicated |
| 11 | 15–19 * | Medical/surgical/Neurosurgical/cardiothoracic | 200–499 | Yes, not ICU-dedicated |
| 12 | 10–14 * | Coronary Unit | 200–499 | Yes, not ICU-dedicated |
| 13 | 15–19 * | Mixed medical/surgical | 200–499 | Yes, not ICU-dedicated |
| 14 | 5–9 * | Burns Unit | 200–499 | Yes, not ICU-dedicated |
| Percentage of Patients | |||||||
|---|---|---|---|---|---|---|---|
| 0% | <25% | 25–50% | 51–75% | >75% | Not Available | Unfamiliar with This Intervention | |
| Intervention used to treat PED | |||||||
| Repetitive swallowing exercises/maneuvers with or without additional resistance (e.g., Mendelsohn or Masako maneuver, supraglottic swallow) | 3 | 1 | 3 | 1 | 1 | 5 | |
| Muscle-strengthening exercises without swallowing (e.g., chin tuck against resistance or Shaker exercise) | 3 | 3 | 0 | 1 | 2 | 5 | |
| Muscle-strengthening exercises using apps on a tablet/iPad | 7 | 1 | 0 | 0 | 1 | 5 | |
| Respiratory exercises [e.g., expiratory muscle strength training (EMST)] | 5 | 0 | 0 | 2 | 3 | 4 | |
| Neuromuscular electrical stimulation (NEMS) of swallowing muscles | 7 | 1 | 1 | 0 | 0 | 1 | 4 |
| Surface EMG (sEMG) biofeedback swallowing training | 8 | 1 | 1 | 4 | |||
| Pharyngeal electrical stimulation (PES) | 8 | 2 | 4 | ||||
| Survey Item | Proportion in Agreement | Mean (Standard Deviation) | Median (IQR) | Modal Value (Appearance Times) |
|---|---|---|---|---|
| Oropharyngeal dysphagia influences ICU length of stay | 12/14 (85.7%) | 5.64 (1.33) | 6 (1) | 6 (7) |
| Oropharyngeal dysphagia influences hospital length of stay | 10/14 (71.4%) | 4.57 (2.4) | 6 (5) | 6 (6) |
| Oropharyngeal dysphagia influences the delay in return to independent physical functioning after critical illness | 14/14 (100%) | 6.57 (0.64) | 7 (1) | 7 (9) |
| Oropharyngeal dysphagia influences the need for care at long-term facilities or nursing homes after critical illness | 14/14 (100%) | 6.35 (0.63) | 6 (1) | 6 (7) |
| The presence of oropharyngeal dysphagia influences the risk of ICU-readmission | 10/14 (71.4%) | 4.85 (2.03) | 5 (5) | 2, 5, 7 (4 each) |
| Water Swallow Test (Including the Yale Swallow Protocol) |
| Gugging Swallowing Screen (GUSS) |
| Volume-viscosity swallow test |
| Oral mechanism exam |
| Methylene (Evan’s) blue dye test |
| Cervical auscultation |
| Video fluoroscopic swallowing study (VFSS) |
| Fiberoptic endoscopic evaluation of swallowing (FEES) |
| No Need for Dysphagia-Specific Treatment, the Dysphagia will Disappear when the Patient’s Strength Increases |
| Protocolized changing in fluid consistency and texture |
| Protocolized postural changes (chin down, etc.) |
| Repetitive swallowing exercises/maneuvers (e.g., Mendelsohn or Masako maneuver, supraglottic swallow) |
| Muscle-strengthening exercises without swallowing (e.g., chin tuck against resistance or Shaker exercise) |
| Muscle-strengthening exercises using apps on a tablet/iPad |
| Respiratory exercises (e.g., expiratory muscle strength training (EMST)) |
| Smaller bore gastric feeding tube |
| Change to PEG-tube |
| If tracheostomy is present, replace with a smaller cannula tube |
| If tracheostomy is present only because of managing airway secretions, remove entirely |
| Neuromuscular electrical stimulation (NEMS) of swallowing muscles |
| Surface EMG biofeedback swallowing training |
| Pharyngeal electrical stimulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpouzika, M.; Iordanou, S.; Kyranou, M.; Iliopoulou, K.; Parissopoulos, S.; Kalafati, M.; Karanikola, M.; Papathanassoglou, E. Strategies of Screening and Treating Post-Extubation Dysphagia: An Overview of the Situation in Greek-Cypriot ICUs. Healthcare 2023, 11, 2283. https://doi.org/10.3390/healthcare11162283
Mpouzika M, Iordanou S, Kyranou M, Iliopoulou K, Parissopoulos S, Kalafati M, Karanikola M, Papathanassoglou E. Strategies of Screening and Treating Post-Extubation Dysphagia: An Overview of the Situation in Greek-Cypriot ICUs. Healthcare. 2023; 11(16):2283. https://doi.org/10.3390/healthcare11162283
Chicago/Turabian StyleMpouzika, Meropi, Stelios Iordanou, Maria Kyranou, Katerina Iliopoulou, Stelios Parissopoulos, Maria Kalafati, Maria Karanikola, and Elizabeth Papathanassoglou. 2023. "Strategies of Screening and Treating Post-Extubation Dysphagia: An Overview of the Situation in Greek-Cypriot ICUs" Healthcare 11, no. 16: 2283. https://doi.org/10.3390/healthcare11162283
APA StyleMpouzika, M., Iordanou, S., Kyranou, M., Iliopoulou, K., Parissopoulos, S., Kalafati, M., Karanikola, M., & Papathanassoglou, E. (2023). Strategies of Screening and Treating Post-Extubation Dysphagia: An Overview of the Situation in Greek-Cypriot ICUs. Healthcare, 11(16), 2283. https://doi.org/10.3390/healthcare11162283









