Rare or Overlooked Cases of Acute Acalculous Cholecystitis in Young Patients with Central Nervous System Lesion
Abstract
1. Introduction
2. Case Presentation
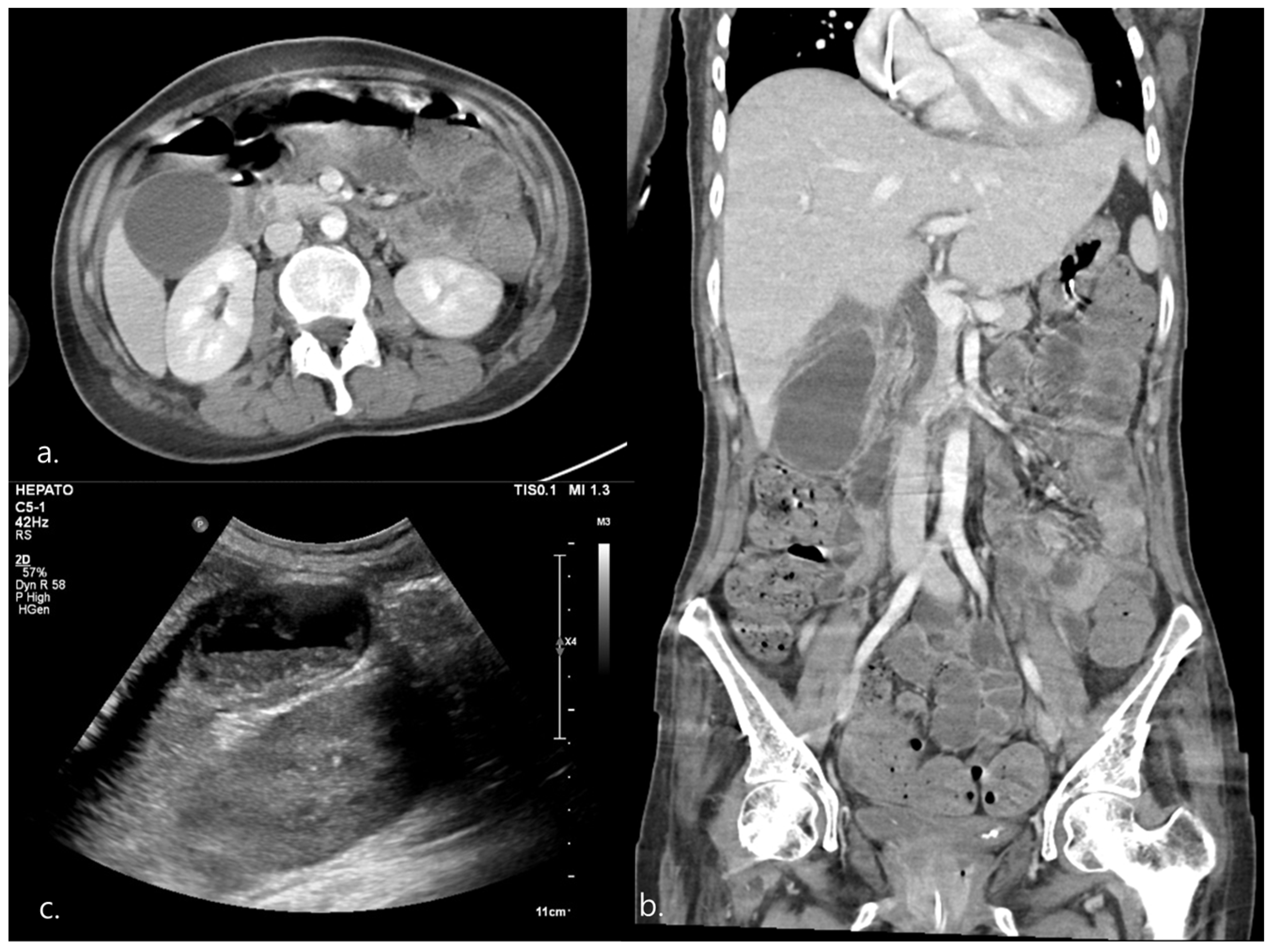
2.1. Case 1
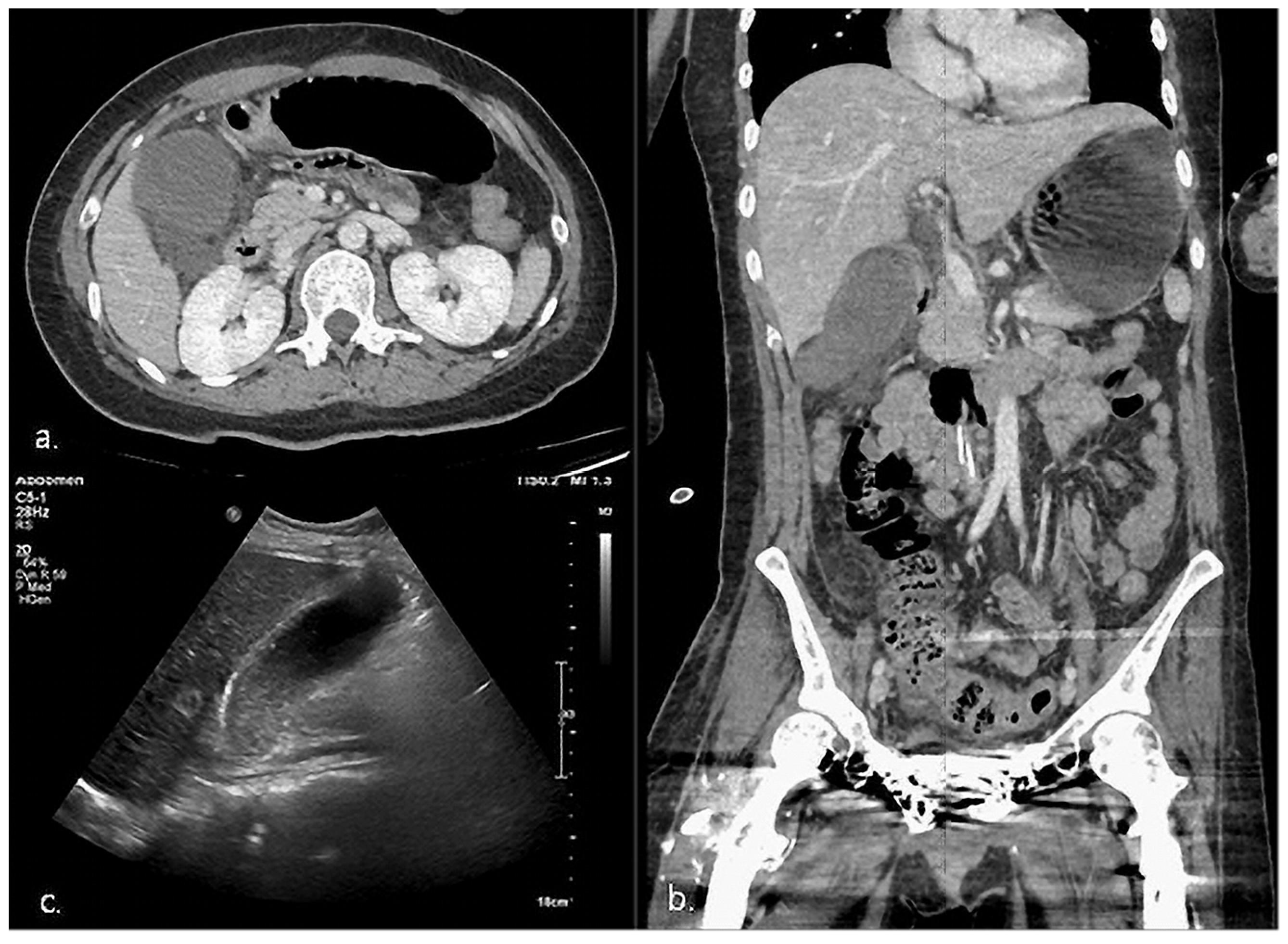
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Housset, C.; Chrétien, Y.; Debray, D.; Chignard, N. Functions of the Gallbladder. Compr. Physiol. 2016, 6, 1549–1577. [Google Scholar] [CrossRef] [PubMed]
- Barie, P.S.; Eachempati, S.R. Acute acalculous cholecystitis. Curr. Gastroenterol. Rep. 2003, 5, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Eguchi, S.; Muto, Y. Pathophysiology and pathology of acute cholecystitis: A secondary publication of the Japanese version from 1992. J. Hepatobiliary Pancreat. Sci. 2022, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.M.; Jung, S.E. Differentiation of acute cholecystitis from chronic cholecystitis: Determination of useful multidetector computed tomography findings. Medicine 2018, 97, e11851. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Takada, T.; Kawarada, Y.; Nimura, Y.; Miura, F.; Hirata, K.; Mayumi, T.; Yoshida, M.; Strasberg, S.; Pitt, H.; et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J. Hepatobiliary Pancreat. Surg. 2007, 14, 78–82. [Google Scholar] [CrossRef]
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Iwashita, Y.; Hibi, T.; Pitt, H.A.; Umezawa, A.; et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J. Hepatobiliary Pancreat. Sci. 2018, 25, 55–72. [Google Scholar] [CrossRef]
- Vaccari, S.; Lauro, A.; Cervellera, M.; Casella, G.; D’Andrea, V.; Di Matteo, F.M.; Santoro, A.; Panarese, A.; Gulotta, E.; Cirocchi, R.; et al. Early versus delayed approach in cholecystectomy after admission to an emergency department. A multicenter retrospective study. G. Chir. 2018, 39, 232–238. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Doyle, D.J.; Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Classification. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fialkowski, E.; Halpin, V.; Whinney, R.R. Acute cholecystitis. BMJ Clin. Evid. 2008, 2008, 411. [Google Scholar]
- Barie, P.S.; Eachempati, S.R. Acute acalculous cholecystitis. Gastroenterol. Clin. N. Am. 2010, 39, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Takada, T.; Kawarada, Y.; Nimura, Y.; Hirata, K.; Sekimoto, M.; Yoshida, M.; Mayumi, T.; Wada, K.; Miura, F.; et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J. Hepatobiliary Pancreat. Surg. 2007, 14, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Treinen, C.; Lomelin, D.; Krause, C.; Goede, M.; Oleynikov, D. Acute acalculous cholecystitis in the critically ill: Risk factors and surgical strategies. Langenbecks Arch. Surg. 2015, 400, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Tana, M.; Tana, C.; Cocco, G.; Iannetti, G.; Romano, M.; Schiavone, C. Acute acalculous cholecystitis and cardiovascular disease: A land of confusion. J. Ultrasound 2015, 18, 317–320. [Google Scholar] [CrossRef]
- Lee, S.; Chung, C.W.; Ko, K.H.; Kwon, S.W. Risk factors for the clinical course of cholecystitis in patients who undergo cholecystectomy. Korean J. Hepatobiliary Pancreat. Surg. 2011, 15, 164–170. [Google Scholar] [CrossRef]
- Fagan, S.P.; Awad, S.S.; Rahwan, K.; Hira, K.; Aoki, N.; Itani, K.M.; Berger, D.H. Prognostic factors for the development of gangrenous cholecystitis. Am. J. Surg. 2003, 186, 481–485. [Google Scholar] [CrossRef]
- Hickman, M.S.; Schwesinger, W.H.; Page, C.P. Acute cholecystitis in the diabetic. A case-control study of outcome. Arch. Surg. 1988, 123, 409–411. [Google Scholar] [CrossRef]
- Botaitis, S.; Polychronidis, A.; Pitiakoudis, M.; Perente, S.; Simopoulos, C. Does gender affect laparoscopic cholecystectomy? Surg. Laparosc. Endosc. Percutaneous Tech. 2008, 18, 157–161. [Google Scholar] [CrossRef]
- Merriam, L.T.; Kanaan, S.A.; Dawes, L.G.; Angelos, P.; Prystowsky, J.B.; Rege, R.V.; Joehl, R.J. Gangrenous cholecystitis: Analysis of risk factors and experience with laparoscopic cholecystectomy. Surgery 1999, 126, 680–685; discussion 685–686. [Google Scholar] [CrossRef]
- Kuy, S.; Sosa, J.A.; Roman, S.A.; Desai, R.; Rosenthal, R.A. Age matters: A study of clinical and economic outcomes following cholecystectomy in elderly Americans. Am. J. Surg. 2011, 201, 789–796. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.S.; Yoon, Y.S.; Ahn, K.S. Risk factors for acute cholecystitis and a complicated clinical course in patients with symptomatic cholelithiasis. Arch. Surg. 2010, 145, 329–333; discussion 333. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, Y.; Imazato, D.; Yamazaki, K.; Kasuya, H. Acute cholecystitis in patients with stroke. Neurol. India 2019, 67, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.C.; Yoo, S.D.; Chon, J.; Han, Y.R.; Lee, S.A. Acute cholecystitis as a rare and overlooked complication in stroke patients: A retrospective monocentric study. Medicine 2019, 98, e14492. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Sheu, E.G. Biliary Tract. In Current Diagnosis & Treatment: Surgery, 15th ed.; Doherty, G.M., Ed.; McGraw Hill LLC: New York, NY, USA, 2020. [Google Scholar]
- Akhan, O.; Akinci, D.; Ozmen, M.N. Percutaneous cholecystostomy. Eur. J. Radiol. 2002, 43, 229–236. [Google Scholar] [CrossRef]
- Lee, M.J.; Saini, S.; Brink, J.A.; Hahn, P.F.; Simeone, J.F.; Morrison, M.C.; Rattner, D.; Mueller, P.R. Treatment of critically ill patients with sepsis of unknown cause: Value of percutaneous cholecystostomy. AJR Am. J. Roentgenol. 1991, 156, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- vanSonnenberg, E.; D’Agostino, H.B.; Goodacre, B.W.; Sanchez, R.B.; Casola, G. Percutaneous gallbladder puncture and cholecystostomy: Results, complications, and caveats for safety. Radiology 1992, 183, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.R.; Hong, K.S.; Seo, E.K. Acute Cholecystitis as a Cause of Fever in Aneurysmal Subarachnoid Hemorrhage. Korean J. Crit. Care Med. 2017, 32, 190–196. [Google Scholar] [CrossRef]
- Fukuoka, T.; Hayashi, T.; Kato, Y.; Ohe, Y.; Deguchi, I.; Maruyama, H.; Horiuchi, Y.; Sano, H.; Nagamine, Y.; Tanahashi, N. Clinical review of 24 patients with acute cholecystitis after acute cerebral infarction. Intern. Med. 2014, 53, 1321–1323. [Google Scholar] [CrossRef]
- Ushiyama, M.; Koike, J.; Zenisaka, H.; Seguchi, K.; Ikeda, S.; Yanagisawa, N. Acute acalculous cholecystitis as a complication of cerebrovascular disease. Rinsho Shinkeigaku 1997, 37, 218–223. [Google Scholar]
- Angelico, M.; Della Guardia, P. Review article: Hepatobiliary complications associated with total parenteral nutrition. Aliment. Pharmacol. Ther. 2000, 14 (Suppl. S2), 54–57. [Google Scholar] [CrossRef]
- Sevastos, N.; Savvas, S.P.; Rafailidis, P.I.; Manesis, E.K. Cholestasis in acute stroke: An investigation on its prevalence and etiology. Scand. J. Gastroenterol. 2005, 40, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Hachinski, V.C.; Gibson, C.J.; Ciriello, J. Changes in plasma catecholamine levels after insula damage in experimental stroke. Brain Res. 1986, 375, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Toouli, J. Sphincter of Oddi: Function, dysfunction, and its management. J. Gastroenterol. Hepatol. 2009, 24 (Suppl. S3), S57–S62. [Google Scholar] [CrossRef] [PubMed]


| Case | Age (yrs) | Sex | Diagnosis of CNS Lesion | Time | BBS | MBI | MMSE | mRS | Diet |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | F | Hypoxic brain injury | Admission (15 August 2021) | 0 | 0 | 0 | 5 | L-tube |
| RM transfer (30 September 2021) | 0 | 0 | 11 | 5 | Soft diet | ||||
| AC diagnosis (20 October 2021) | 3 | 5 | 17 | 4 | Regular diet | ||||
| 2 | 32 | F | Autoimmune encephalitis | Admission (29 January 2021) | 0 | 0 | 0 | 5 | L-tube |
| RM transfer (13 May 2021) | 0 | 0 | 0 | 5 | L-tube | ||||
| AC diagnosis (15 May 2021) | 0 | 0 | 0 | 5 | L-tube |
| Case | Time to Symptom Onset (Days) | Time to Diagnosis (Days) | Symptom | Image Findings | Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Local | Systemic | Diagnostic Tool | GB Size (mm) | Wall Thickness (mm) | Biliary Sludge | GB Stone | ||||
| 1 | HD 65 | 1 | Epigastric discomfort RLQ tenderness Murphy’s sign * | Fever * Elevated CRP *, WBC * | CT, US | 93 × 41 * | 3.76 | (+) * | (−) | Definite AC |
| 2 | HD 105 | 4 | RUQ tenderness * | Fever Elevated CRP * | CT, US | 78 × 49 | 4.1 * | (+) * | (−) | Definite AC |
| Case | Diagnosis | Severity | General Condition | Plan | |
|---|---|---|---|---|---|
| CCI Score [9] | ASA-PS Classification [10] | ||||
| 1 | Definite AC | Grade II (Moderate) | Total score 3 | ASA IV | 1st. Early GB drainage (PTGBD) 2nd. Delayed/elective LC |
| 2 | Definite AC | Grade III (Severe) | Total score 3 | ASA IV | 1st. Early GB drainage (PTGBD) 2nd. Observation |
| Case | The Initial Consecutive Fasting Time (Days) | The Total Fasting Time (Days) |
|---|---|---|
| 1 | 9 | 16 |
| 2 | 4 | >30 |
| Reference value | 5.38 ± 2.78 * | 15.85 ± 2.85 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lim, M.-G.; Han, J.-S.; Ahn, C.-H.; Jung, T.-D. Rare or Overlooked Cases of Acute Acalculous Cholecystitis in Young Patients with Central Nervous System Lesion. Healthcare 2023, 11, 1378. https://doi.org/10.3390/healthcare11101378
Kim S-H, Lim M-G, Han J-S, Ahn C-H, Jung T-D. Rare or Overlooked Cases of Acute Acalculous Cholecystitis in Young Patients with Central Nervous System Lesion. Healthcare. 2023; 11(10):1378. https://doi.org/10.3390/healthcare11101378
Chicago/Turabian StyleKim, Seong-Hun, Min-Gyu Lim, Jun-Sang Han, Chang-Hwan Ahn, and Tae-Du Jung. 2023. "Rare or Overlooked Cases of Acute Acalculous Cholecystitis in Young Patients with Central Nervous System Lesion" Healthcare 11, no. 10: 1378. https://doi.org/10.3390/healthcare11101378
APA StyleKim, S.-H., Lim, M.-G., Han, J.-S., Ahn, C.-H., & Jung, T.-D. (2023). Rare or Overlooked Cases of Acute Acalculous Cholecystitis in Young Patients with Central Nervous System Lesion. Healthcare, 11(10), 1378. https://doi.org/10.3390/healthcare11101378






