Virtual Feedback for Arm Motor Function Rehabilitation after Stroke: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
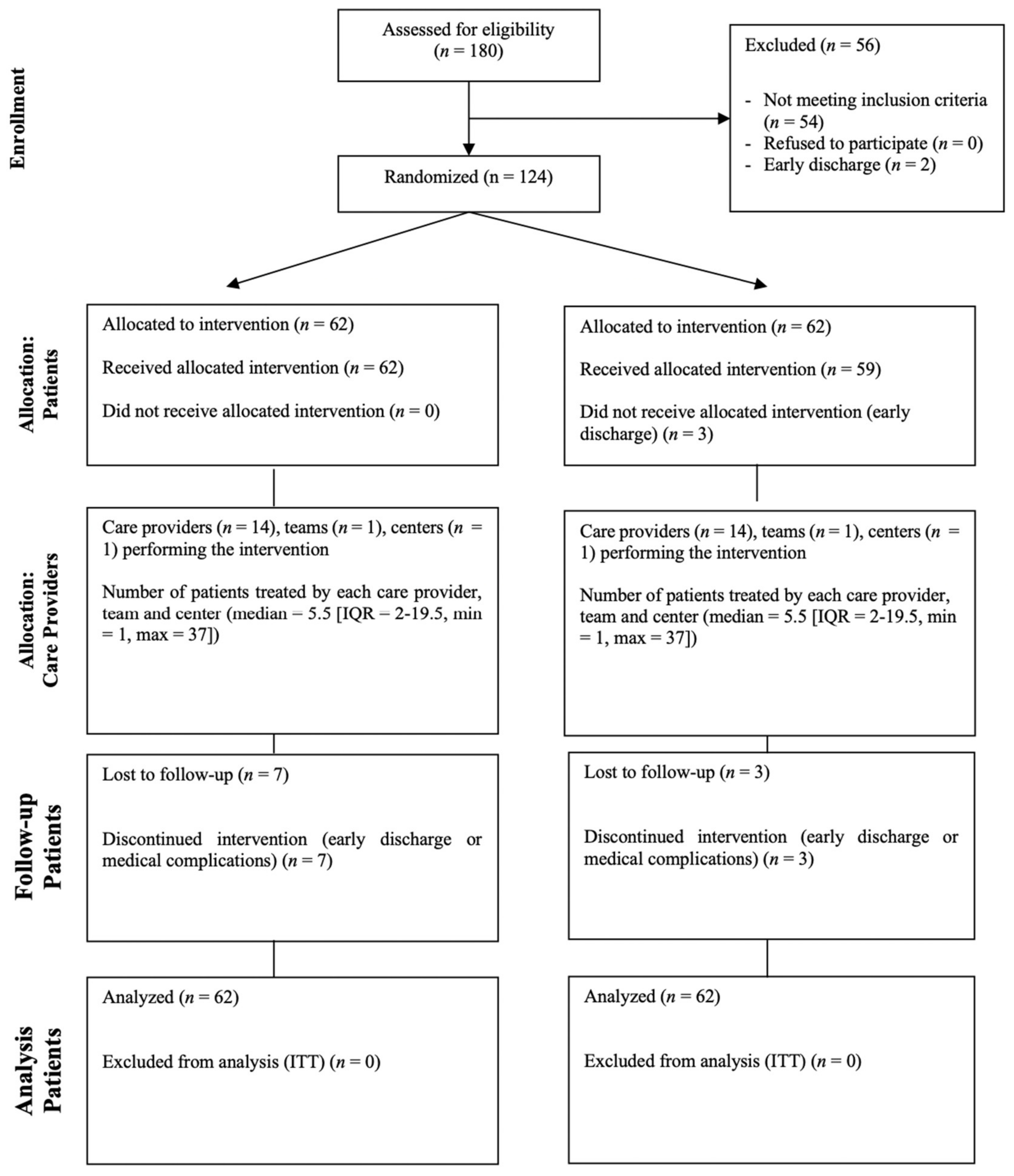
2.1. Trial Design and Population
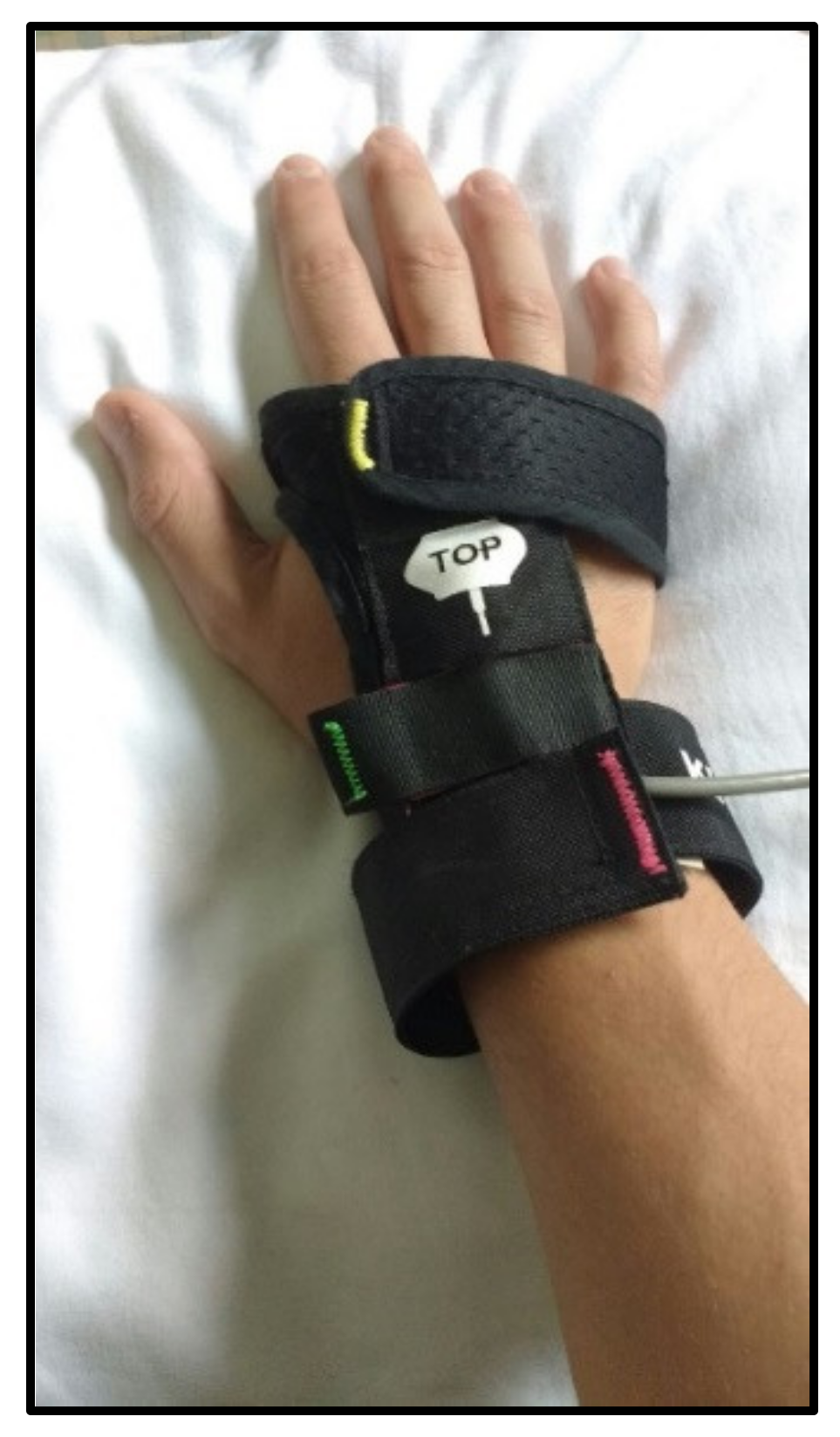
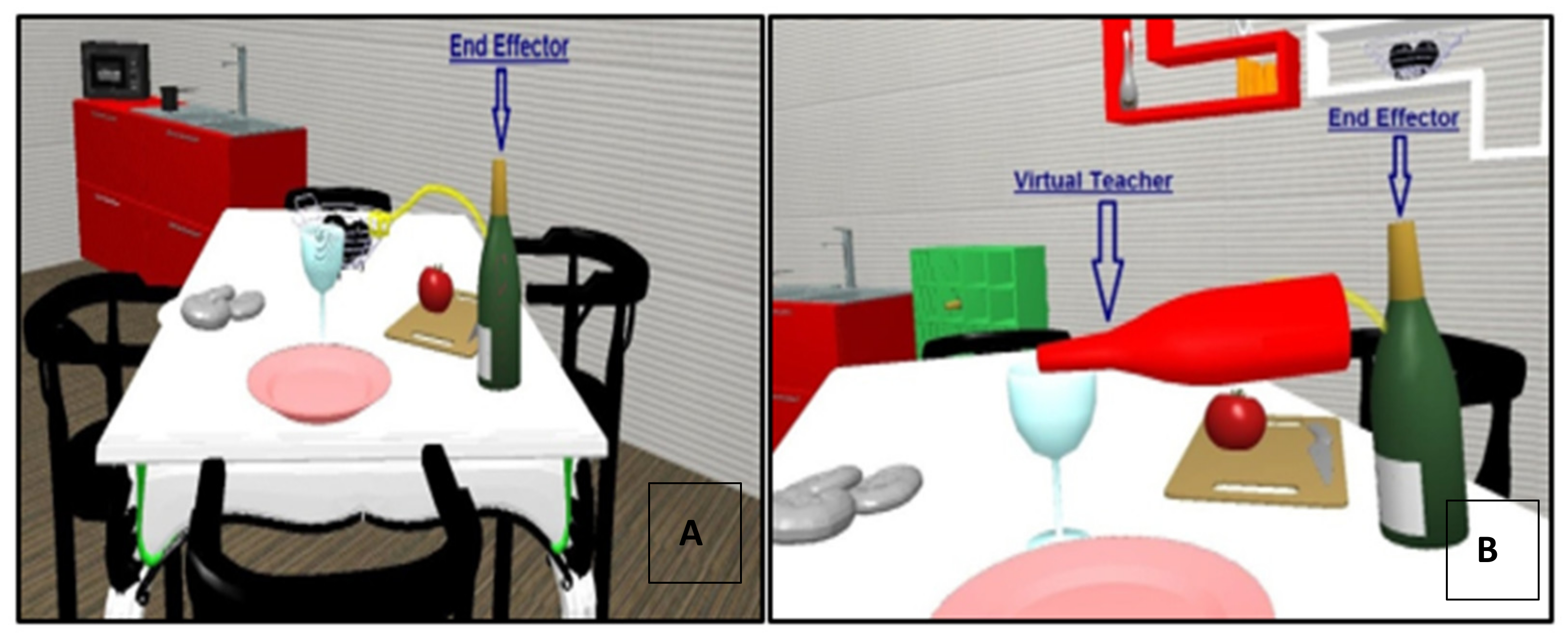
2.2. The Virtual Reality Rehabilitation System
2.3. Assessment
2.4. Intervention
2.4.1. Conventional Therapy
2.4.2. Virtual Reality Rehabilitation
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Neuromotor Interpretation
4.2. Clinical and Pragmatic Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; Van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11, CD010820. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Rose, D.K.; Tan, S.M.; Lewthwaite, R.; Chui, H.C.; Azen, S.P. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch. Phys. Med. Rehabil. 2004, 85, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Raghavan, P. Upper Limb Motor Impairment After Stroke. Phys. Med. Rehabil. Clin. 2015, 26, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Nudo, R.J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef] [Green Version]
- Doya, K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr. Opin. Neurobiol. 2000, 10, 732–739. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Diedrichsen, J.; Flanagan, J.R. Principles of sensorimotor learning. Nat. Rev. Neurosci. 2011, 12, 739–751. [Google Scholar] [CrossRef]
- Reinkensmeyer, D.J.; Burdet, E.; Casadio, M.; Krakauer, J.W.; Kwakkel, G.; Lang, C.E.; Swinnen, S.P.; Ward, N.S.; Schweighofer, N. Computational neurorehabilitation: Modeling plasticity and learning to predict recovery. J. Neuroeng. Rehabil. 2016, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Todorov, E.; Shadmehr, R.; Bizzi, E. Augmented Feedback Presented in a Virtual Environment Accelerates Learning of a Difficult Motor Task. J. Mot. Behav. 1997, 29, 147–158. [Google Scholar] [CrossRef]
- Arya, K.N.; Pandian, S.; Verma, R.; Garg, R.K. Movement therapy induced neural reorganization and motor recovery in stroke: A review. J. Bodyw. Mov. Ther. 2011, 15, 528–537. [Google Scholar] [CrossRef]
- Piron, L.; Tonin, P.; Piccione, F.; Iaia, V.; Trivello, E.; Dam, M. Virtual Environment Training Therapy for Arm Motor Rehabilitation. Presence Teleoperators Virtual Environ. 2005, 14, 732–740. [Google Scholar] [CrossRef]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Turolla, A.; Dam, M.; Ventura, L.; Tonin, P.; Agostini, M.; Zucconi, C.; Kiper, P.; Cagnin, A.; Piron, L. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. J. Neuroeng. Rehabil. 2013, 10, 85. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [Green Version]
- Laver, K.E.; George, S.; Thomas, S.; Deutsch, J.E.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2011, 9, CD008349. [Google Scholar] [CrossRef] [Green Version]
- Testa, M.; Rossettini, G. Enhance placebo, avoid nocebo: How contextual factors affect physiotherapy outcomes. Man. Ther. 2016, 24, 65–74. [Google Scholar] [CrossRef]
- Rossettini, G.; Camerone, E.M.; Carlino, E.; Benedetti, F.; Testa, M. Context matters: The psychoneurobiological determinants of placebo, nocebo and context-related effects in physiotherapy. Arch. Physiother. 2020, 10, 11. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [Green Version]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P.; Group, C.N. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Mathiowetz, V.W.K.; Kashman, N.; Volland, G. Adult Norms For The Nine Hole Peg Test Of Finger Dexterity. Occup. Ther. J. Res. 1985, 5, 24–38. [Google Scholar] [CrossRef]
- Levin, M.F.; Desrosiers, J.; Beauchemin, D.; Bergeron, N.; Rochette, A. Development and validation of a scale for rating motor compensations used for reaching in patients with hemiparesis: The reaching performance scale. Phys. Ther. 2004, 84, 8–22. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Granger, C.V. Advances in functional assessment for medical rehabilitation. Top. Geriatr. Rehabil. 1986, 1, 59–74. [Google Scholar] [CrossRef]
- Gaskin, C.J.; Happell, B. Power, effects, confidence, and significance: An investigation of statistical practices in nursing research. Int. J. Nurs. Stud. 2014, 51, 795–806. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beninato, M.; Gill-Body, K.M.; Salles, S.; Stark, P.C.; Black-Schaffer, R.M.; Stein, J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.K.; Banina, M.C.; Turolla, A.; Levin, M.F. Reaching performance scale for stroke—Test-retest reliability, measurement error, concurrent and discriminant validity. PMR 2021, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Chen, C.C.; Hsueh, I.P.; Huang, S.L.; Hsieh, C.L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabilit. Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R [Internet]; RStudio, Inc.: Boston, MA, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 1 March 2020).
- Latash, M.L. Motor synergies and the equilibrium-point hypothesis. Motor Control 2010, 14, 294–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of virtual reality as a tool for upper limb rehabilitation: Incorporation of motor control and motor learning principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef] [Green Version]

| Demographics | Overall (n = 124) | Teacher Group (n = 62) | No-Teacher Group (n = 62) | p-Value |
|---|---|---|---|---|
| Sex Total Male (%)/Female (%) | 79 (64%)/ 45 (36%) | 42 (68%)/ 20 (32%) | 37 (60%)/ 25 (40%) | p = 0.4 |
| Age Mean ± SD/Median (range) | 62.58 ± 14.01/ 64 (19–92) | 63.944 ± 13.58/ 66 (24–84) | 61.21 ± 15.12/ 62 (19–84) | p = 0.3 |
| Type of stroke Ischemic (%)/Hemorrhagic (%) | 93 (75%)/ 31 (25%) | 49 (79%)/ 13 (21%) | 44 (71%)/ 18 (29%) | p = 0.3 |
| Hemisphere Right (%)/Left (%) | 65 (52%)/ 59 (48%) | 34 (55%)/ 28 (45%) | 31 (50%)/ 31(50%) | p = 0.6 |
| Months from injury Mean ± SD/Median (range) | 7.36 ± 14.65/ 3 (1–144) | 5.71 ± 8.69/ 3 (1–60) | 9.01 ± 18.78/ 4 (1–144) | p = 0.2 |
| Outcome Measures | Teacher (n = 62) | No Teacher (n = 62) | Between Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | Within Group | Effect Size | T0 | T1 | Within Group | Effect Size | ||
| FMA-UE Mean ± SD/Median (range) | 40.77 ± 14.27 43 (14–62) | 47.06 ± 14 50 (17–66) | p < 0.01 * | 1.14 | 36.45 ± 14.27 36 (0–62) | 41.97 ± 15.61 43 (13–66) | p < 0.01 * | 0.92 | p = 0.4 |
| FMA-sensation Mean ± SD/Median (range) | 18.42 ± 6.84 21 (0–24) | 20.06 ± 5.75 23 (4–24) | p < 0.01 * | 0.42 | 18.53 ± 6.25 21 (0–24) | 20.66 ± 5 22 (0–24) | p < 0.01 * | 0.53 | p = 0.3 |
| FMA-pain/rom Mean ± SD/Median (range) | 42.08 ± 7.04 44 (17–48) | 43.58 ± 5 45 (17–48) | p < 0.01 * | 0.31 | 41.73 ± 5.29 43 (27–48) | 43.03 ± 4 43 (30–48) | p < 0.01 * | 0.36 | p = 0.5 |
| RPS Mean ± SD/Median (range) | 23.66 ± 11 28 (0–36) | 28.1 ± 9 32 (0–36) | p < 0.01 * | 0.83 | 20.6 ± 11.23 22 (0–36) | 24.08 ± 10 24 (4–36) | p < 0.01 * | 0.57 | p = 0.2 |
| NHPT Mean ± SD/Median (range) | 0.12 ± 0.3 0.02 (0–2) | 0.15 ± 0.2 0.06 (0–0.56) | p < 0.01 * | 0.08 | 0.12 ± 0.2 0 (0–0.76) | 0.16 ± 0.2 0.01 (0–0.75) | p < 0.01 * | 0.27 | p = 0.4 |
| FIM Mean ± SD/Median (range) | 93.32 ± 20.79 100 (38–123) | 103 ± 18 100 (64–126) | p < 0.01 * | 0.91 | 91.56 ± 21.89 96 (33–123) | 99.10 ± 21.69 103 (42–126) | p < 0.01 * | 0.73 | p = 0.7 |
| MAS biceps brachii Mean ± SD/Median (range) | 0.78 ± 1 1 (0–4) | 0.58 ± 0.86 0 (0–4) | p < 0.01 * | 0.30 | 0.84 ± 0.94 1 (0–4) | 0.76 ± 0.88 1 (0–3) | p = 0.84 | 0.10 | p = 0.2 |
| Overall Population (n = 124) | |||
| Outcome | Model | R2 | Residuals S-W test |
| ΔFMA-UE | 25.54 + 0.13 FMA-UE T0 + 0.30 FMA sensation T0 + 0.72 FIM T0—2.44 MAS biceps brachii T0 | 0.80 | p = 0.02 |
| Teacher group (n = 62) | |||
| Outcome | Model | R2 | Residuals S-W test |
| FMA-UE T1 | 10.72 + 0.89 FMA-UE T0 | 0.85 | p = 0.03 |
| No-Teacher group (n = 62) | |||
| Outcome | Model | R2 | Residuals S-W test |
| ΔFMA-UE | 13.62 + 0.3 FMA-UE T0 + 0.82 FIM T0 | 0.81 | p = 0.3 |
| FIM T1 | 13.62 + 0.30 FMA-UE T0 + 0.82 FIM T0 | 0.81 | p = 0.32 |
| Responders FMA-UE (n = 68) | |||
| Outcome | Model | R2 | Residuals S-W test |
| FMA-UE T1 | 13.80 + 0.91 FMA-UE T0 | 0.91 | p = 0.02 |
| Non-Responders FMA-UE (n = 56) | |||
| Outcome | Model | R2 | Residuals S-W test |
| FMA-UE T1 | 1.07 + 1.72 sex M + 0.98 FMA-UE T0 -0.68 MAS flexor carpi T0 | 0.99 | p = 0.08 |
| FIM T1 | 13.00 + 4.89 sex M + 0.15 FMA-UE T0 + 0.33 FMA sensation T0 + 0.76 FIM T0 | 0.88 | p = 0.82 |
| Responders RPS (n = 43) | |||
| Outcome | Model | R2 | Residuals S-W test |
| RPS T1 | 12.37 + 0.91 FMA-UE T0—0.77 MAS tot T0 | 0.80 | p = 1 |
| Non-responders RPS (n = 81) | |||
| Outcome | Model | R2 | Residuals S-W test |
| RPS T1 | 10.84—0.09 age + 2.52 sex M + 0.62 FMA-UE T0 + 0.58 RPS T0 | 0.91 | p = 1 |
| Outcome Measures | Responder (n = 68) | Non-Responder (n = 56) | Between Groups | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | Within Group | T0 | T1 | Within Group | ||
| FMA-UE Mean ± SD/ Median (range) | 39 ± 14 39 (8–60) | 49 ± 13 50 (15–66) | p < 0.01 * | 38 ± 15 36 (13–62) | 39 ± 15 38 (13–65) | p = 0.01 * | p < 0.01 * |
| FMA-sensation Mean ± SD/ Median (range) | 19 ± 6 21 (0–24) | 21 ± 4 24 (7–24) | p < 0.01 * | 18 ± 7 21 (0–24) | 19 ± 5 22 (0–24) | p = 0.01 * | p < 0.01 * |
| FMA-pain/rom Mean ± SD/ Median (range) | 42 ± 6 44 (17–48) | 44 ± 4 45 (30–48) | p < 0.01 * | 42 ± 6 43 (17–48) | 42 ± 5 43 (17–48) | p = 0.3 | p = 0.02 * |
| RPS Mean ± SD/ Median (range) | 24 ± 11 28 (0–36) | 29 ± 10 32 (4–36) | p < 0.01 * | 20 ± 11 21 (0–36) | 23 ± 10 21 (0–36) | p < 0.01 * | p = 0.03 * |
| NHPT Mean ± SD/ Median (range) | 0.12 ± 0.23 0.08 (1–0.5) | 0.22 ± 0.19 0.18 (0–0.75) | p < 0.01 * | 0.11 ± 0.29 0 (0–2) | 0.08 ± 0.19 0 (0–0.41) | p = 0.3 | p < 0.01 * |
| FIM Mean ± SD/ Median (range) | 92 ± 22 99 (33–123) | 103 ± 20 112 (42–126) | p < 0.01 * | 93 ± 21 96 (38–123) | 98 ± 19 100 (51–124) | p < 0.01 * | p < 0.01 * |
| MAS biceps brachii Mean ± SD/ Median (range) | 1 ± 1 0 (0–3) | 1 ± 1 0 (0–3) | p = 0.09 | 1 ± 1 1 (0–4) | 1 ± 1 1 (0–4) | p = 0.2 | p = 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvalaggio, S.; Kiper, P.; Pregnolato, G.; Baldan, F.; Agostini, M.; Maistrello, L.; Turolla, A. Virtual Feedback for Arm Motor Function Rehabilitation after Stroke: A Randomized Controlled Trial. Healthcare 2022, 10, 1175. https://doi.org/10.3390/healthcare10071175
Salvalaggio S, Kiper P, Pregnolato G, Baldan F, Agostini M, Maistrello L, Turolla A. Virtual Feedback for Arm Motor Function Rehabilitation after Stroke: A Randomized Controlled Trial. Healthcare. 2022; 10(7):1175. https://doi.org/10.3390/healthcare10071175
Chicago/Turabian StyleSalvalaggio, Silvia, Pawel Kiper, Giorgia Pregnolato, Francesca Baldan, Michela Agostini, Lorenza Maistrello, and Andrea Turolla. 2022. "Virtual Feedback for Arm Motor Function Rehabilitation after Stroke: A Randomized Controlled Trial" Healthcare 10, no. 7: 1175. https://doi.org/10.3390/healthcare10071175
APA StyleSalvalaggio, S., Kiper, P., Pregnolato, G., Baldan, F., Agostini, M., Maistrello, L., & Turolla, A. (2022). Virtual Feedback for Arm Motor Function Rehabilitation after Stroke: A Randomized Controlled Trial. Healthcare, 10(7), 1175. https://doi.org/10.3390/healthcare10071175








