Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications
Abstract
:1. Introduction
2. Thermodynamic Model
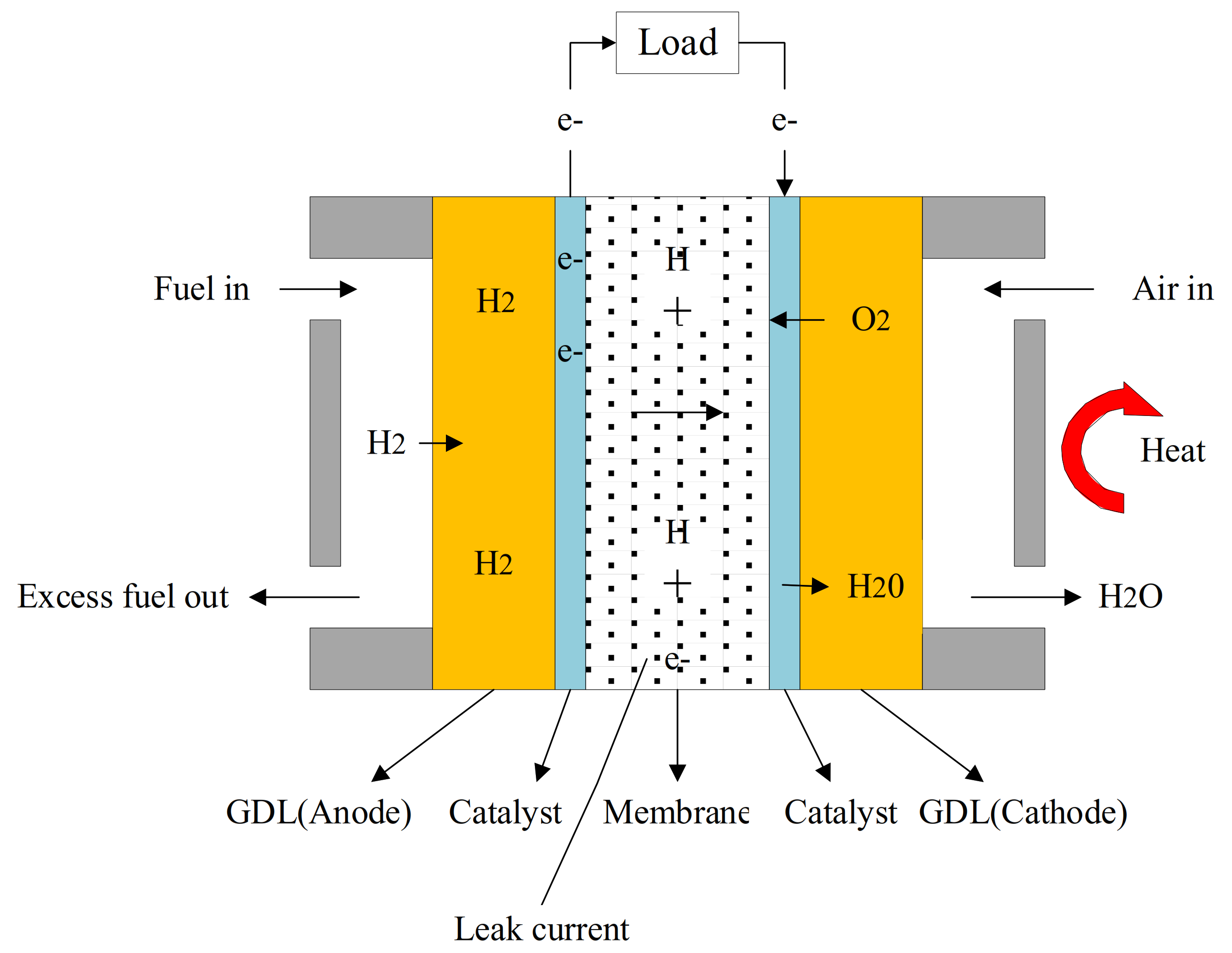
2.1. Internal Processess of HT-PEMFC
2.2. Reversible Output Voltage of HT-PEMFC
2.3. Irreversible Loss of HT-PEMFC
2.3.1. Polarization Phenomenon
2.3.2. Leakage Current
2.4. Irreversible Output Voltage
2.5. Power Density and Efficiency of HT-PEMFC
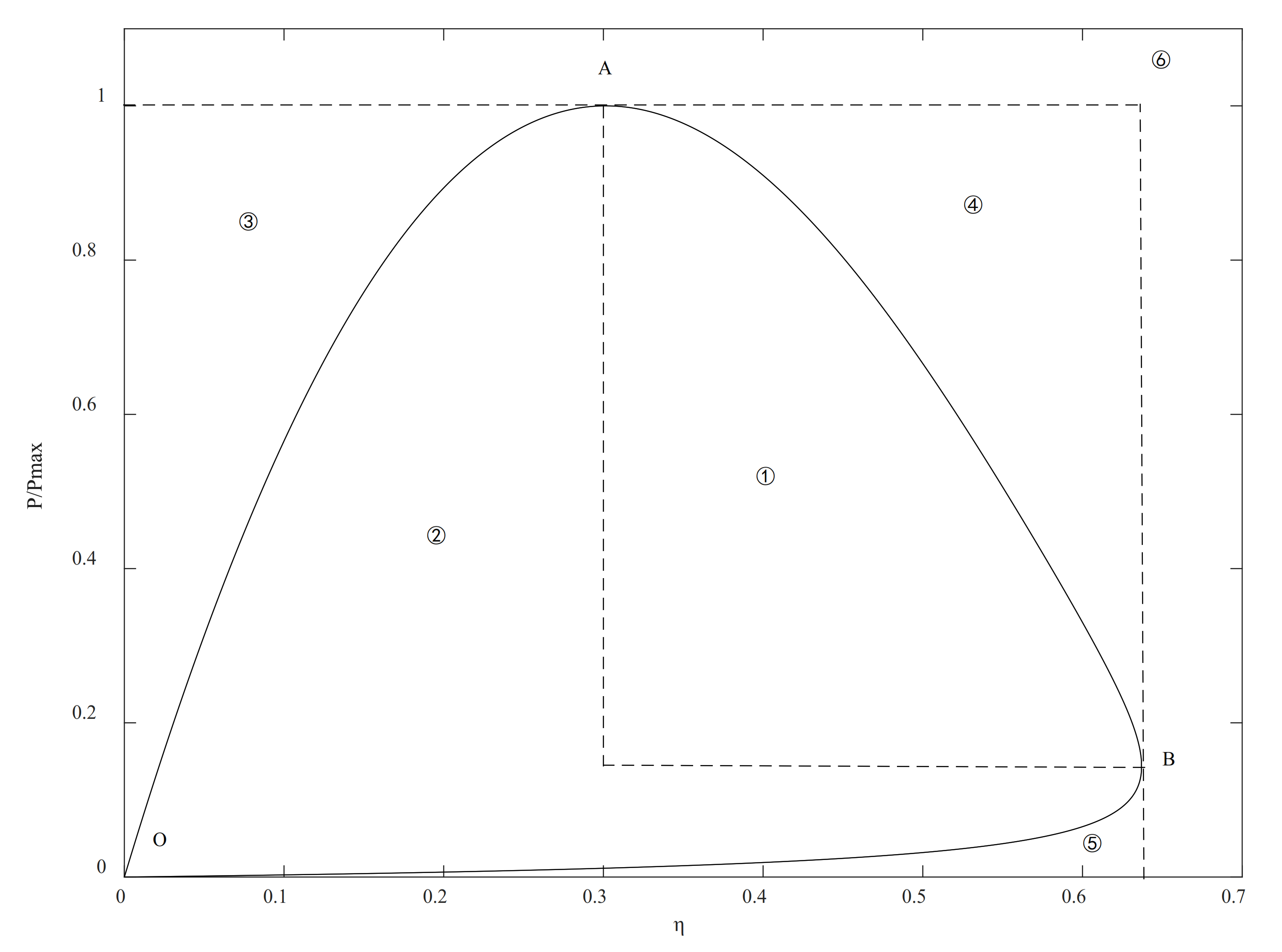
2.6. Thermodynamic Optimization
3. Results and Discussion
3.1. Model Validation
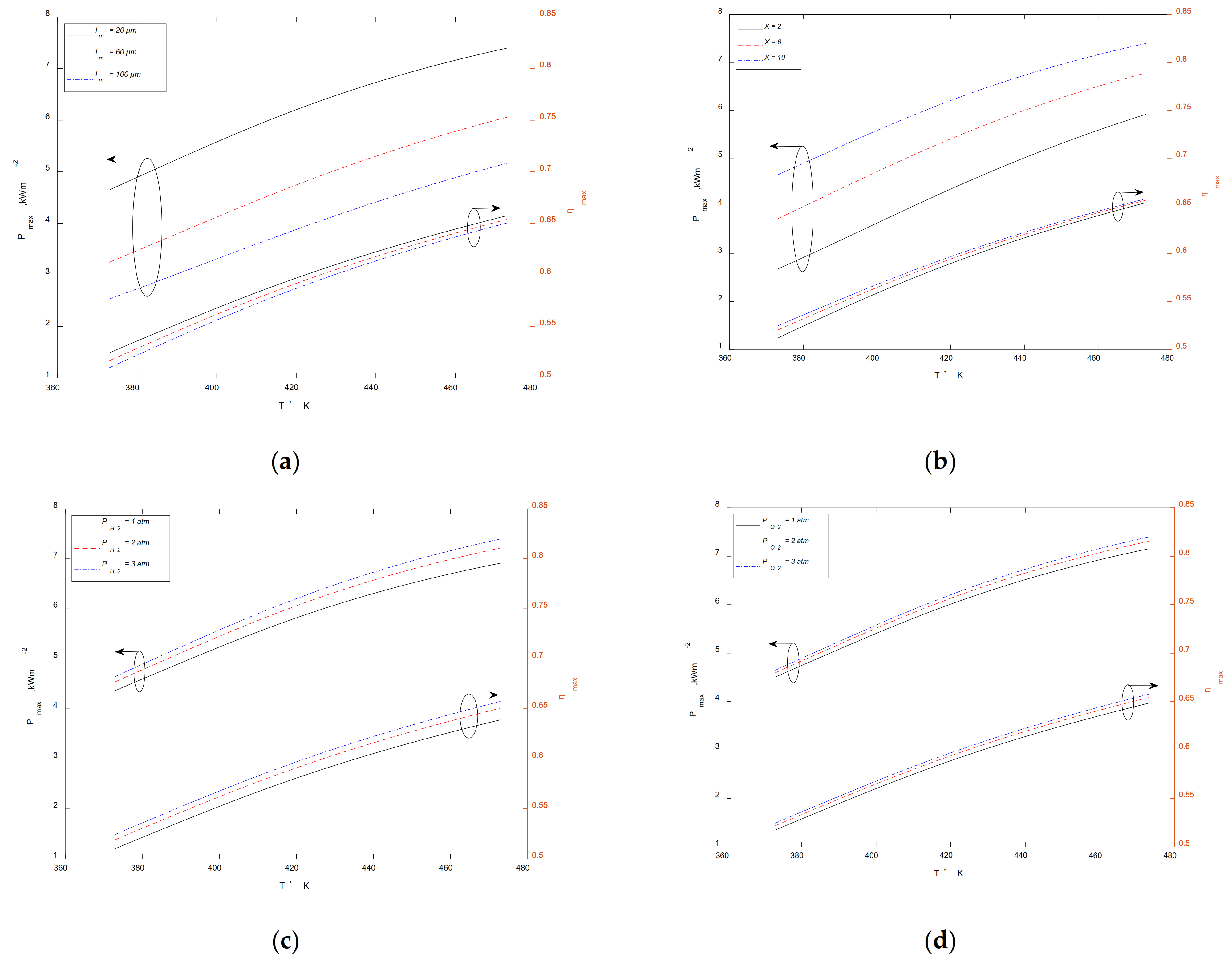
3.2. Maximum Output Performance at a Given Temperature
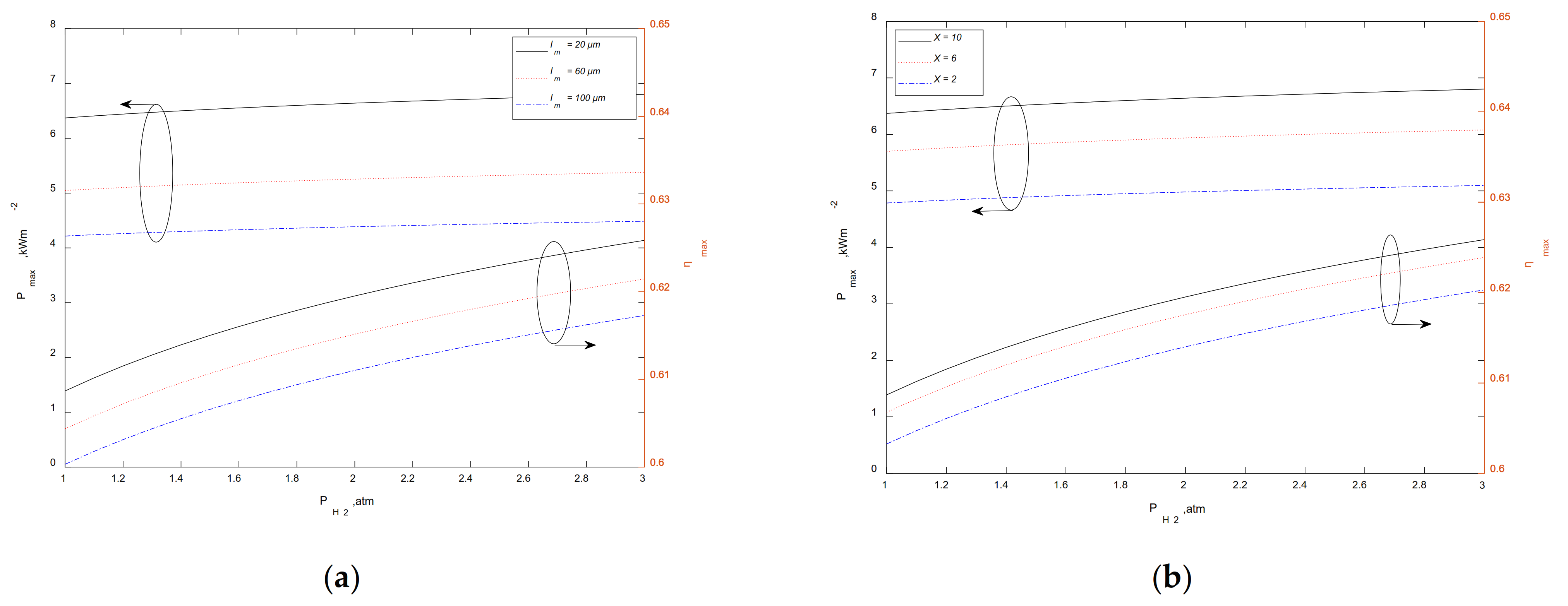
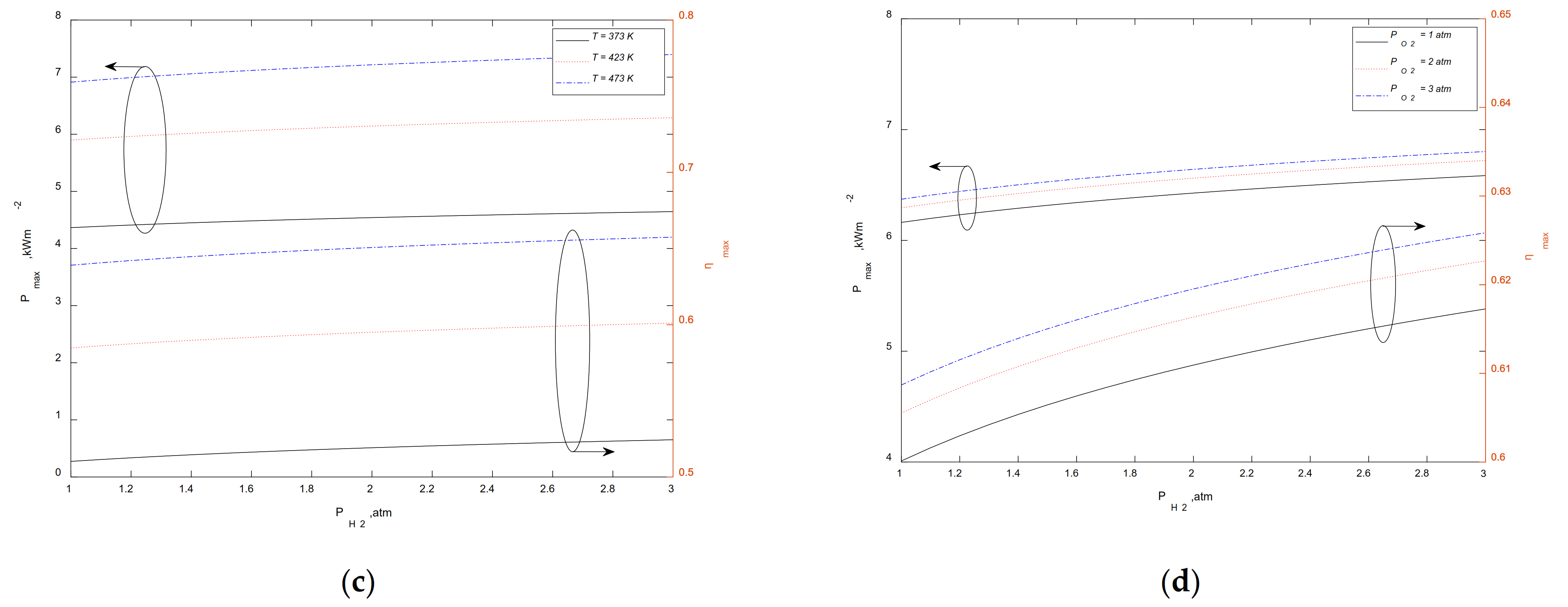
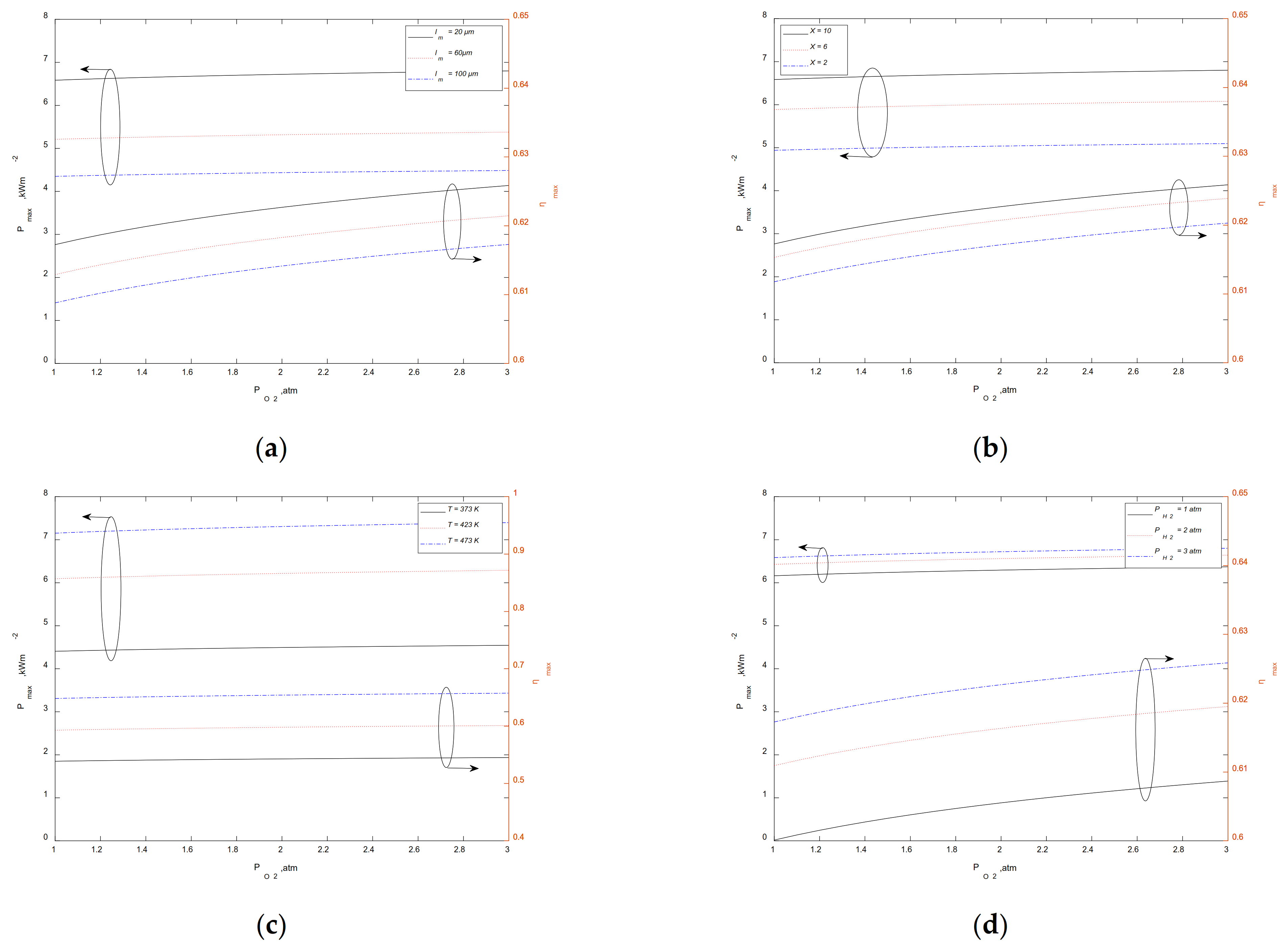
3.3. Maximum Output Performance at a Given
3.4. Maximum Output Performance at a Given
3.5. Maximum Output Performance at a Given
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.Q.; Jia, L.M. Review on sustainable development of forest-based biodiesel. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 44, 216–224. [Google Scholar]
- Yao, J.J.; Feng, X.Q.; Xiao, H.; Zheng, Y.; Zhang, C.L. Improvement effects of different solid waste and their disposal by products on saline-alkali soil in Huanghua Port. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 45–52. [Google Scholar] [CrossRef]
- Zhang, Z.G. Researches on green features and category architecture of green strategies of renewable-resource-based enterprises: A case study of forestry enterprise. J. Nanjing For. Univ. Nat. Sci. Ed. 2020, 44, 1–8. [Google Scholar] [CrossRef]
- Sun, Y.F. High Temperature Proton Exchange Membrane Technology Improvement Research. Appl. Energy Technol. 2018, 6, 50–52. [Google Scholar]
- Cheddie, D.; Munroe, N. Analytical correlations for intermediate temperature PEM fuel cells. J. Power Sources 2006, 160, 299–304. [Google Scholar] [CrossRef]
- Hu, J.W.; Zhang, H.M.; Hu, J.; Zhai, Y.F.; Yi, B.L. Two dimensional modeling study of PBI/H3PO4 high temperature PEMFCs based on electrochemical methods. J. Power Sources 2006, 160, 1026–1034. [Google Scholar] [CrossRef]
- Cheddie, D.F.; Munroe, N.D.H. A two-phase model of an intermediate temperature PEM fuel cell. Int. J. Hydrogen Energy 2007, 32, 832–841. [Google Scholar] [CrossRef]
- Scott, K.; Pilditch, S.; Mamlouk, M. Modelling and experimental validation of a high temperature polymer electrolyte fuel cell. J. Appl. Electrochem. 2007, 37, 1245–1259. [Google Scholar] [CrossRef]
- Kang, T.; Kim, M.; Kim, J.; Sohn, Y.J. Numerical modeling of the degradation rate for membrane electrode assemblies in high temperature proton exchange membrane fuel cells and analyzing operational effects of the degradation. Int. J. Hydrogen Energy 2015, 40, 5444–5455. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.; Kim, J.; Sohn, Y.J. One-dimensional modeling and analysis for performance degradation of high temperature proton exchange membrane fuel cell using PA doped PBI membrane. Solid State Ionics 2014, 262, 319–323. [Google Scholar] [CrossRef]
- Cheddie, D.; Munroe, N. Mathematical model of a PEMFC using a PBI membrane. Energy Convers. Manag. 2006, 47, 1490–1504. [Google Scholar] [CrossRef]
- Qin, W.X.; Qin, X.Y.; Chen, L.G. Finite time thermodynamics optimization of an irreversible KCS-34 cycle coupled to variable temperature heat reservoirs. Energy Conserv. 2018, 37, 69–74. [Google Scholar]
- He, S.; Lin, L.Y.; Wu, Z.X.; Chen, Z.M. Application of Finite Element Analysis in Properties Test of Finger-jointed Lumber. J. Bioresour. Bioprod. 2020, 5, 124. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, Y.J.; Fu, H.Y.; Wang, Y.L.; Wang, Z. Deflection test and modal analysis of lightweight timber floors. J. Bioresour. Bioprod. 2021, 6, 266–278. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.C.; Zhou, L.; Zhang, F.S.; Jing, Y.; Huang, M.Z.; Liu, H.B. Quality-related monitoring of papermaking wastewater treatment processes using dynamic multiblock partial least squares. J. Bioresour. Bioprod. 2021. [Google Scholar] [CrossRef]
- Hu, T.P.; Yu, Z.; Guo, L.; Xu, C.Y. Thermodynamic self-consistent dynamic model of wood dust explosion. J. For. Eng. 2019, 4, 29–34. [Google Scholar]
- Stanley, J.; Watowich, R.; Stephen, B. Optimal current paths for model electrochemical systems. J. Phys. Chem. B 1986, 90, 4624–4631. [Google Scholar]
- Li, D.; Li, S.; Ma, Z.; Xu, B.; Lu, Z.; Li, Y.; Zheng, M. Ecological Performance Optimization of a High Temperature Proton Exchange Membrane Fuel Cell. Mathematics 2021, 9, 1332. [Google Scholar] [CrossRef]
- Sieniutycz, S. Thermodynamics of Power Production in Fuel Cells. Chem. Process. Eng.-Inz. 2010, 31, 81–105. [Google Scholar]
- Sieniutycz, S.; Poswiata, A. Thermodynamic aspects of power production in thermal, chemical and electrochemical systems. Energy 2012, 45, 62–70. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, Y.; Xu, B.; Ma, Z.S. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes 2019, 7, 419. [Google Scholar] [CrossRef] [Green Version]
- Muthuraja, P.; Prakash, S.; Shanmugam, V.M.; Radhakr sihnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membranes for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772. [Google Scholar] [CrossRef]
- Miansari, M.; Sedighi, K.; Amidpour, M.; Alizadeh, E.; Miansari, M. Experimental and thermodynamic approach on proton exchange membrane fuel cell performance. J. Power Sources 2009, 190, 356–361. [Google Scholar] [CrossRef]
- Peng, X.R.; Zhang, Z.K.; Zhao, L.Y. Analysis of Raman spectroscopy and XPS of plasma modified polypropylene decorative film. J. For. Eng. 2020, 5, 45–51. [Google Scholar]
- Yu, P.J.; Zhang, W.; Chen, M.Z.; Zhou, X.Y. Plasma-treated thermoplastic resin film as adhesive for preparing environmentally-friendly plywood. J. For. Eng. 2020, 5, 41–47. [Google Scholar]
- Lobato, J.; Rodrigo, M.A.; Linares, J.J.; Scott, K. Effect of the catalytic ink preparation method on the performance of high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2006, 157, 284–292. [Google Scholar] [CrossRef]
- Guo, X.R.; Zhang, H.C.; Zhao, J.P.; Wang, F.; Wang, J.T.; Miao, H.; Yuan, J.L. Performance evaluation of an integrated high-temperature proton exchange membrane fuel cell and absorption cycle system for power and heating/cooling cogeneration. Energy Convers. Manag. 2019, 181, 292–301. [Google Scholar] [CrossRef]
- Chan, S.H.; Khor, K.A.; Xia, Z.T. A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness. J. Power Sources 2001, 93, 130–140. [Google Scholar] [CrossRef]
- Andreadis, G.M.; Podias, A.K.M.; Tsiakaras, P.E. The effect of the parasitic current on the direct ethanol PEM fuel cell operation. J. Power Sources 2008, 181, 214–227. [Google Scholar] [CrossRef]
- Haji, S. Analytical modeling of PEM fuel cell i-V curve. Renew. Energy 2011, 36, 451–458. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, P.F.; Yao, J.; Tan, P.; Xu, H.R.; Chen, B.; Yang, F.S.; Zhang, Z.X.; Ni, M. Thermo-economic modeling and analysis of an NG-fueled SOFC-WGS-TSA-PEMFC hybrid energy conversion system for stationary electricity power generation. Energy 2020, 192, 116613. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Ou, C.J.; Chen, J.C. A new analytical approach to model and evaluate the performance of a class of irreversible fuel cells. Int. J. Hydrogen Energy 2008, 33, 4161–4170. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Liao, T.J.; Zhou, Y.H.; Zhao, Y.R.; Chen, J.C. Exploiting the waste heat from an alkaline fuel cell via electrochemical cycles. Energy 2018, 142, 983–990. [Google Scholar] [CrossRef]
- Zhou, S.J.; Wang, P.; Zhang, M.; Chen, S.Z.; Xu, W.; Zhu, L.T.; He, X.Q.; Gong, S.R. Effects of atmospheric acid deposition on root physiological characteristics of Pinus massoniana seedlings. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 44, 111–118. [Google Scholar] [CrossRef]
- Xiong, G.K.; Li, Y.Q.; Xiong, Y.Q.; Duan, A.G.; Cao, D.C.; Sun, J.J.; Nie, L.Y.; Sheng, W.T. Effects of low stand density afforestation on the growth, stem-form and timber assortment structure of Cunninghamia lanceolata plantations. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 165–173. [Google Scholar] [CrossRef]
- Ji, X.L.; Yang, P. The exploration of the slope displacement with vegetation protection under different rainfall intensity. J. For. Eng. 2020, 5, 152–156. [Google Scholar]
- Li, X.S.; Deng, T.T.; Wang, M.H.; Ju, S.; Li, X.C.; Li, M. Linear positioning algorithm improvement of wood acoustic emission source based on wavelet and signal correlation analysis methods. J. For. Eng. 2020, 5, 138–143. [Google Scholar]
- Guo, Y.H.; Guo, X.R.; Zhang, H.C.; Hou, S.J. Energetic, exergetic and ecological analyses of a high-temperature proton exchange membrane fuel cell based on a phosphoric-acid-doped polybenzimidazole membrane. Sustain. Energy Technol. 2020, 38, 100671. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| current density, | 0–20,000 [27] |
| operating temperature, | 373–473 [20] |
| intake pressure, | 1–3 [27] |
| thickness of membrane, | 20, 60, 100 [27] |
| doping level, | 2, 6, 10 [27] |
| electronic number, | 2 |
| faraday constant, | 96,485 |
| ambient temperature, | 298.15 |
| transfer coefficient, | 0.25 [20] |
| diffusion layer thickness, | 2.6 [20] |
| electron conductivity, | 53 [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Li, D.; Ma, Z.; Zheng, M.; Li, Y. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics 2021, 9, 1792. https://doi.org/10.3390/math9151792
Xu B, Li D, Ma Z, Zheng M, Li Y. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics. 2021; 9(15):1792. https://doi.org/10.3390/math9151792
Chicago/Turabian StyleXu, Bing, Dongxu Li, Zheshu Ma, Meng Zheng, and Yanju Li. 2021. "Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications" Mathematics 9, no. 15: 1792. https://doi.org/10.3390/math9151792
APA StyleXu, B., Li, D., Ma, Z., Zheng, M., & Li, Y. (2021). Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics, 9(15), 1792. https://doi.org/10.3390/math9151792






