New Multiscale Approach of Complex Modelling Chordae Tendineae Considering Strain-Dependent Modulus of Elasticity
Abstract
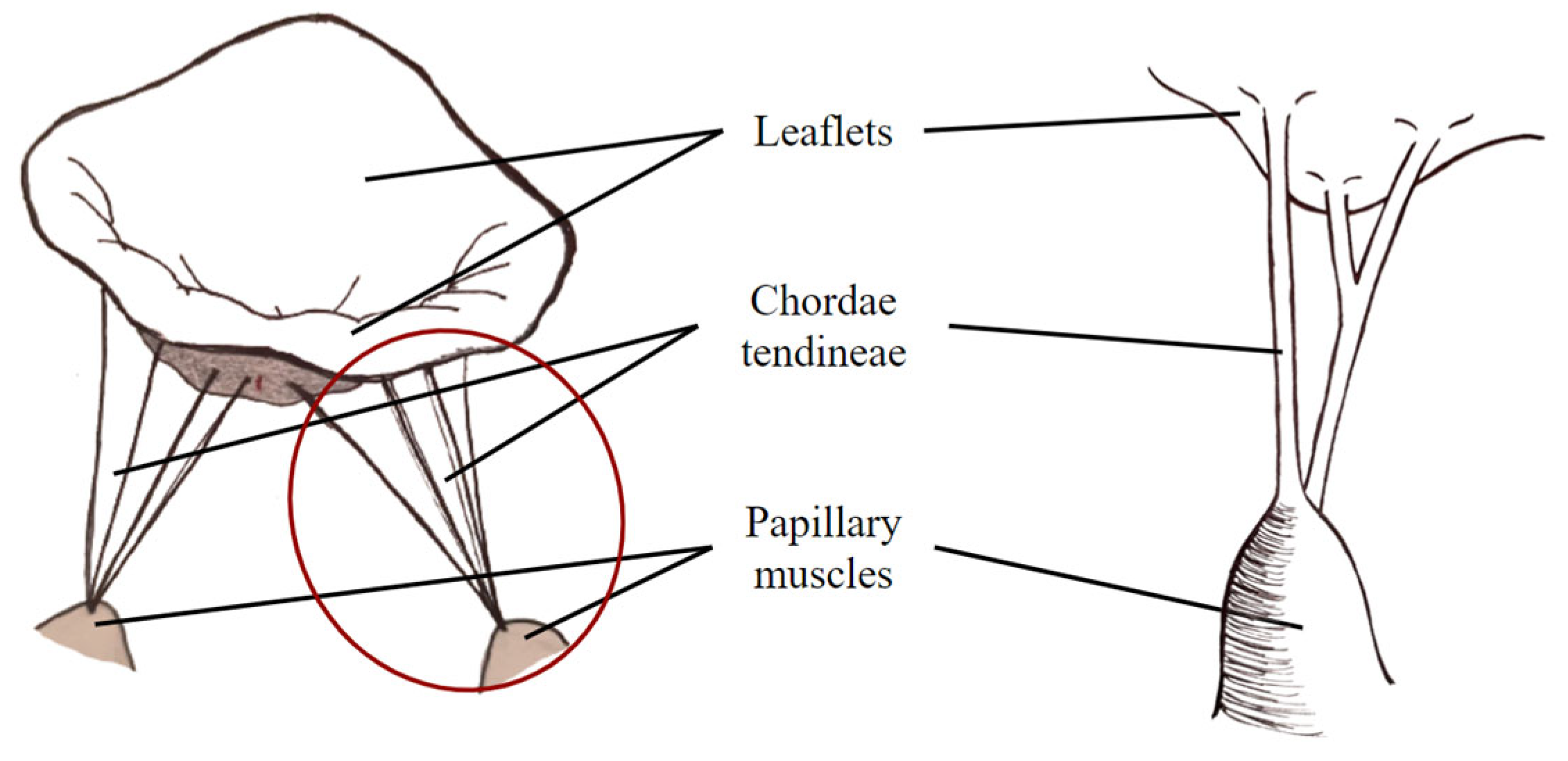
1. Introduction
2. Materials and Methods
2.1. Three-Dimensional Formulation
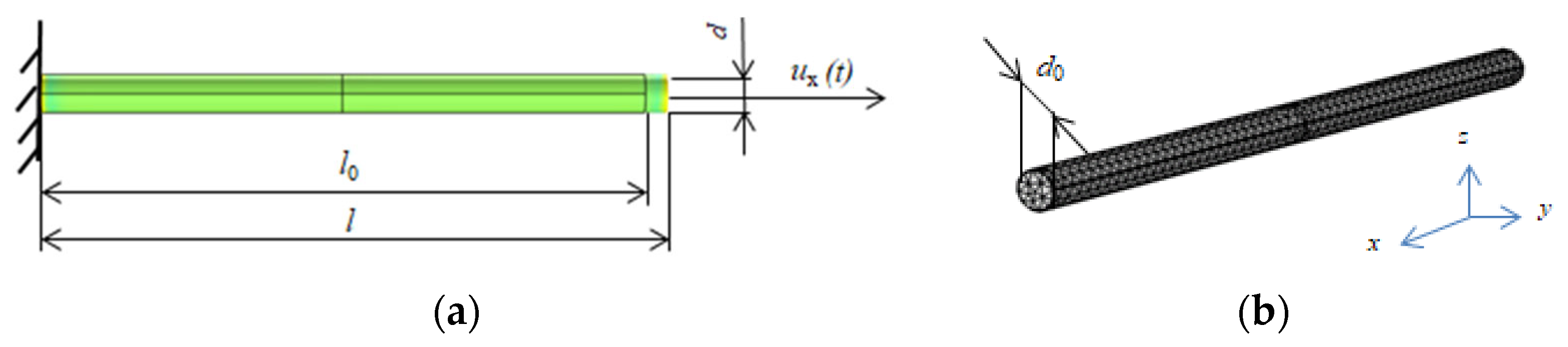
2.2. One-Dimensional Formulation
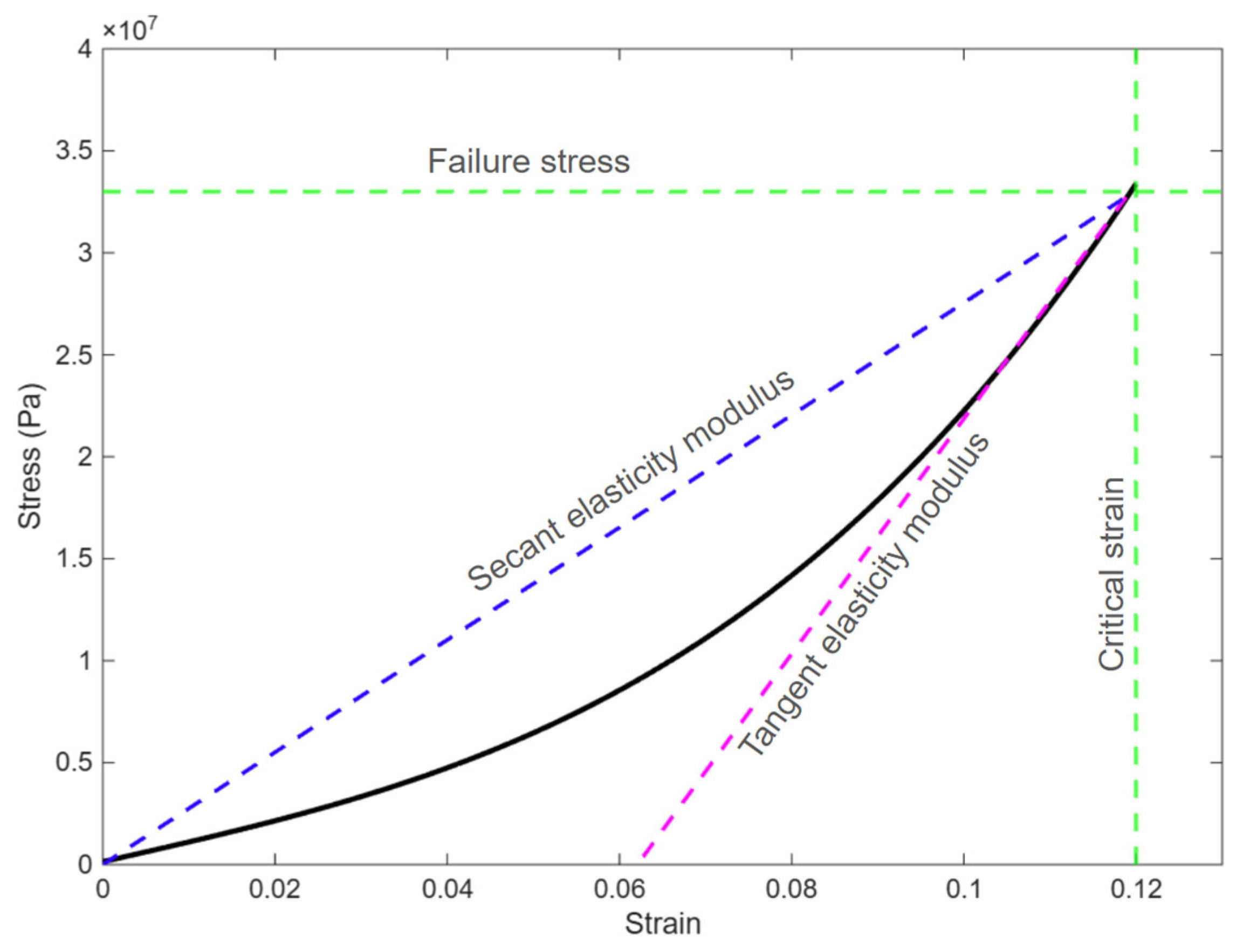
2.2.1. Material Nonlinearity
2.2.2. Geometrically Nonlinear Model
2.3. Finite Element Model
2.3.1. Development of 3D Finite Element Model
2.3.2. FE Methodology
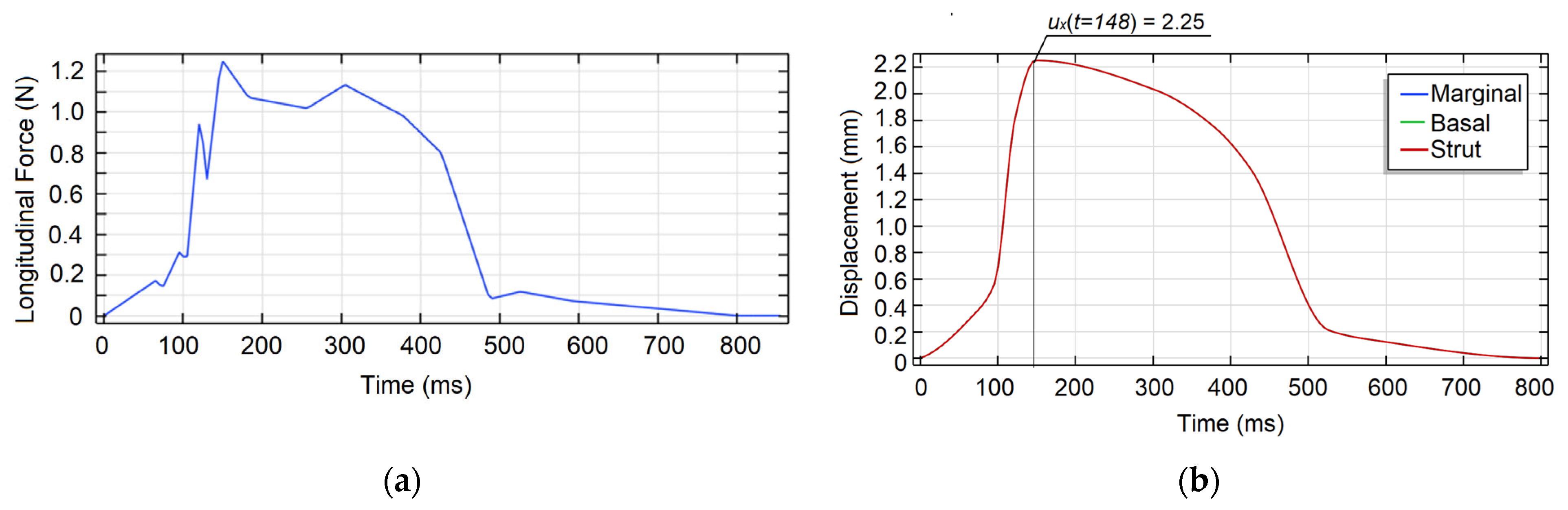
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MV | Mitral Valve |
| CT | Chordae Tendineae |
| FSI | Fluid–Structure Interaction |
| FEM | Finite Element Methods |
| CFD | Computational Fluid Dynamics |
| MA | Mitral Annulus |
References
- Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Gherbesi, E.; Faletra, F.F. Anatomy of Mitral Valve Complex as Revealed by Non-Invasive Imaging: Pathological, Surgical and Interventional Implications. J. Cardiovasc. Dev. Dis. 2020, 7, 49. [Google Scholar] [CrossRef]
- Zuo, K.; Pham, T.; Li, K.; Martin, C.; He, Z.; Sun, W. Characterization of Biomechanical Properties of Aged Human and Ovine Mitral Valve Chordae Tendineae. J. Mech. Behav. Biomed. Mater. 2016, 62, 607–618. [Google Scholar] [CrossRef]
- Koivumäki, J.T.; Hoffman, J.; Maleckar, M.M.; Einevoll, G.T.; Sundnes, J. Computational Cardiac Physiology for New Modelers: Origins, Foundations, and Future. Acta Physiol. 2022, 236, e13865. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Wisneski, A.D.; Sandhu, A.; Wang, Z.; Mahadevan, V.S.; Nguyen, T.C.; Guccione, J.M. Looking towards the Future: Patient-Specific Computational Modeling to Optimize Outcomes for Transcatheter Mitral Valve Repair. Front. Cardiovasc. Med. 2023, 10, 1140379. [Google Scholar] [CrossRef]
- Le, T.B.; Usta, M.; Aidun, C.; Yoganathan, A.; Sotiropoulos, F. Computational Methods for Fluid-Structure Interaction Simulation of Heart Valves in Patient-Specific Left Heart Anatomies. Fluids 2022, 7, 94. [Google Scholar] [CrossRef]
- Toma, M.; Einstein, D.R.; Bloodworth, C.H.; Cochran, R.P.; Yoganathan, A.P.; Kunzelman, K.S. Fluid-Structure Interaction and Structural Analyses Using a Comprehensive Mitral Valve Model with 3D Chordal Structure. Int. J. Numer. Methods Biomed. Eng. 2016, 33, e2815. [Google Scholar] [CrossRef]
- Abbas, S.S.; Nasif, M.S.; Al-Waked, R. State-of-the-Art Numerical Fluid–Structure Interaction Methods for Aortic and Mitral Heart Valves Simulations. Simulation 2022, 98, 3–34. [Google Scholar] [CrossRef]
- Gao, H.; Ma, X.; Qi, N.; Berry, C.; Griffith, B.E.; Luo, X. A Finite Strain Nonlinear Human Mitral Valve Model with Fluid-Structure Interaction. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 1597–1613. [Google Scholar] [CrossRef]
- Kunzelman, K.; Cochran, R.; Chuong, C.; Ring, W.; Verrier, E.; Eberhart, R. Finite Element Analysis of the Mitral Valve. J. Heart Valve Dis. 1993, 3, 326–340. [Google Scholar]
- Ambrosi, D.; Deorsola, L.; Turzi, S.; Zoppello, M. Elementary mechanics of the mitral valve. SIAM J. Appl. Math. 2022, 82, 75–94. [Google Scholar] [CrossRef]
- Liao, J.; Vesely, I. A Structural Basis for the Size-Related Mechanical Properties of Mitral Valve Chordae Tendineae. J. Biomech. 2003, 36, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Mangine, N.R.; Laurence, D.W.; Sabin, P.M.; Wu, W.; Herz, C.; Zelonis, C.N.; Unger, J.S.; Pinter, C.; Lasso, A.; Maas, S.A.; et al. Effect of Parametric Variation of Chordae Tendineae Structure on Simulated Atrioventricular Valve Closure. arXiv 2024, arXiv:2411.09599. [Google Scholar] [CrossRef]
- Crispino, A.; Bennati, L.; Vergara, C. Cardiac Hemodynamics Computational Modeling Including Chordae Tendineae, Papillaries, and Valves Dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ross, C.J.; Zheng, J.; Ma, L.; Wu, Y.; Lee, C.H. Mechanics and Microstructure of the Atrioventricular Heart Valve Chordae Tendineae: A Review. Bioengineering 2020, 7, 25. [Google Scholar] [CrossRef]
- Ogden, R. Large Deformation Isotropic Elasticity—On the Correlation of Theory and Experiment for Incompressible Rubberlike Solids. Proc. R. Soc. Lond. A Math. Phys. Sci. 1972, 326, 565–584. [Google Scholar] [CrossRef]
- Votta, E.; Arnoldi, A.; Invernizzi, A.; Ponzini, R.; Veronesi, F.; Tamborini, G.; Pepi, M.; Alamanni, F.; Redaelli, A.; Caiani, E.G. Mitral Valve Patient-Specific Finite Element Modeling from 3-D Real Time Echocardiography: A Potential New Tool for Surgical Planning. In Proceedings of the CI2BM09—MICCAI Workshop on Cardiovascular Interventional 370 Imaging and Biophysical Modelling, London, UK, 20 September 2009; 9p. [Google Scholar] [CrossRef]
- Lim, K.O.; Boughner, D.R. Mechanical Properties of Human Mitral Valve Chordae Tendineae: Variation with Size and Strain Rate. Can. J. Physiol. Pharmacol. 2011, 53, 330–339. [Google Scholar] [CrossRef]
- Liu, L.W.; Lee, C.H.; Aggarwal, A.; Chao, C.M.; Ross, C.J.; Liao, Y.K. Viscoelastic Modelling of the Tricuspid Valve Chordae Tendineae Tissue. Appl. Math. Model. 2022, 105, 648–669. [Google Scholar] [CrossRef] [PubMed]
- Gun, M.; Barry, M.; Tribouilloy, C.; Bohbot, Y.; Touati, G. Anatomical Mitral Valve Repair: Mathematical Prediction of Artificial Chordae Length in Para-Commissural Regions. Curr. Probl. Cardiol. 2024, 49, 102063. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K. Characterization of Biomechanical Properties of Mitral Valve Chordae Tendineae. Ph.D. Thesis, University of Connecticut, Storrs, CT, USA, 2014. [Google Scholar]
- Bonet, J.; Wood, R.D. Nonlinear Continuum Mechanics for Finite Element Analysis; Cambridge University Press: Cambridge, UK, 1997; ISBN 0-521-57272-X. [Google Scholar]
- Gaidulis, G.; Suresh, K.S.; Xu, D.; Padala, M. Patient-Specific Three-Dimensional Ultrasound Derived Computational Modeling of the Mitral Valve. Ann. Biomed. Eng. 2022, 50, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sari, C.R.; Gao, H.; Lei, Y.; Segers, P.; De Beule, M.; Wang, G.; Ma, X. Mechanical and Morphometric Study of Mitral Valve Chordae Tendineae and Related Papillary Muscle. J. Mech. Behav. Biomed. Mater. 2020, 111, 104011. [Google Scholar] [CrossRef]
- Fatemifar, F.; Feldman, M.D.; Oglesby, M.; Han, H.C. Comparison of Biomechanical Properties and Microstructure of Trabeculae Carneae, Papillary Muscles, and Myocardium in the Human Heart. J. Biomech. Eng. 2019, 141, 0210071. [Google Scholar] [CrossRef]
- Stevanella, M.; Maffessanti, F.; Conti, C.A.; Votta, E.; Arnoldi, A.; Lombardi, M.; Parodi, O.; Caiani, E.G.; Redaelli, A. Mitral Valve Patient-Specific Finite Element Modeling from Cardiac MRI: Application to an Annuloplasty Procedure. Cardiovasc. Eng. Technol. 2011, 2, 66–76. [Google Scholar] [CrossRef]
- Lam, J.H.; Ranganathan, N.; Wigle, E.D.; Silver, M.D. Morphology of the Human Mitral Valve. Circulation 1970, 41, 449–458. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Cochran, R. Mechanical Properties of Basal and Marginal Mitral Valve Chordae Tendineae. ASAIO Trans. 1990, 3, M405–M408. [Google Scholar]
- Constable, M.; Northeast, R.; Lawless, B.M.; Burton, H.E.; Gramigna, V.; Goh, K.L.; Buchan, K.G.; Espino, D.M. Mechanical Testing of Glutaraldehyde Cross-Linked Mitral Valves. Part Two: Elastic and Viscoelastic Properties of Chordae Tendineae. Proc. Inst. Mech. Eng. H 2021, 235, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ott, R.; Öner, A.; Bellé, P.H.; Borowski, F.; Schmitz, K.P.; Stiehm, M. Validation of a Fluid-Structure Interaction Model for the Characterization of Transcatheter Mitral Valve Repair Devices. Curr. Dir. Biomed. Eng. 2021, 7, 605–608. [Google Scholar] [CrossRef]
- Paulsen, M.J.; Imbrie-Moore, A.M.; Wang, H.; Bae, J.H.; Hironaka, C.E.; Farry, J.M.; Lucian, H.J.; Thakore, A.D.; MacArthur, J.W.; Cutkosky, M.R.; et al. Mitral Chordae Tendineae Force Profile Characterization Using a Posterior Ventricular Anchoring Neochordal Repair Model for Mitral Regurgitation in a Three-Dimensional-Printed Ex Vivo Left Heart Simulator. Eur. J. Cardio-Thorac. Surg. 2020, 57, 535–544. [Google Scholar] [CrossRef] [PubMed]
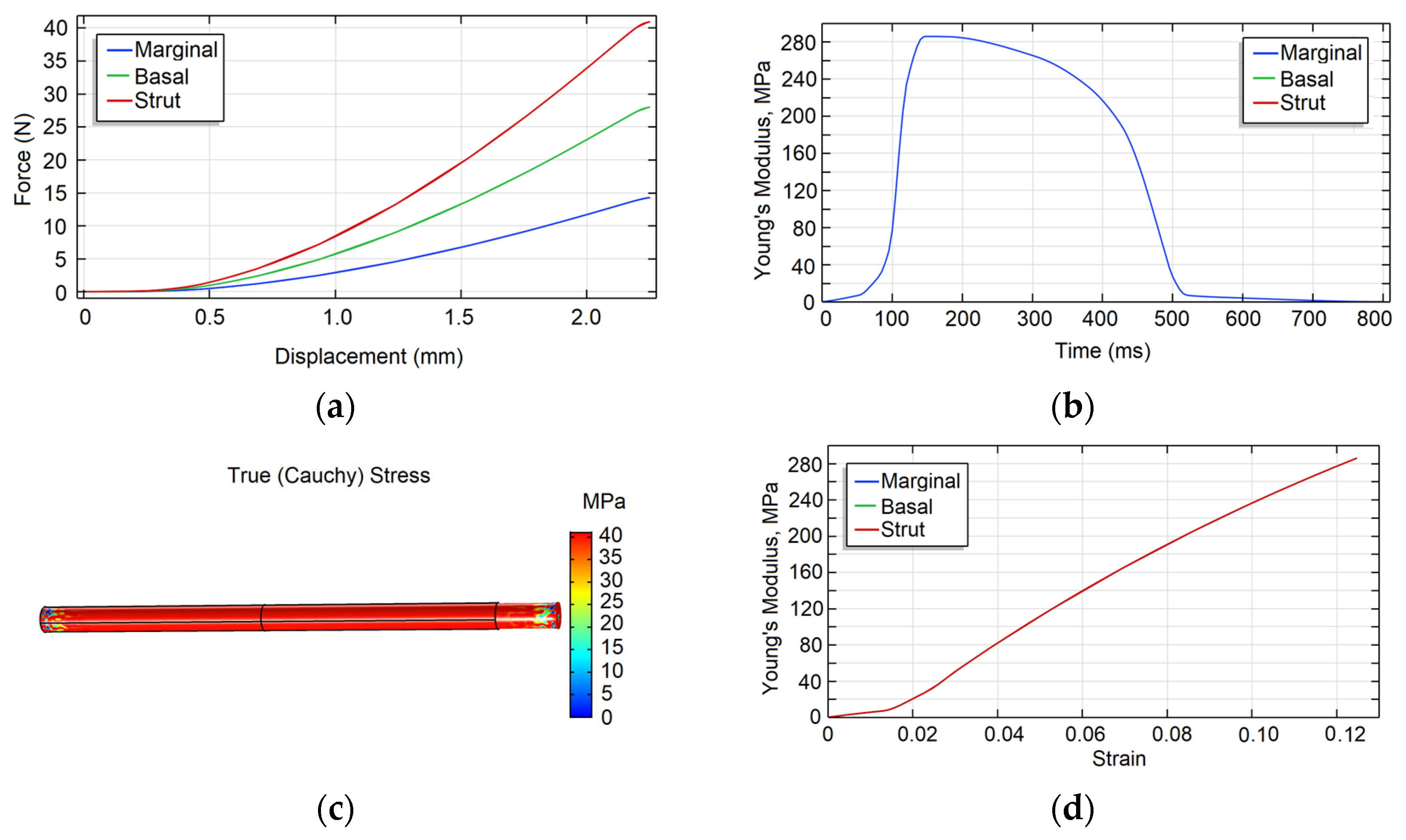
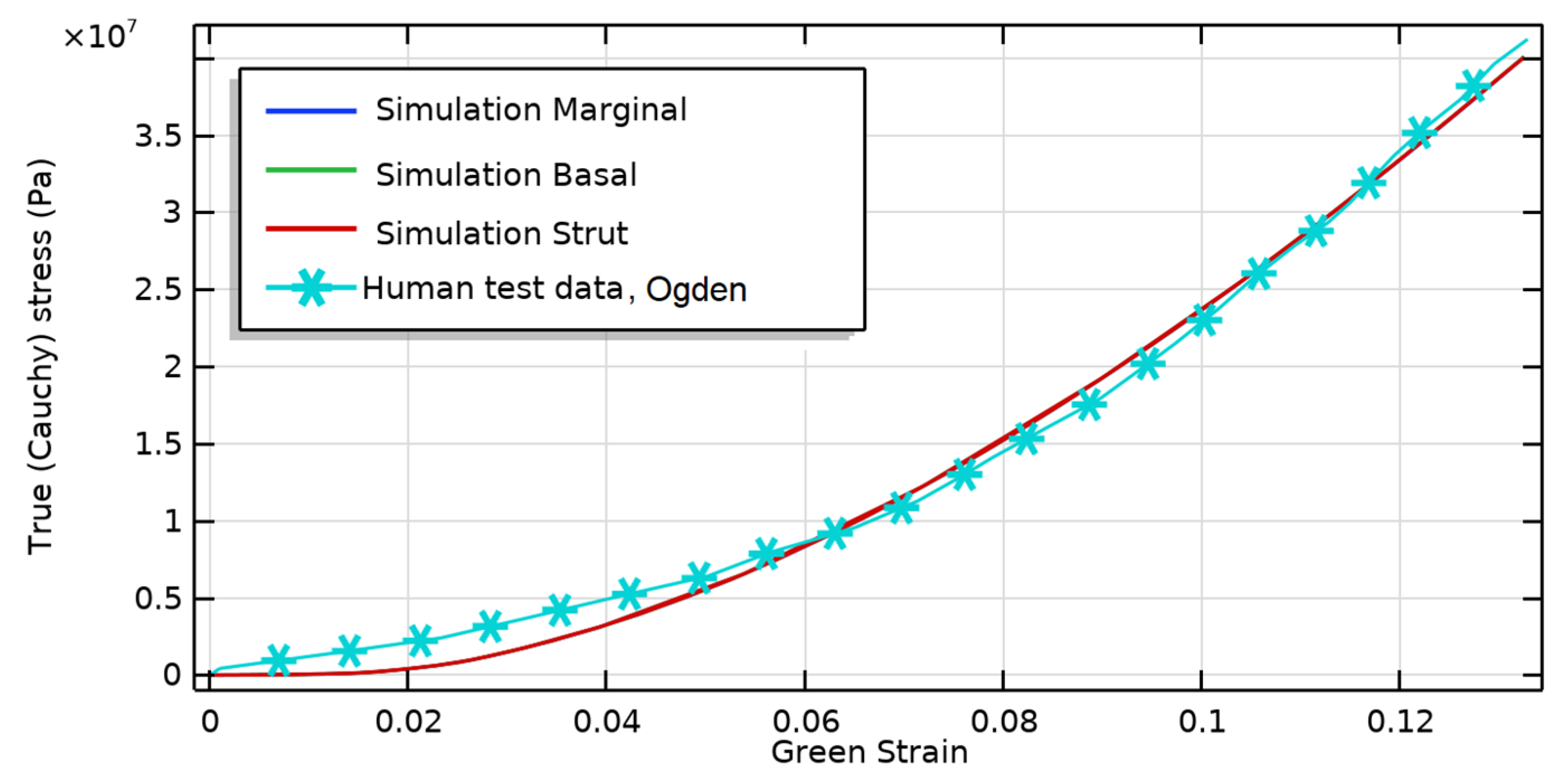
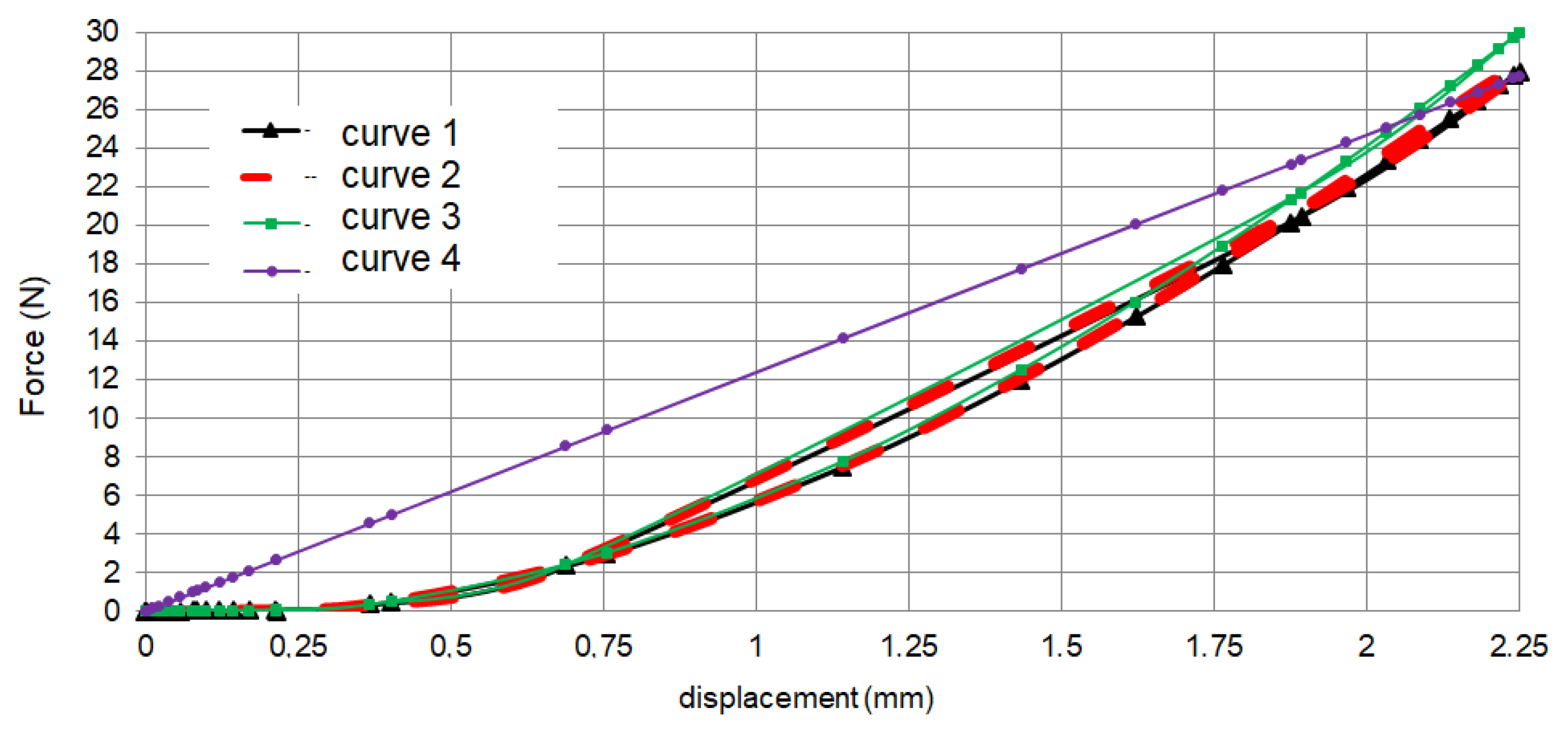
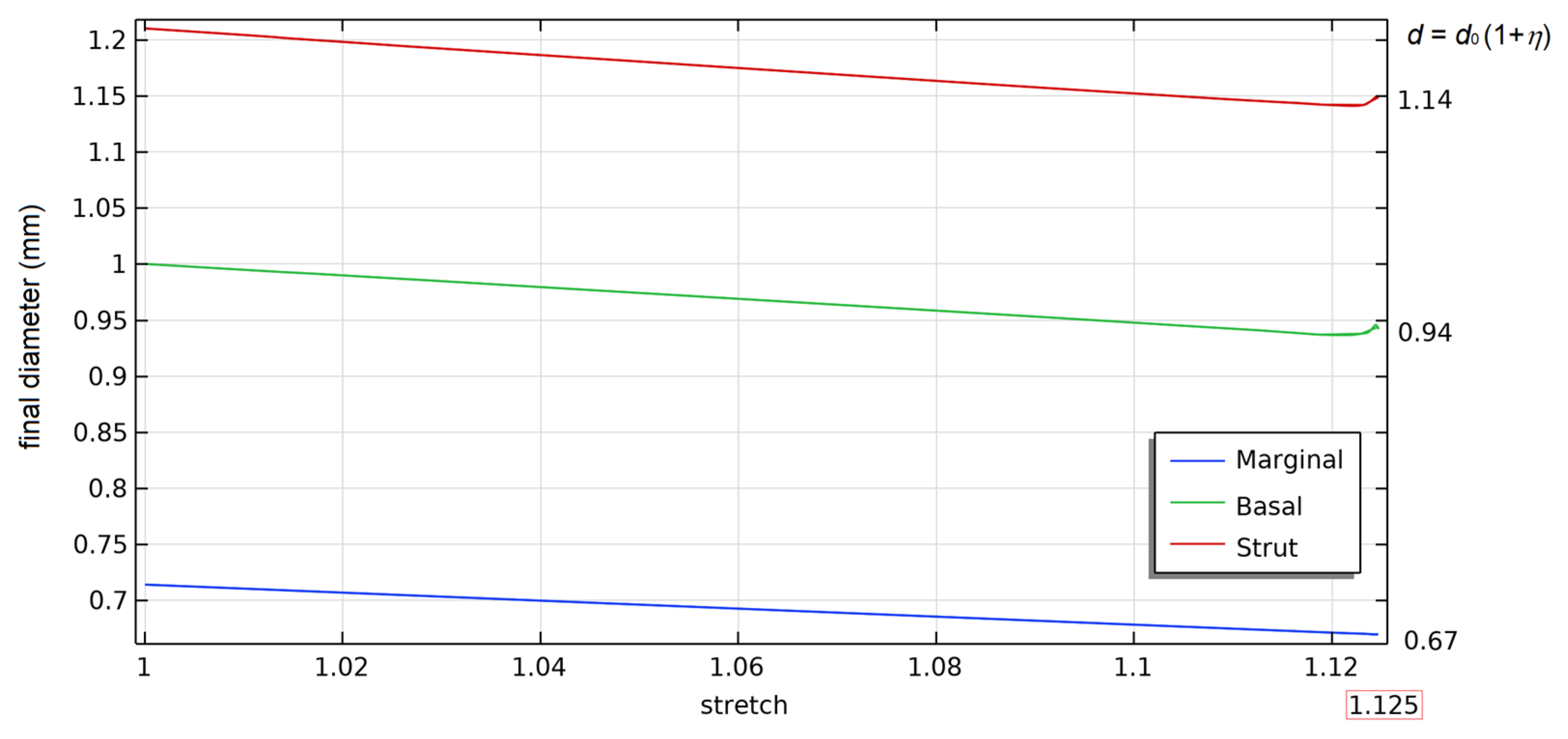
| a5 | a4 | a3 | a2 | a1 | a | |
|---|---|---|---|---|---|---|
| Prior to the TP | −30 × 106 | 10 × 106 | −2 × 106 | 121,676 | −690.91 | 1.69565 |
| After to the TP | −7521.8 | 3640.3 | −50.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez Hurtado, A.; Borodinas, S.; Chabarova, O.; Selivonec, J.; Stupak, E. New Multiscale Approach of Complex Modelling Chordae Tendineae Considering Strain-Dependent Modulus of Elasticity. Mathematics 2025, 13, 3331. https://doi.org/10.3390/math13203331
Menéndez Hurtado A, Borodinas S, Chabarova O, Selivonec J, Stupak E. New Multiscale Approach of Complex Modelling Chordae Tendineae Considering Strain-Dependent Modulus of Elasticity. Mathematics. 2025; 13(20):3331. https://doi.org/10.3390/math13203331
Chicago/Turabian StyleMenéndez Hurtado, Alicia, Sergejus Borodinas, Olga Chabarova, Jelena Selivonec, and Eugeniuš Stupak. 2025. "New Multiscale Approach of Complex Modelling Chordae Tendineae Considering Strain-Dependent Modulus of Elasticity" Mathematics 13, no. 20: 3331. https://doi.org/10.3390/math13203331
APA StyleMenéndez Hurtado, A., Borodinas, S., Chabarova, O., Selivonec, J., & Stupak, E. (2025). New Multiscale Approach of Complex Modelling Chordae Tendineae Considering Strain-Dependent Modulus of Elasticity. Mathematics, 13(20), 3331. https://doi.org/10.3390/math13203331








