1. Introduction
Respiratory Syncytial Virus (RSV) is a prevalent respiratory pathogen first identified in 1956. Its incidence typically increases during the winter months, presenting with symptoms that resemble those of the common cold. While RSV infections are generally self-limiting and resolve within two weeks, the virus can pose significant health risks to specific populations. In particular, it may lead to complications such as bronchiolitis and, in more severe cases, pneumonia. Moreover, RSV has the potential to aggravate pre-existing chronic conditions, including asthma and congestive heart failure, and may negatively impact cardiovascular health [
1]. It is estimated that, globally, around 30 million cases occur annually among children under the age of five, with fewer than 10% requiring hospitalization [
2]. Statistics indicate that 80% of bronchiolitis cases in children under one year are due to this virus [
2,
3,
4]. The virus was first identified in 1956 when it was isolated from a chimpanzee with respiratory symptoms and was initially named the “chimpanzee coryza agent”; in the following decade, it was renamed “Respiratory Syncytial Virus” (RSV) to reflect the large syncytial cell formations it causes in tissue cultures [
3,
4]. Epidemiological studies from that period showed that RSV was among the most common causes of respiratory complications in newborns and infants [
3,
4]. Although RSV infections in adults were reported as early as the 1960s, the burden in this population was not fully appreciated until the 1990s, when the clinical impact among the elderly, individuals with chronic cardiac or pulmonary disease, long-term care facility residents, and immunocompromised patients became clearer [
4]. Recurrent RSV infections are common, though typically less severe than primary infections [
5]. Recent evidence indicates that recurrent infections can still impose a notable disease burden even in previously healthy adults [
6]. RSV is responsible for 50–90% of hospitalizations due to bronchiolitis, 5–40% of pneumonia cases, and 10–30% of bronchitis cases; primary infections rarely occur without symptoms [
6]. Epidemiologically, RSV exhibits a clear seasonal pattern, typically beginning to spread in the fall and peaking during winter in temperate regions while remaining active year-round in tropical or hot climates, especially during rainy periods [
2]. Environmental factors such as air pollution, tobacco smoke, and indoor crowding are major contributors to RSV spread [
2]. The likelihood of outbreaks increases in hospitals during peak seasons—particularly in crowded pediatric wards—where 30–50% of healthcare workers may be at risk of nosocomial infection [
3]. Transmission is not limited to healthcare settings; households also play a role, with older siblings often introducing the virus and increasing the infection risk for infants [
3,
6].
Mathematical modeling is a fundamental tool for understanding and analyzing complex phenomena and systems. A substantial body of research has shown that it provides a powerful and effective framework for integrating concepts from science, technology, engineering, and mathematics into real-world applications [
7,
8,
9,
10,
11,
12].
Recent work has increasingly focused on mathematical models of Respiratory Syncytial Virus (RSV) transmission [
1], with extensions that incorporate fractional-order dynamics [
13,
14,
15,
16].
To the best of our knowledge, the optimal control of the RSV infection model in [
1] has not yet been investigated in depth. In this paper, we apply Pontryagin’s maximum principle (PMP) to derive and analyze optimal control strategies for a two-strain RSV model, building on the foundational works in [
17,
18,
19,
20,
21,
22].
The remainder of this paper is structured as follows:
Section 2 formulates the mathematical model.
Section 3 presents the computation of the basic reproduction number and investigates both local and global stability.
Section 4 examines the occurrence of forward bifurcation.
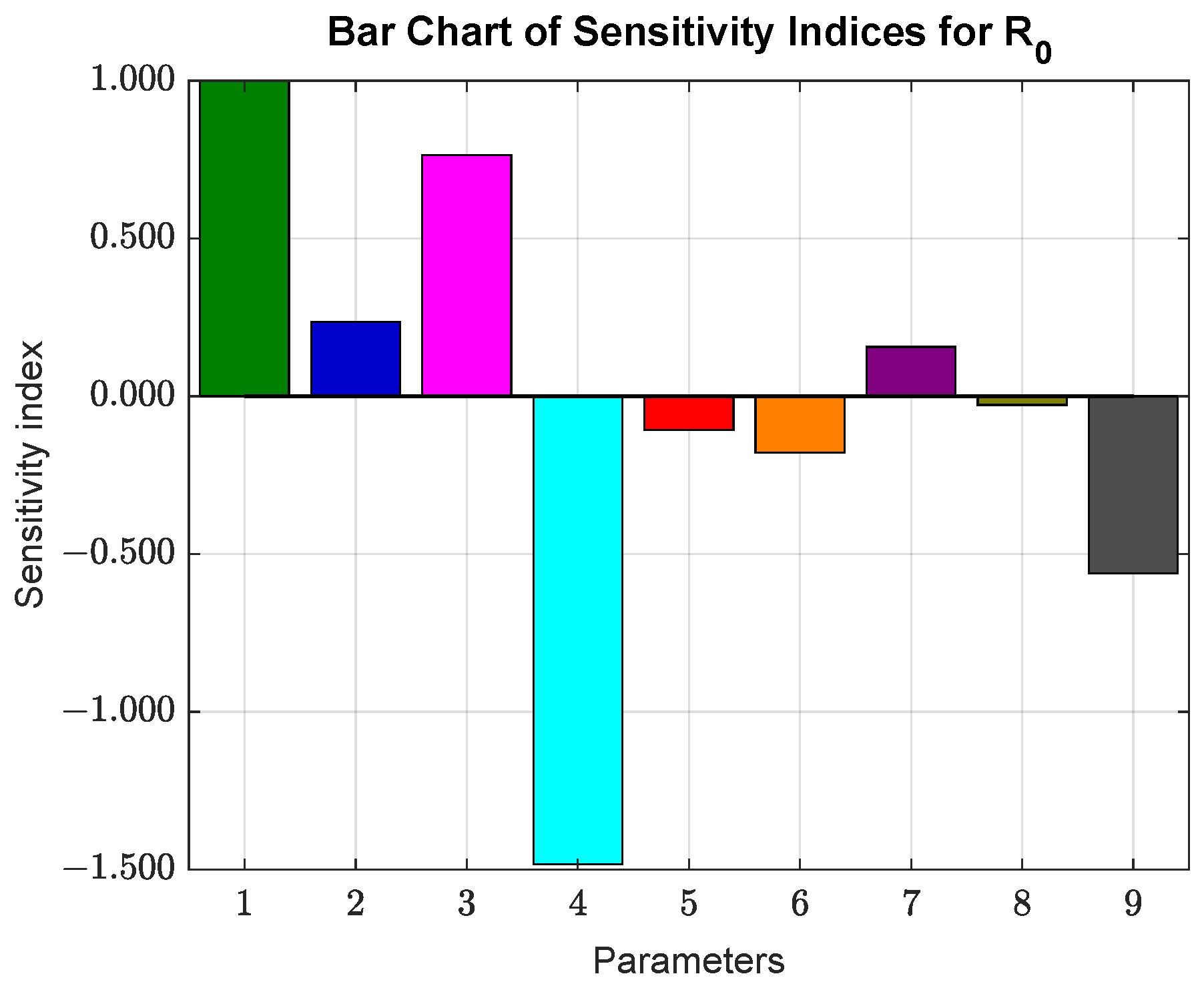
Section 5 is devoted to sensitivity analysis.
Section 6 focuses on the formulation and analysis of the optimal control problem.
Section 7 presents numerical simulations illustrating the effectiveness of the proposed optimal control strategies.
Section 8 investigates the cost-effective analysis. Finally,
Section 9 gives the conclusion.
6. Optimal Control Application
In this study, we consider three time-dependent control functions in the Respiratory Syncytial Virus (RSV) transmission model: represents the isolation of infected individuals, denotes the treatment of infected individuals, and corresponds to the vaccination of susceptible individuals.
The corresponding Respiratory Syncytial Virus (RSV) transmission model is given by
The ultimate objective is to determine the optimal level of intervention aimed at minimizing the infection and the cost of controls. We consider the objective functional as
where
T denotes the final time, and
,
, and
are balancing constants associated with the susceptible, acutely infected, and chronically infected populations, respectively. Similarly,
,
, and
represent the weight coefficients corresponding to each individual control measure. The diagram representing the effect of Respiratory Syncytial Virus in the presence of control is shown in
Figure 3.
The following equation gives the Lagrangian for the optimal control problem:
We apply Pontryagin’s maximum principle (PMP) [
35,
36] to derive the necessary optimality conditions for the control. This principle converts the system (
6) and the Lagrangian into a problem of minimizing point-wise Hamiltonian (H), with respect to
,
, and
as
where
,
are the adjoint variables associated with
, and
R.
The adjoint equation and transversality conditions are standard results from Pontryagin’s maximum principle. We differentiate Hamiltonian with respect to state
, and
R, respectively, which then gives the following adjoint system:
By Pontryagin’s maximum principle, the first-order stationarity conditions
yield the unconstrained critical controls
:
where
are the costate variables, and
are the control weights. Enforcing the bounds
gives the optimal controls as the projection of
onto
:
equivalently,
7. Numerical Simulation
This section presents numerical simulations that evaluate the effects of optimal control strategies on RSV transmission. The model is integrated using the classical fourth-order Runge–Kutta method coupled with the forward–backward sweep algorithm to assess the impact of various control combinations. In each iteration of the forward–backward sweep, the state equations are solved forward in time from the given initial conditions; the adjoint equations are then solved backward in time using the transversality conditions; finally, the controls are updated via the characterization, and the procedure is repeated until convergence [
36].
The key parameter values employed in our model are summarized in
Table 1 and were adopted from Sungchasit et al. [
1], who investigated a comparable RSV transmission model. The optimal strategy is obtained by simulating the optimality system comprising the controlled model (
6), the adjoint equations (
7), and the associated transversality conditions. Simulations are performed over an 18-day horizon, and we assume that isolation, treatment, and vaccination controls are applied on
days. The baseline basic reproduction number is
. The weights used in the objective functional are
,
,
,
,
, and
. To ensure reproducibility, we specify the numerical methods and their parameters as follows.
We solved the optimality system by a forward–backward sweep on
days using the classical fourth-order Runge–Kutta (RK4) scheme with a fixed step
for both the forward state solve and the backward adjoint solve. After each sweep, the controls were updated via the standard projection of the characterization onto
, with relaxation
and
. The iteration was terminated when the relative change in all three controls satisfied
or when a maximum of 1000 outer iterations was reached.
In this paper, we consider three controls: the first is the isolation control , the second is the treatment control , and the third is the vaccination control , but to determine which of the controls is more efficient, they should be divided into seven strategies, divided into three categories: the first category consists of one control, the second category consists of two controls, and the final category consists of all controls as follows:
Implementation of single control:
- –
Strategy 1: practising isolation control protocols only
- –
Strategy 2: practising treatment control protocols only
- –
Strategy 3: practising vaccination control protocols only
The use of double controls:
- –
Strategy 4: treatment and vaccination control protocols
- –
Strategy 5: isolation and vaccination control protocols
- –
Strategy 6: isolation and treatment control protocols
Implementation of triple control:
- –
Strategy 7: isolation, treatment, and vaccination controls
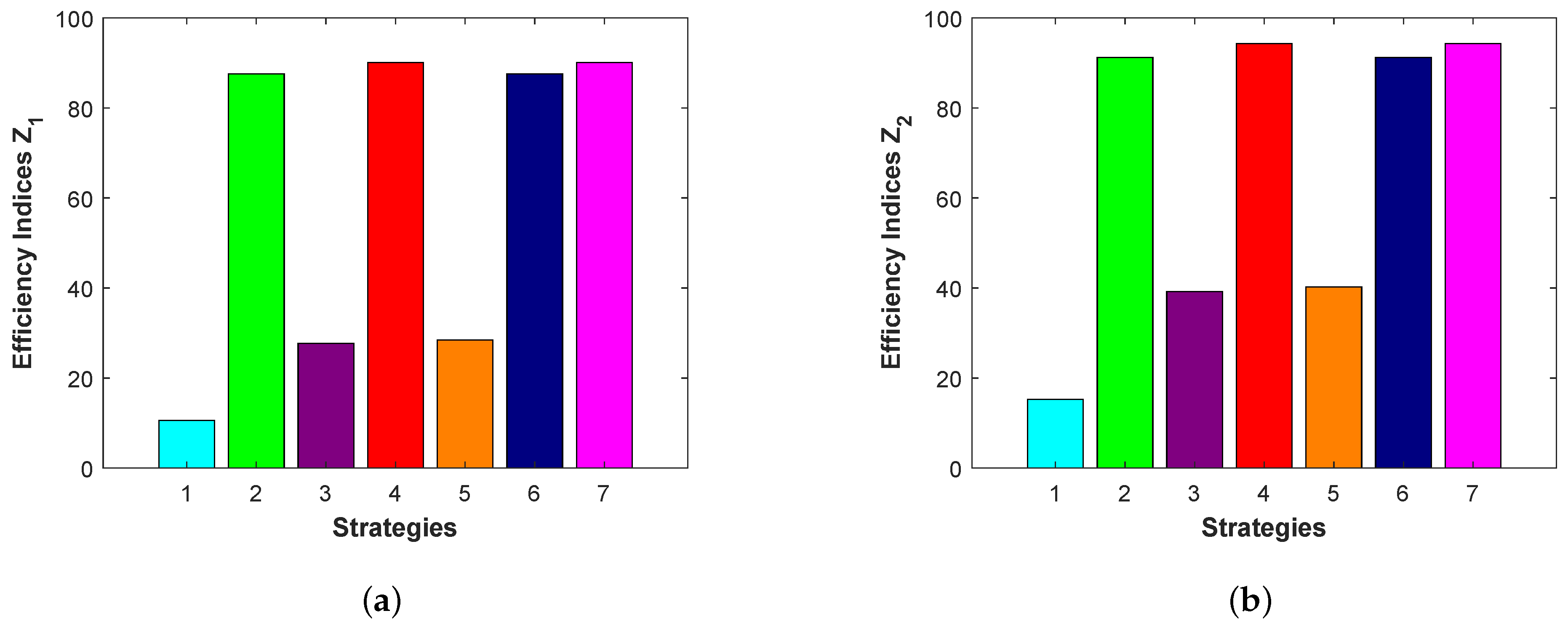
To determine the best control strategy among seven strategies, we have to calculate the efficiency index
, where
and
are the cumulative number of infected individuals with control and without control, respectively. The preferred strategy is the one with the highest efficiency index [
37,
38].
We will study the cumulative number of acutely infected populations and chronically infected populations during the time interval
. The acute infected population is determined by
. The values of alternating current
and efficiency index
for seven strategies are given in
Table 3. Similarly, the chronic infected population is determined by
. Here, we have
. The values of alternating current (
) and the efficiency index (E.I.) for the seven strategies are presented in
Table 4.
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10 illustrate (a) the time series of acutely infected individuals (
), (b) the time series of chronically infected individuals (
) with and without control, and (c) the optimal control trajectory over the time interval
.
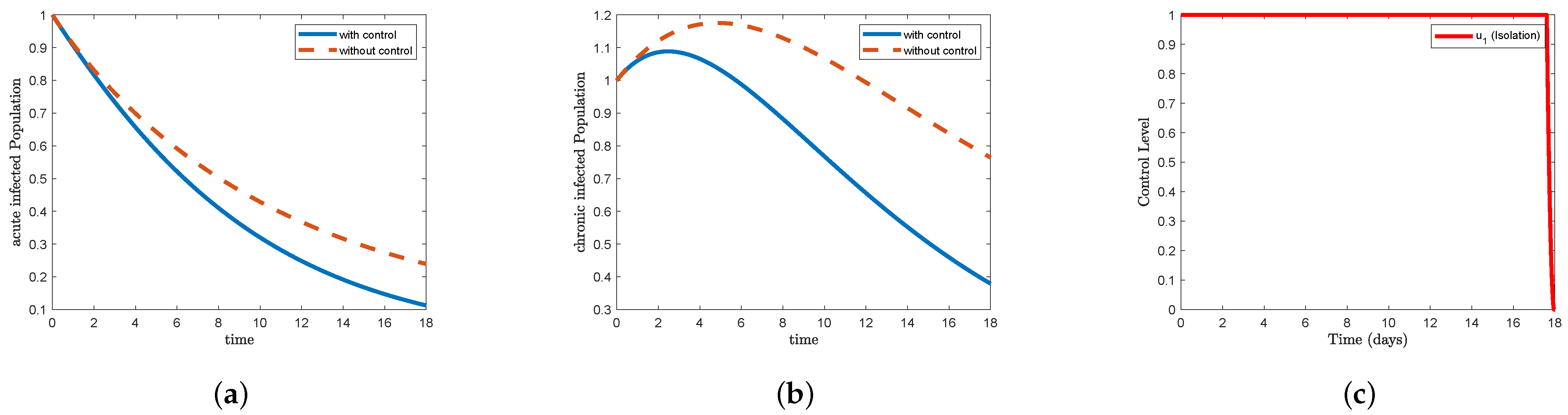
In
Figure 4, which represents STR-1, we find that the form (a) acute infected in the control condition decreases faster than the non-control condition, reaching close to
at the end of the study period. We also find that the form (b) chronic infected increases to reach nearly
on the third day and then begins to decrease to less than
at the end of the study period. In
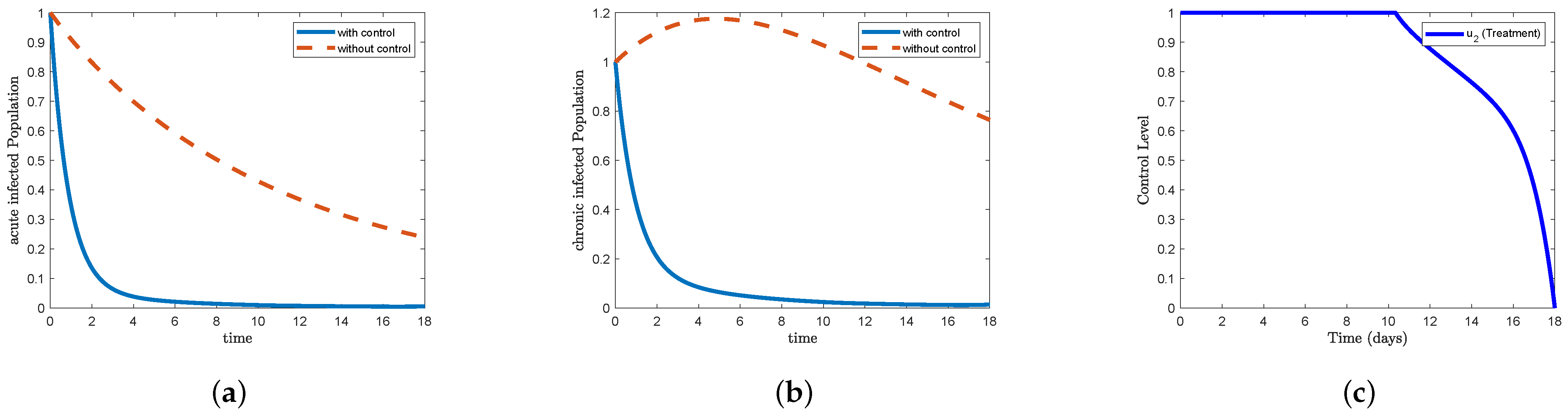
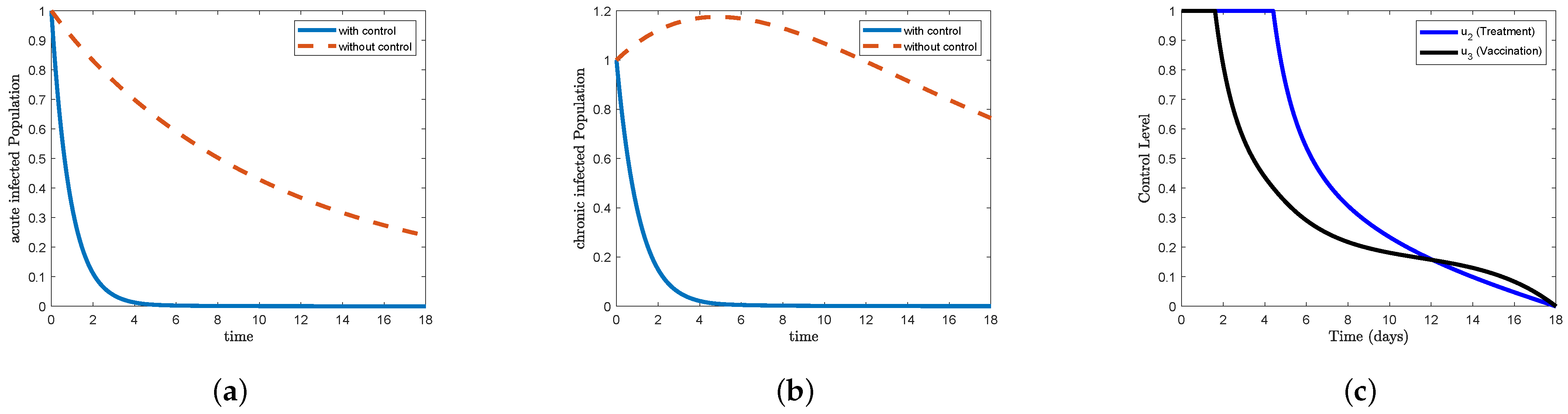
Figure 5, which represents STR-2, and
Figure 9, which represents STR-6, we note that the form (a) acute infected in the control condition decreases sharply to reach
on the third day and gradually decreases to eliminate the disease after 13 days. We also note that the form (b) chronic infected decreases sharply to reach
on the third day and gradually decreases to reach nearly
on the 13th day and continues until the end of the study period. In
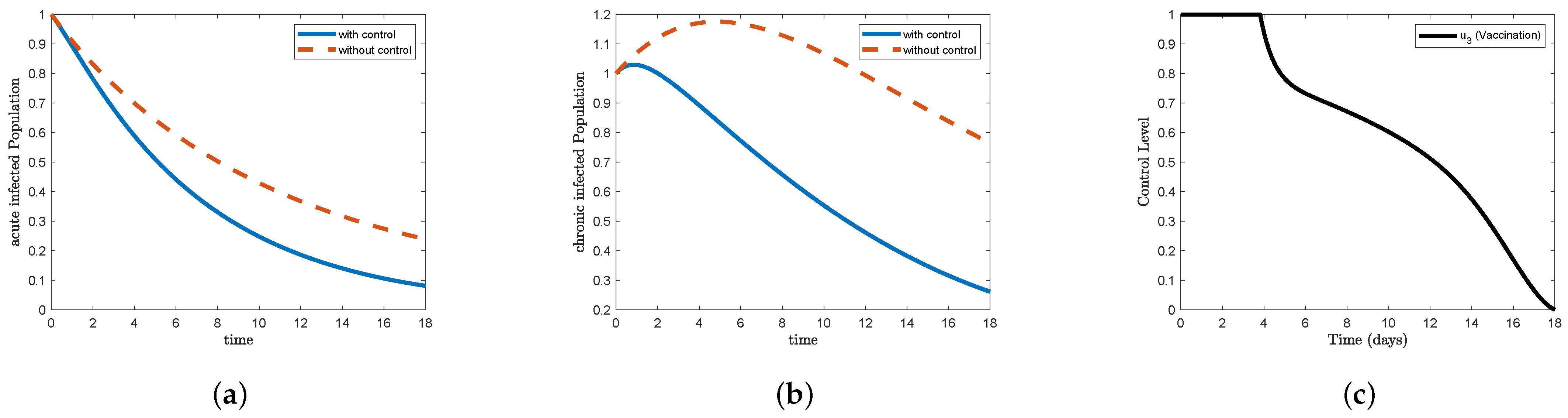
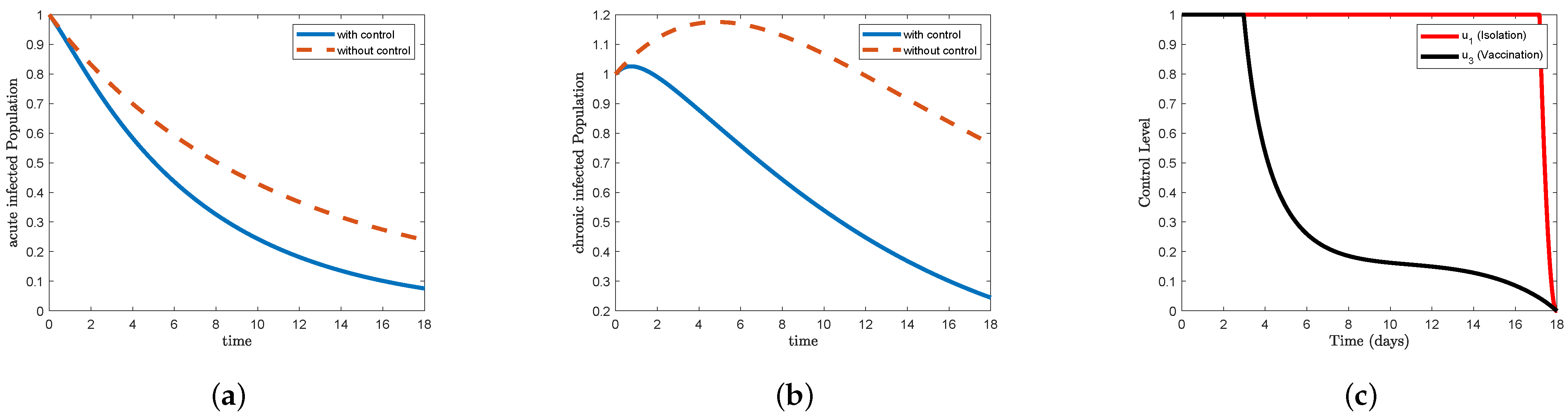
Figure 6, which represents STR-3, and
Figure 8, which represents STR-5, we note that the form (a) acute infected in the control condition decreases faster than the non-control condition, reaching less than
at the end of the study period. For the (b) chronic infected form, we find that it increased to approximately
on the first day and then began to gradually decrease to less than
at the end of the study period. In
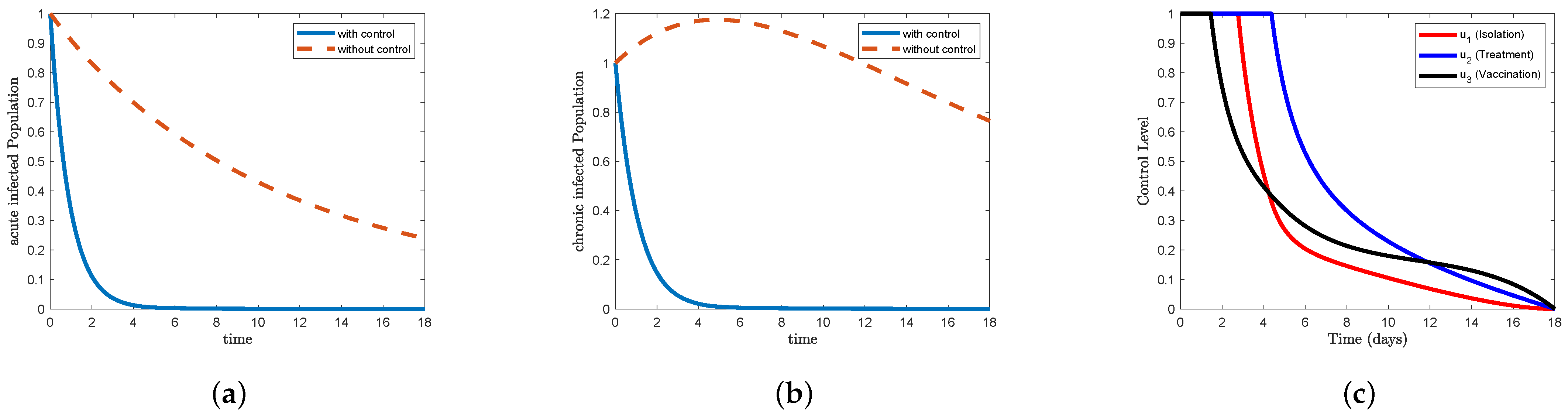
Figure 7, which represents STR-4, and
Figure 10, which represents STR-7, we find that the (a) acute infected form in the control condition can eliminate the disease before approximately the fifth day. We also note that the (b) chronic infected form in the control condition can eliminate the disease immediately after the fifth day. Whereas if we do not use the control, we find that the disease in all strategies in Figure (a) acute infected decreases gradually to reach
, and in Figure (b) chronic infected, we find that it increased to approximately
on the fifth day and then began to decrease to less than
at the end of the study period.
In
Figure 4c, we find that the control isolation
maintained its maximum level for more than 17 days and then decreased sharply to the minimum at the end of the study period. In
Figure 5c, we find that the control treatment
maintained its maximum level for more than 10 days, then began to gradually decrease on day 16 to reach
, and then decrease sharply to reach the minimum at the end of the study period. In
Figure 6c, we find that the control vaccination
maintained its highest level for less than 4 days and then began to decline sharply on the fifth day to reach less than
, after which the decline became gradual to reach the minimum at the end of the study period. In
Figure 7c, the data indicate that the control treatment
maintained its maximum level for more than
days, then began to decline sharply on the seventh day to reach approximately
, and then gradually further declined to reach the minimum at the end of the study period, while the control vaccination
maintained its highest level for more than
days, then began to decline sharply on the fifth day to reach
, and then gradually further declined to reach the lowest point at the end of the study period. In
Figure 8c, the data indicate that the control isolation
maintained its maximum level for 17 days and then decreased sharply to reach the minimum at the end of the study period, while the control vaccination
maintained the highest level for 3 days, then began to decrease sharply on the sixth day to reach
, and then gradually further declined to reach the lowest point at the end of the study period. In
Figure 9c, the data indicate that the control isolation
maintained its maximum level for less than 15 days and then began to decline sharply to reach the minimum at the end of the study period, while the control treatment
maintained its maximum level for more than 8 days, then began to decline gradually until approximately day 15 to reach less than
, and then sharply further decreased to reach the minimum point at the end of the study period. In
Figure 10c, the data indicate that the control isolation
maintained its maximum level for less than 3 days and then began to decline sharply until day 5, where it reached less than
, and then gradually reached the minimum after 17 days, while the control treatment
maintained its maximum level for more than
days and then began to decline sharply until day 7 to reach approximately
and then gradually to reach the minimum at the end of the study period, while the control vaccination
maintained its maximum level for more than
days, then began to decline sharply until day 5 to reach
, and then gradually further declined to reach the lowest point at the end of the study period.
It follows from
Table 3 and
Table 4 that, when using a single control, STR-2 is the best strategy among STR-1, STR-2, and STR-3 for reducing the number of incidents. Treatment is, therefore, more effective than isolation and vaccination. When using two controls, STR-4 is the best strategy among STR-4, STR-5, and STR-6 for reducing the number of incidents. Vaccination with treatment is, therefore, more effective than isolation with treatment and isolation with vaccination. When using all controls, we note that adding the isolation control to the vaccination with treatment has no effect.
8. Cost-Effective Analysis
In this section, we utilize the average cost-effectiveness ratio (ACER) and the incremental cost-effectiveness ratio (ICER) to conduct the cost-effectiveness analysis.
Based on the simulation results of the optimality system, using the parameter values in
Table 1, the average cost-effectiveness ratio (ACER) for each strategy is computed as follows [
37,
38,
39,
40,
41,
42]:
The total number of individuals infected averted during the intervention period T is obtained by using
where
,
, and
represent the infected classes in the absence of control measures, while
E,
, and
denote the corresponding optimal solutions under the implementation of control strategies. The total cost implemented during the period
T is calculated as follows:
Based on this cost-effectiveness analysis, the most cost-effective strategy is the one with the smallest average cost-effectiveness ratio (ACER) [
37,
40,
41].
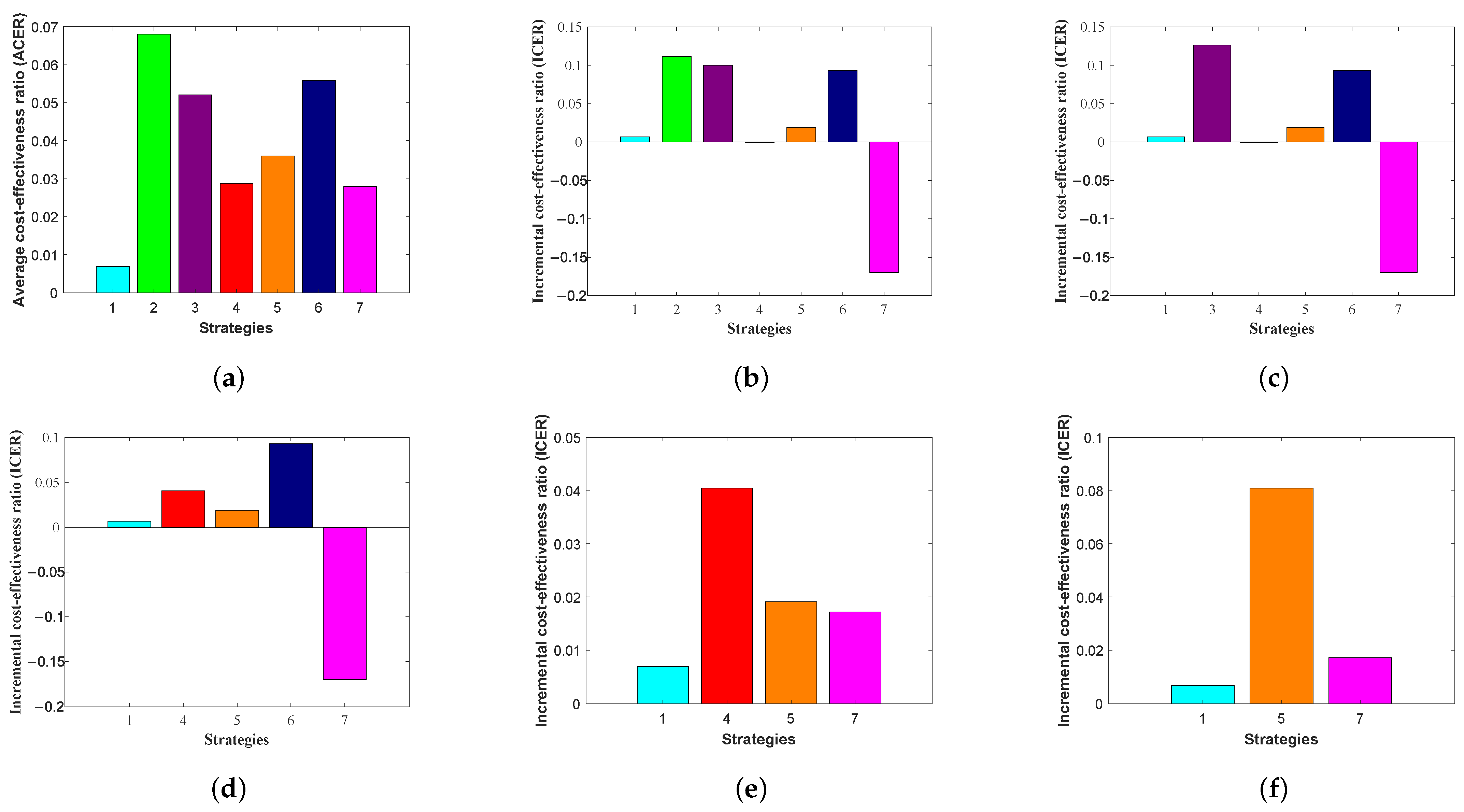
Table 5 shows the number of infections avoided, the total cost of each strategy, and the ACER.
According to the average cost-effectiveness ratio (ACER) method in
Table 5, STR-2 has the highest ACER value of
, and the lowest value is STR-1, which is
. This indicates that STR-2 is the most effective strategy. Therefore, as shown in
Figure 11, the average cost-effectiveness ratio, ranked from the most to the least expensive, is as follows: STR-2, STR-6, STR-3, STR-5, STR-4, STR-7, and STR-1.
The incremental cost-effectiveness ratio (ICER) is a key metric used to compare the cost-effectiveness of two viable interventions. It is calculated by dividing the difference in costs by the difference in their health outcomes or effects and is mathematically represented as [
33,
37,
39,
40,
41]
In
Table 6, we find seven strategies, where the ICER is calculated as follows:
From Equation (
12), we find that
The results obtained from ICER computations are presented in
Table 6.
We note from the results in
Table 6 that STR-2, which is based on the control treatment
, has a higher ICER than STR-3, which relies on the control vaccination
. This means that the individual application of the control treatment
is more expensive and less effective than the control vaccination
. Therefore, we exclude STR-2 from the list of alternative control strategies.
Then, ICER is calculated again for the remaining six strategies as follows:
Calculations of ICER are summarized in
Table 7.
We note from the results in
Table 7 that STR-3, which is based on the control vaccination
, is higher than STR-6, which relies on the control isolation
and the control treatment
. This means that the individual application of the control vaccination
is more expensive and less effective than the control isolation
and the control treatment
. Therefore, we exclude STR-3 from the list of alternative control strategies.
We now need to recalculate ICER for the remaining four strategies. The calculations are performed as follows:
The results obtained from the ICER computations are presented in
Table 8.
We examine the results in
Table 8, which shows that STR-6, which depends on the control isolation
and the control treatment
, is higher than STR-4, which depends on the control treatment
and the control vaccination
. This means that the application of the control isolation
and the control treatment
is more cost-effective and efficient than the control treatment
and the control vaccination
. Therefore, we exclude STR-6 from the search list for alternative control.
We now need to recalculate ICER for the remaining four strategies. The calculations are performed as follows:
The results obtained from the ICER computations are presented in
Table 9.
We examine the results in
Table 9, which shows that STR-4, which depends on the control treatment
and the control vaccination
, is higher than STR-5, which depends on the control isolation
and the control vaccination
. This means that the application of the control treatment
and the control vaccination
is more cost-effective and efficient than the control isolation
and the control vaccination
. Therefore, we exclude STR-4 from the search list for alternative control.
Then, ICER is calculated again for the remaining three strategies as follows:
The results obtained from ICER computations are presented in
Table 10.
We note from the results in
Table 10 that STR-5, which is based on the control isolation
and the control vaccination
, is higher than STR-7, which relies on the control isolation
, the control treatment
, and the control vaccination
. This means that the dual application of the control isolation
and the control vaccination
is more expensive and less effective than the control isolation
, the control treatment
, and the control vaccination
. Therefore, we exclude STR-5 from the list of alternative control strategies.
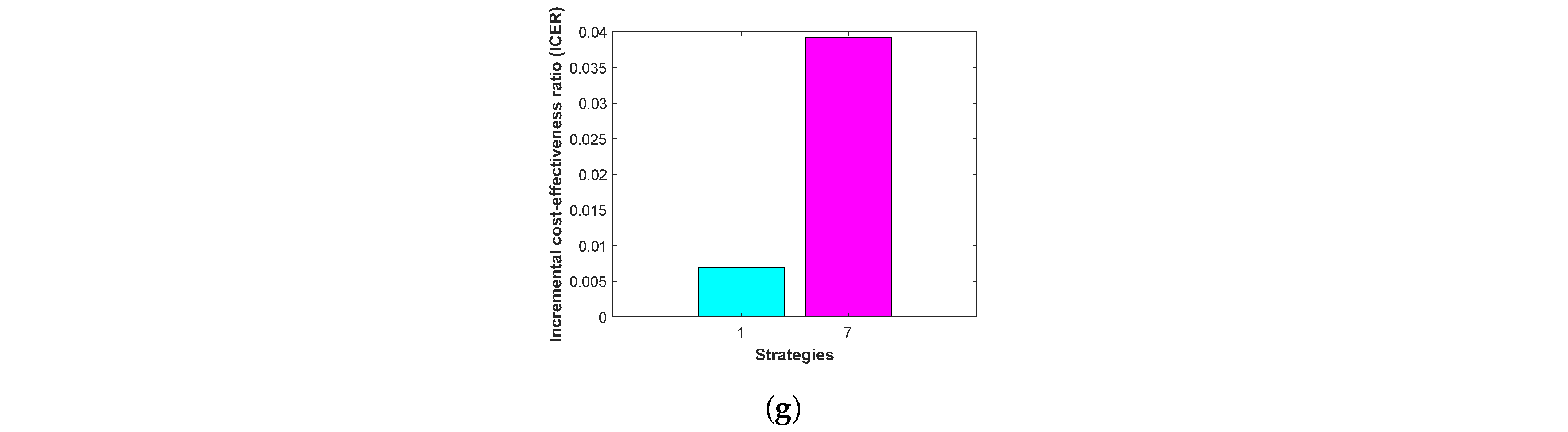
Then, the
is calculated again for the remaining two strategies as follows:
The results obtained from the
computations are presented in
Table 11.
From
Table 11, it is clear that ICER(7) is greater than ICER(1). The lower ICER value obtained for STR-1 means that STR-7 is significantly dominated, indicating that STR-7 is more expensive and less effective to implement than STR-1. Therefore, STR-1, which relies on the control isolation
, is the most cost-effective of the seven strategies analyzed in this work. As illustrated in
Figure 12, the bar charts present the cost–benefit ratio and the incremental cost–benefit ratios across the six successive stages of the elimination process.
9. Conclusions
In conclusion, this research presents a SEIR-type epidemiological model that integrates biological, mathematical, economic, and control aspects in the analysis of the spread of infectious diseases. The model was designed to reflect the transmission dynamics of a contagious viral disease within a homogeneous population, taking into account the progression of epidemic cases from exposed individuals to infected individuals and then recovering through the latency phase. This distinguishes the SEIR model from simpler models. Three time-based control variables representing vital preventive interventions were included: the first simulates the effect of insulation, the second represents the effect of treatment, and the last represents the effect of vaccination. This allows us to study the effectiveness of each intervention individually, as well as of each two interventions in combination, and also of three control variables in reducing the spread of infection.
Analytically, the basic reproduction number was calculated using the next generation matrix methodology, which was interpreted biologically in terms of the rate of generation of new cases and numerically by finding the maximum eigenvalue of the matrix. The results showed that the stability of the system around the disease-free equilibrium point and the endemic equilibrium point is directly related to the value of this number. Using Jacobian analysis and Lyapunov techniques, the study demonstrated that the disease-free equilibrium point is locally and globally stable when , and the endemic equilibrium point is locally and globally stable when . It was also shown that by studying the bifurcation when , the system has a forward bifurcation.
The sensitivity analysis helped identify the parameters that most influence the basic reproduction number . The results indicate that the parameter has the most significant effect on , where a increase in its value leads to a increase in . In contrast, the parameter (death rate) shows a strong negative effect on , where a increase in leads to a decrease in .
The Pontryagin principle was adopted to formulate the optimal control problem, allowing the derivation of optimal conditions that minimize the number of infections and the costs associated with interventions. Seven intervention strategies were simulated, and the results showed that when only one intervention was applied, treatment was superior to other preventive measures. When two interventions were applied, we found that combining vaccination with treatment was more effective than isolation with treatment and isolation with vaccination. When using all controls, we observed that adding an isolation control to vaccination with treatment did not affect effectiveness or economic return.
This conclusion was supported by economic analysis based on two cost-effectiveness indices, the average cost-effectiveness ratio (ACER), and the incremental cost-effectiveness ratio (ICER), which allowed for a precise evaluation of the trade-off between different strategies, not only based on the number of infections averted but also in terms of the cost incurred per infection averted. The results showed that ACER indicates that isolation control, with a value of , is the most economically effective. In contrast, treatment, with a value of , has the highest value and, therefore, is the least financially practical. For ICER, the results indicate that the isolation control, with a value of , is the most cost-effective.
The values of the baseline parameters in the SEIR model were adopted from previous research, with minor modifications made to some parameters to suit the model’s nature and the simulation conditions. These modifications were designed to enhance the accuracy of the results without deviating from the scientific framework of the available data. These modified values contributed to the calculation of the basic reproduction number, which yielded a value of , as well as to the analysis of disease dynamics and the evaluation of the effectiveness of the proposed interventions.
The model developed in this research provides a foundation for building more complex future models that take into account virus mutations, the emergence of new strains, variations in community immunity, and changing climatic and environmental conditions. It also represents a framework that is adaptable to real-world data collected in the field, allowing it to be used as a decision-support tool for health policymakers as they seek to select the optimal strategy to control the spread of the disease and reduce its social and economic impacts. Hence, the importance of mathematical models in the epidemiological field is evident, not only for understanding the internal dynamics of the spread of infection but also for directing interventions towards balanced and practical solutions on both the medical and economic levels.
We acknowledge that our model relies on certain simplifying assumptions for analytical tractability, which may limit its direct applicability to real-world scenarios. Specifically, the model assumes a homogeneous population and constant control efficacy over time. A more realistic approach would incorporate population heterogeneity, such as age-structured groups, and allow for time-dependent control measures to reflect changing public health policies. Additionally, a key limitation stems from the parameter set, which was adopted from a previously published study to maintain consistency. Our sensitivity analysis revealed a high sensitivity of to demographic parameters (), which may be biologically counterintuitive in certain contexts. Future research could explore the model’s dynamics with a wider range of parameter values that are more aligned with various biological settings. These extensions would be valuable for future research and could provide a more nuanced understanding of disease dynamics.