Stress Analysis of the Radius and Ulna in Tennis at Different Flexion Angles of the Elbow
Abstract
1. Introduction
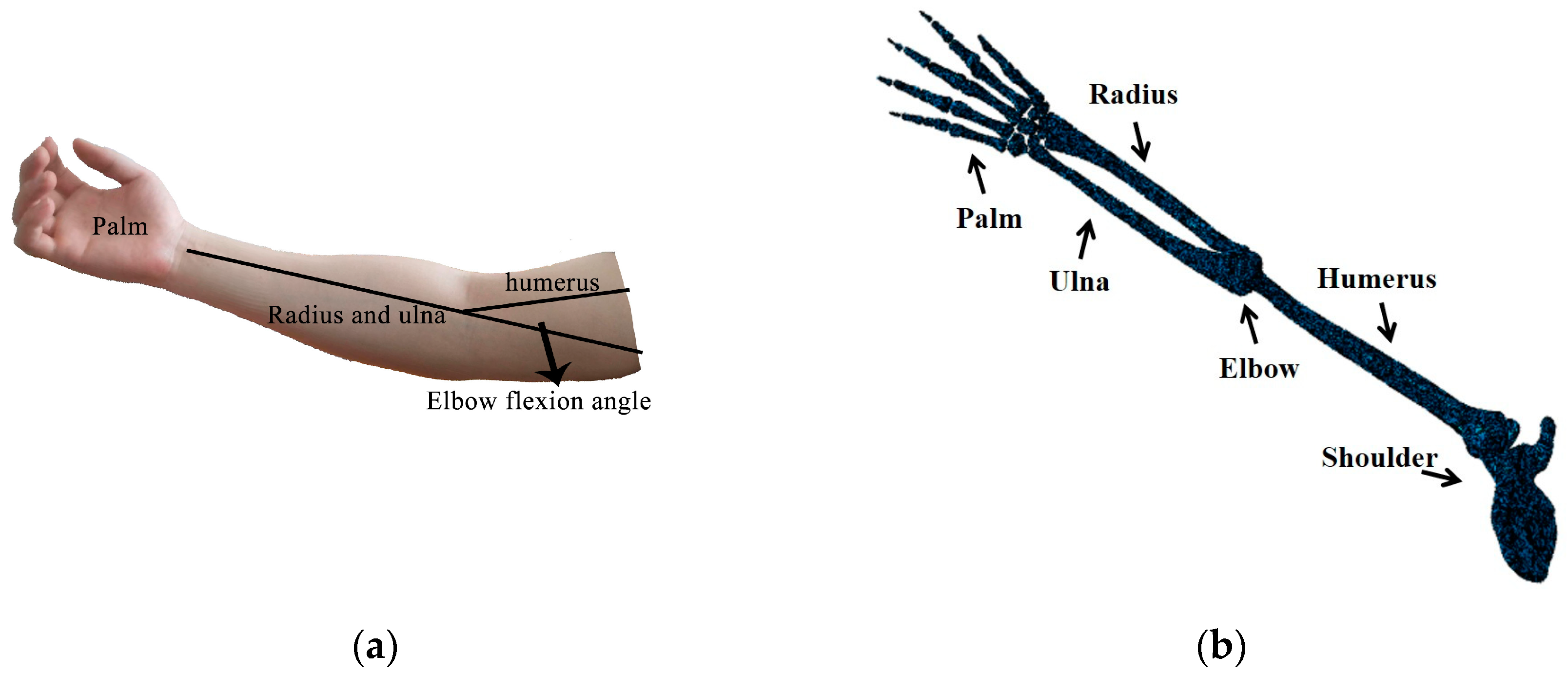
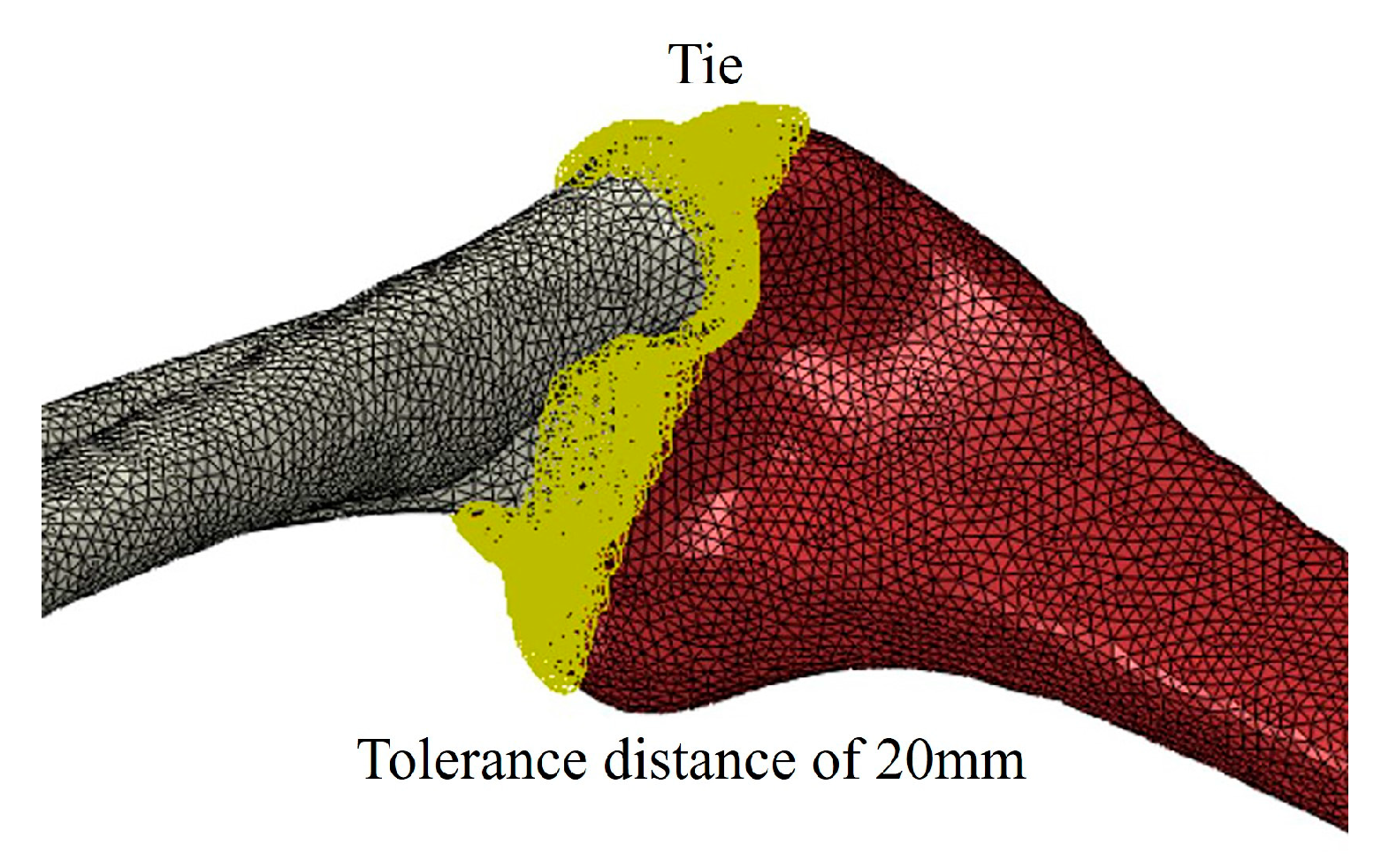
2. Finite Element Method for Human Skeletal Analysis
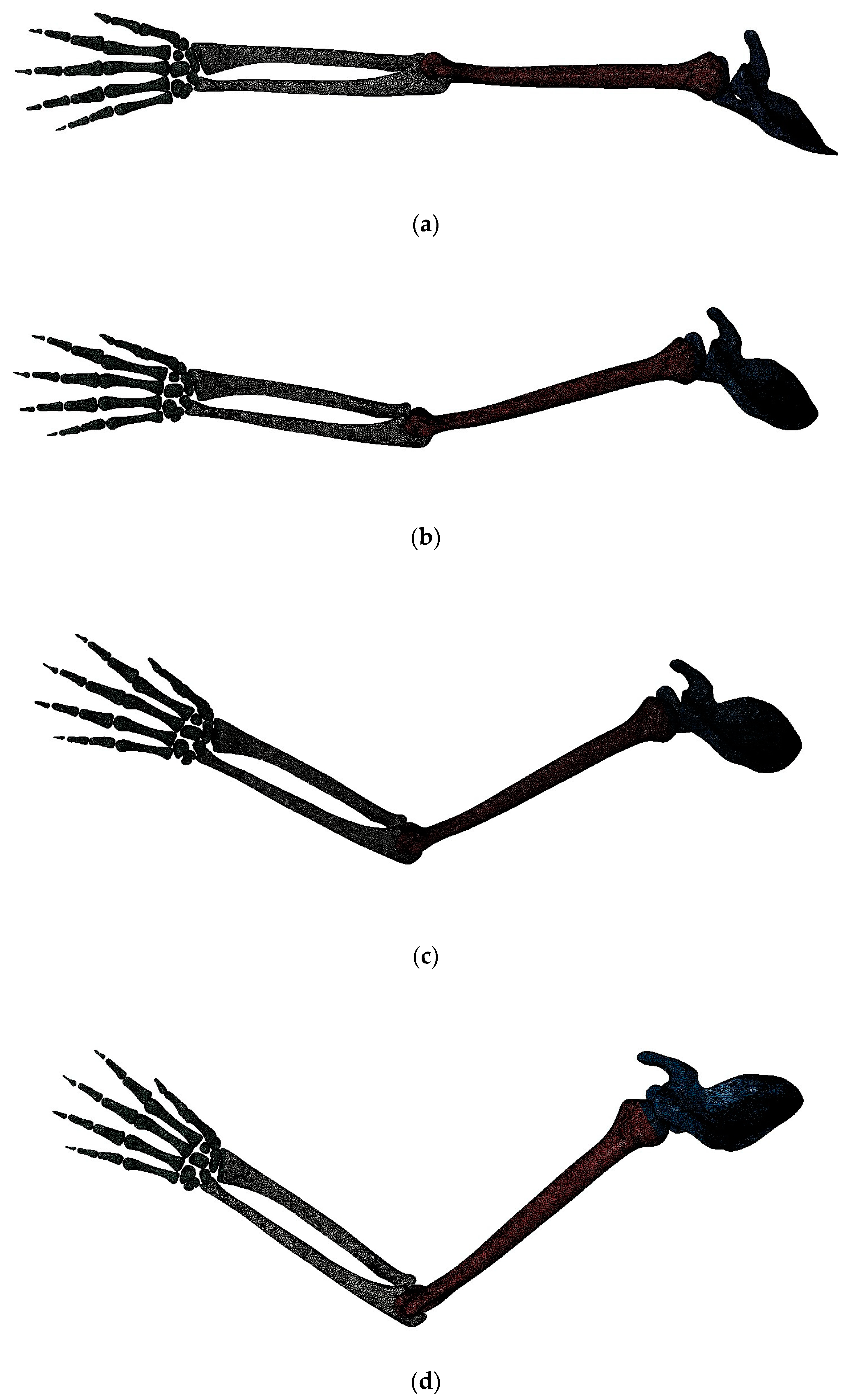
3. Finite Element Modeling of Different Elbow Flexion Angles in Tennis
4. Numerical Results and Analysis
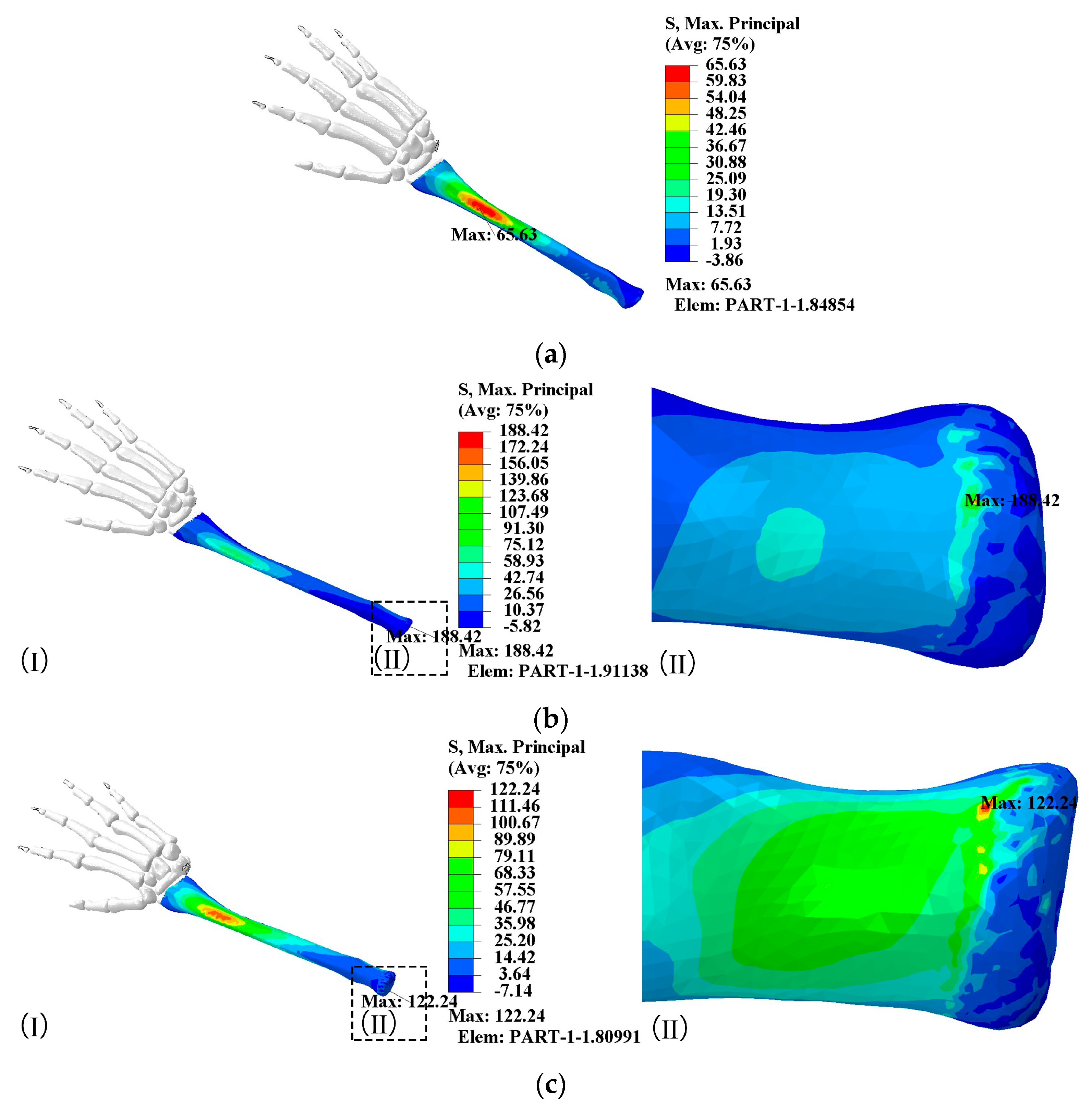
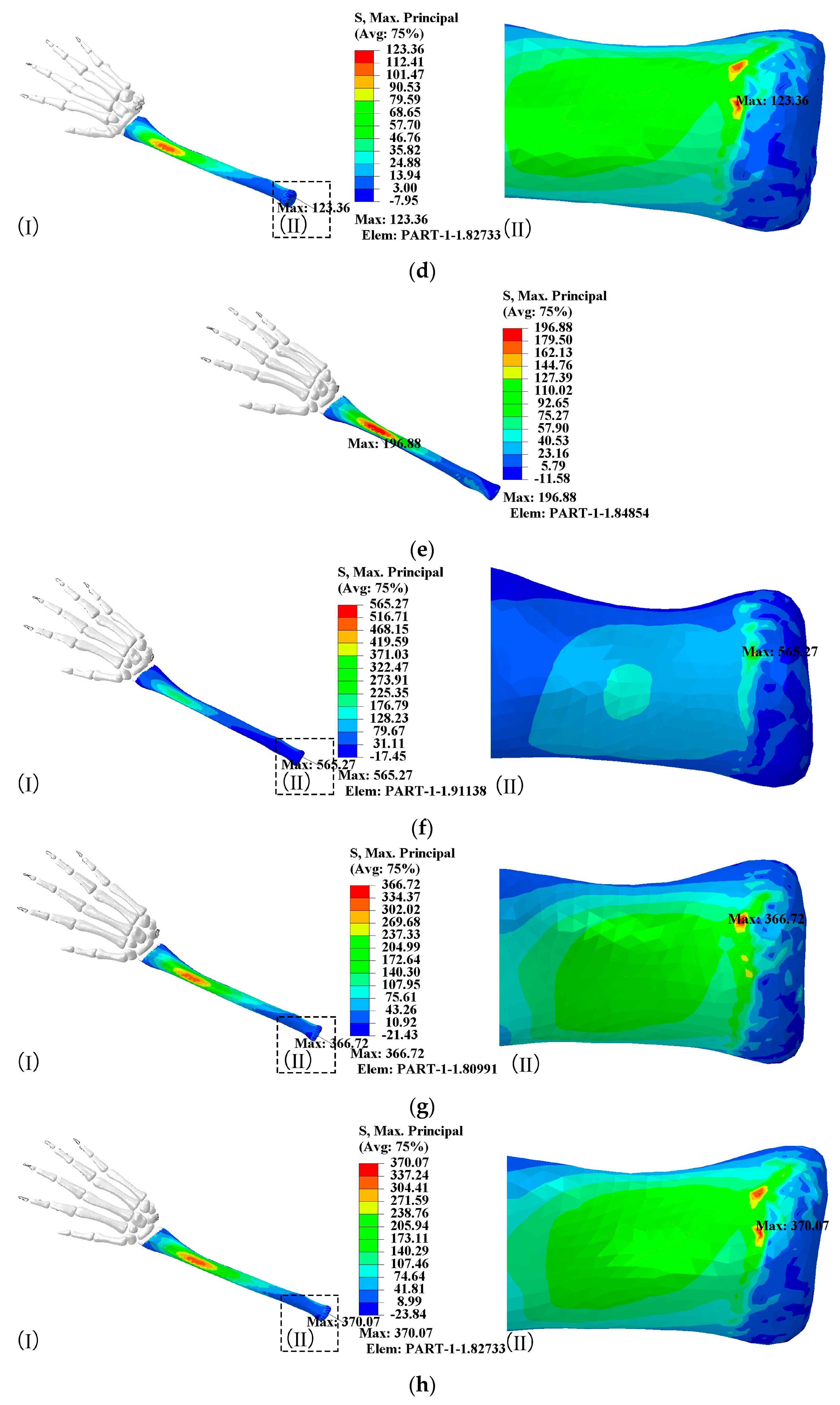
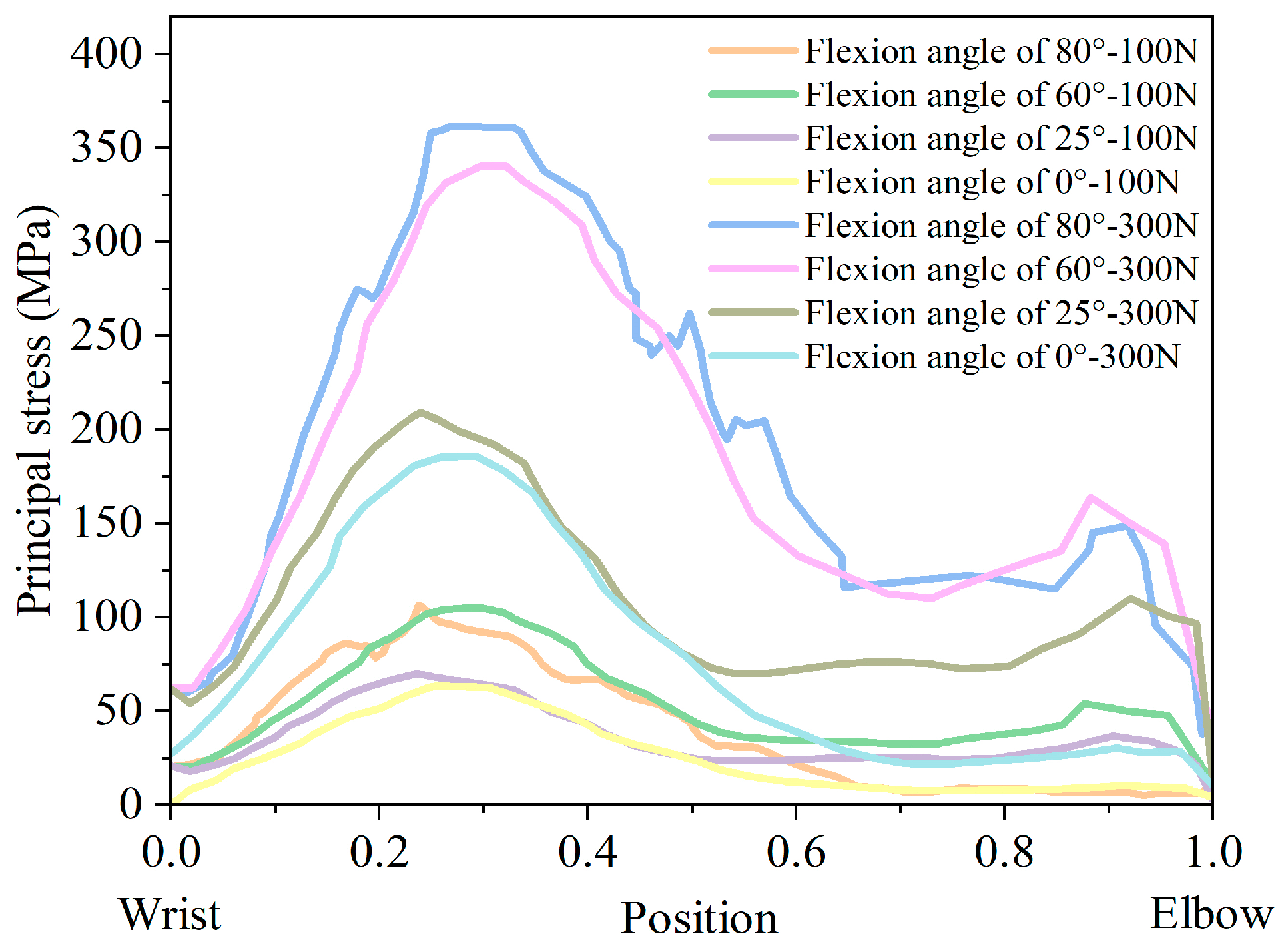
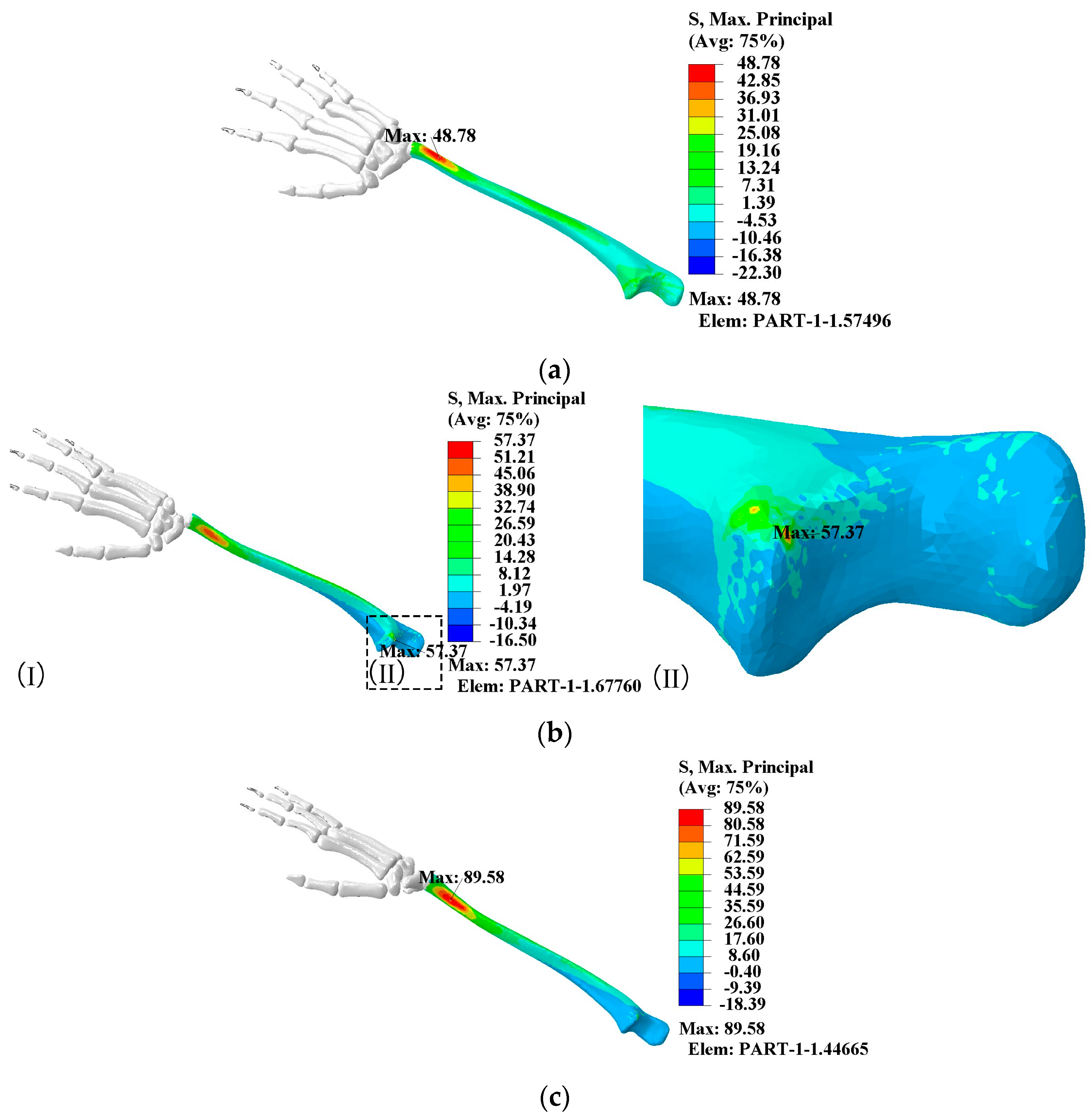
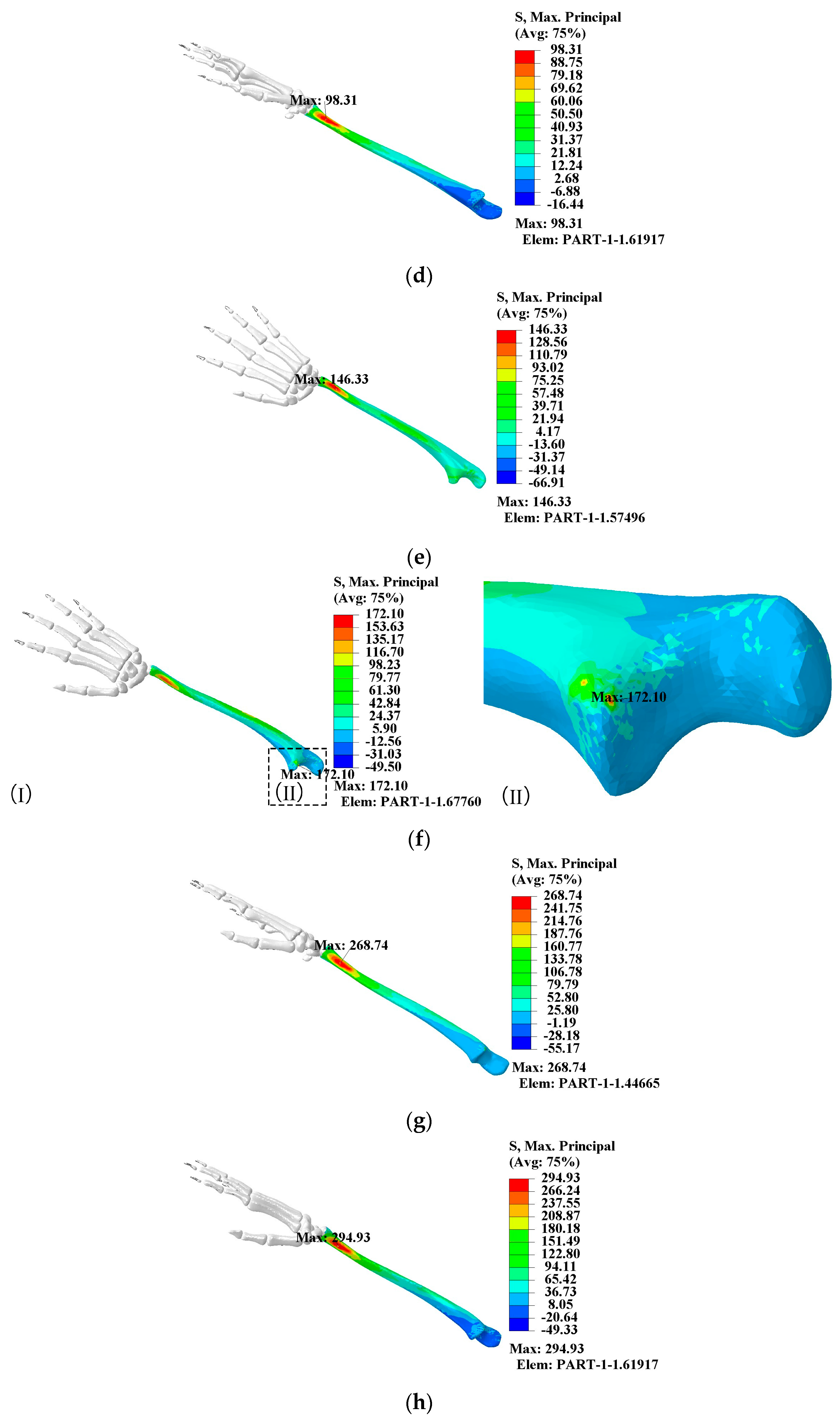
- When the elbow flexion is 0°, the maximum principal stress is generated at about 1/4th of the radius. At the elbow flexion is 25–80° range of elbow flexion, the maximum principal stress acts at the elbow joint as compressive stress;
- The maximum principal compressive stress is generated at 1/10th of the ulna at 0° and 60–80° elbow flexion. At elbow flexion is 25°, the maximum principal stress is located at the elbow joint on the length of the ulna, with the region of concentration of the principal stress at 1/10th and at the elbow joint;
- A change in hitting force does not change the principal stress distribution, which increases directly to the hitting force.
5. Conclusions
- The radius is most vulnerable at a quarter of its length, and when the elbow flexion is 25–80°, it is more likely to injury. For better joint protection in the radius and elbow joints, hitting the ball with a straight arm motion is recommended more frequently. Additionally, wearing quarter-position impact-resistant arm sleeves and elbow pads can provide further protection;
- Injuries to the ulna bone are most likely to occur near the wrist. To minimize the risk of such injuries, it is recommended to wear wrist guards with reinforcements of radial and dorsal hand-side.
- In recent years, meshless methods have developed rapidly, especially the element-free Galerkin (EFG) method and the corresponding improved methods [43,44,45]. Based on the moving least-squares approximation, the EFG method can obtain solutions with great precision, because the least-squares method in mathematics results in the best approximation [46,47,48]. With the development of bone mechanics, the muscle and skin should be considered when analyzing the stress analysis of the radius and ulna in tennis. Then the large deformation of bone, muscle, and skin will be considered. The meshless method has advantages over the finite element method when analyzing large deformation problems. Then meshless method will be applied to the stress analysis of the radius and ulna in tennis in the future.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warden, S.J.; Galley, M.R.; Hurd, A.L.; Wallace, J.M.; Gallant, M.A.; Richard, J.S.; George, L.A. Elevated mechanical loading when young provides lifelong benefits to cortical bone properties in female rats independent of a surgically induced menopause. Endocrinology 2013, 154, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Sogo, N.; Nagasawa, S.; Kato, T.; Umemura, Y. Bones benefits gained by jump training are preserved after detraining in young and adult rats. Am. Physiol. Soc. 2008, 105, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Ducher, G.; Prouteau, S.; Courteix, D.; Benhamou, C.-L. Cortical and trabecular bone at the forearm show different adaptation patterns in response to tennis playing—ScienceDirect. J. Clin. Densitom. 2004, 7, 399–405. [Google Scholar] [CrossRef]
- Ducher, G.; Tournaire, N.; Meddahi-Pellé, A.; Benhamou, C.-L.; Courteix, D. Short-term and long-term site-specific effects of tennis playing on trabecular and cortical bone at the distal radius. J. Bone Miner. Metab. 2006, 24, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Warden, S.J.; Wright, C.; Fuchs, R.K. Bone microarchitecture and strength adaptation to physical activity: A within-subject controlled HRpQCT study. Med. Sci. Sports Exerc. 2021, 53, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Pluim, B.M.; Staal, J.B.; Windler, G.E.; Jayanthi, N. Tennis injuries: Occurrence, aetiology, and prevention. Br. J. Sports Med. 2006, 40, 415–423. [Google Scholar] [CrossRef]
- Abrams, G.D.; Renstrom, P.A.; Safran, M.R. Epidemiology of musculoskeletal injury in the tennis player. Br. J. Sports Med. 2012, 46, 492. [Google Scholar] [CrossRef]
- Sallis, R.E.; Jones, K.; Sunshine, S.; Smith, G.; Simon, L. Comparing sports injuries in men and women. J. Sports Med. 2001, 22, 420–423. [Google Scholar] [CrossRef]
- Kaiser, P.; Stock, K.; Benedikt, S.; Ellenbecker, T.; Arora, R. Acute tennis injuries in the recreational tennis player. Orthop. J. Sports Med. 2021, 9, 232596712097367. [Google Scholar] [CrossRef]
- Chung, K.C.; Lark, M.E. Upper extremity injuries in tennis players: Diagnosis, treatment, and management. Hand Clin. 2017, 33, 175–186. [Google Scholar] [CrossRef]
- Mircea, M.; Mereuta, C.; Iordan, D.A. Injuries resulting from practicing performance sports in table tennis and tennis. Ann. Dunarea Jos Univ. Galati 2021, 2, 12–13. [Google Scholar]
- Kramer, D.E. Elbow pain and injury in young athletes. J. Pediatr. Orthop. 2010, 30, 7–12. [Google Scholar] [CrossRef]
- Patrick, N.C.; Hammert, W.C. Hand and wrist tendinopathies. Clin. Sports Med. 2020, 39, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.J.; Dapena, J. Contributions of joint rotations to racquet speed in the tennis serve. J. Sports Sci. 2006, 24, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Gross, M.T.; Prentice, W.E.; Yu, B. Comparison of ball-and-racquet impact force between two tennis backhand stroke techniques. J. Orthop. Sports Phys. Ther. 2000, 31, 247–254. [Google Scholar] [CrossRef][Green Version]
- Bahamonde, R.E. Changes in angular momentum during the tennis serve. J. Sports Sci. 2000, 18, 579–592. [Google Scholar] [CrossRef]
- Ireland, A.; Degens, H.; Maffulli, N.; Rittweger, J. Tennis service stroke benefits humerus bone: Is torsion the cause? Calcif. Tissue Int. 2015, 97, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.; Maden-Wilkinson, T.; Mcphee, J.; Cooke, K.; Narici, M.; Degens, H.; Rittweger, J. Upper limb muscle-bone asymmetries and bone adaptation in elite youth tennis players. Med. Sci. Sports Exerc. 2013, 45, 1749–1758. [Google Scholar] [CrossRef]
- Bollen, S.R.; Robinson, D.G.; Crichton, K.J.; Cross, M.J. Stress fractures of the ulna in tennis players using a double-handed backhand stroke. Am. J. Sports Med. 1993, 21, 751–752. [Google Scholar] [CrossRef]
- Jones, G.L. Upper extremity stress fractures. Clin. Sports Med. 2006, 25, 159–174. [Google Scholar] [CrossRef]
- Lavallee, M.E.; Sears, K.; Corrigan, A. The evaluation and treatment of elbow injuries. Prim. Care Clin. Off. Pract. 2013, 40, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.W. Imaging of upper extremity stress fractures in the athlete. Clin. Sports Med. 2006, 25, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Kekelekis, A.; Nikolaidis, P.T.; Moore, I.S.; Rosemann, T.J.; Knechtle, B. Risk factors for upper limb injury in tennis players: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 2744. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B. Biomechanics and tennis. Br. J. Sports Med. 2006, 40, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Rnm, A.; Mks, A.; Vk, B. Biomechanical analysis of human femur using finite element method: A review study. Mater. Today Proc. 2022, 56, 384–389. [Google Scholar]
- Gíslason, M.K.; Stansfield, B.; Nash, D.H. Finite element model creation and stability considerations of complex biological articulation: The human wrist joint. Med. Eng. Phys. 2010, 32, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Marqués, R.; Melchor, J.; Sánchez-Montesinos, I.; Roda, O.; Rus, G.; Hernández-Cortés, P. Biomechanical finite element method model of the proximal carpal row and experimental validation. Front. Physiol. 2021, 12, 749372. [Google Scholar] [CrossRef]
- Ganadhiepan, G.; Miramini, S.; Patel, M.; Mendis, P.; Zhang, L. Bone fracture healing under Ilizarov fixator: Influence of fixator configuration, fracture geometry, and loading. Int. J. Numer. Method Biomed. Eng. 2019, 35, e3199. [Google Scholar] [CrossRef]
- Ghimire, S.; Miramini, S.; Richardson, M.; Mendis, P.; Zhang, L. Effects of dynamic loading on fracture healing under different locking compression plate configurations: A finite element study. J. Mech. Behav. Biomed. Mater. 2019, 94, 74–85. [Google Scholar] [CrossRef]
- Zhang, L.; Richardson, M.; Mendis, P. Role of chemical and mechanical stimuli in mediating bone fracture healing. Clin. Exp. Pharmacol. Physiol. 2012, 39, 706–710. [Google Scholar] [CrossRef]
- Gíslason, M.K.; Nash, D.H. Finite element modelling of a multi-bone joint: The human wrist. In Finite Element Analysis; Farzad, E., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 83–85. [Google Scholar]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite element analysis of fracture fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef]
- Imai, K.; Ohnishi, I.; Bessho, M.; Nakamura, K. Nonlinear finite element model predicts vertebral bone strength and fracture site. Spine 2006, 31, 1789–1794. [Google Scholar] [CrossRef]
- Schileo, E.; Taddei, F.; Cristofolini, L.; Viceconti, M. Subject-specific finite element models implementing a maximum principal strain criterion are able to estimate failure risk and fracture location on human femurs tested in vitro. J. Biomech. 2008, 41, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Yamamoto, I.; Morita, R. Fracture simulation of the femoral bone using the finite-element method: How a fracture initiates and proceeds. Bone Min. Metab. 1999, 17, 108–112. [Google Scholar] [CrossRef]
- Koutromanos, I. Fundamentals of Finite Element Analysis: Linear Finite Element Analysis; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Chen, L.; Cheng, Y.M. Reproducing kernel particle method with complex variables for elasticity. Acta Phys. Sin. 2008, 57, 1–10. [Google Scholar] [CrossRef]
- Sun, F.X.; Wang, J.F.; Cheng, Y.M. An improved interpolating element-free Galerkin method for elasticity. Chin. Phys. B 2013, 22, 120203. [Google Scholar] [CrossRef]
- Celik, A.; Kovaci, H.; Saka, G.; Kaymaz, I. Numerical investigation of mechanical effects caused by various fixation positions on a new radius intramedullary nail. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Landlinger, J.; Stöggl, T.; Lindinger, S.; Wagner, H.; Müller, E. Differences in ball speed and accuracy of tennis groundstrokes between elite and high-performance players. Eur. J. Sport Sci. 2012, 12, 301–308. [Google Scholar] [CrossRef]
- Vaverka, F.; Nykodym, J.; Hendl, J.; Zhanel, J.; Zahradnik, D. Association between serve speed and court surface in tennis. Int. J. Perform. Anal. Sport 2018, 18, 262–272. [Google Scholar] [CrossRef]
- Seeley, M. Tennis forehand kinematics change as post-impact ball speed is altered. Sports Biomech. 2011, 10, 415–426. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Wang, J.F.; Bai, F.N. A new complex variable element-free Galerkin method for two-dimensional potential problems. Chin. Phys. B 2012, 21, 090203. [Google Scholar] [CrossRef]
- Sun, F.X.; Wang, J.F.; Cheng, Y.M. An improved interpolating element-free Galerkin method for elastoplasticity via nonsingular weight functions. Int. J. Appl. Mech. 2016, 8, 1650096. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, M.J.; Cheng, Y.M. A fast complex variable element-free Galerkin method for three-dimensional wave propagation problems. Int. J. Appl. Mech. 2017, 9, 1750090. [Google Scholar] [CrossRef]
- Cheng, J. Analysis of the factors influencing industrial land leasing in Beijing of China based on the district-level data. Land Use Policy 2022, 122, 106389. [Google Scholar] [CrossRef]
- Cheng, J.; Luo, X.W. Analyzing the land leasing behavior of the government of Beijing, China, via the multinomial logit model. Land 2022, 11, 376. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, P. Analysis of the complex network of the urban function under the lockdown of COVID-19: Evidence from Shenzhen in China. Mathematics 2022, 10, 2412. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Du, Q.; Yin, X.; Fu, R.; Zhu, Y. Stress Analysis of the Radius and Ulna in Tennis at Different Flexion Angles of the Elbow. Mathematics 2023, 11, 3524. https://doi.org/10.3390/math11163524
Chen Y, Du Q, Yin X, Fu R, Zhu Y. Stress Analysis of the Radius and Ulna in Tennis at Different Flexion Angles of the Elbow. Mathematics. 2023; 11(16):3524. https://doi.org/10.3390/math11163524
Chicago/Turabian StyleChen, Yan, Qiang Du, Xiyang Yin, Renjie Fu, and Yiyun Zhu. 2023. "Stress Analysis of the Radius and Ulna in Tennis at Different Flexion Angles of the Elbow" Mathematics 11, no. 16: 3524. https://doi.org/10.3390/math11163524
APA StyleChen, Y., Du, Q., Yin, X., Fu, R., & Zhu, Y. (2023). Stress Analysis of the Radius and Ulna in Tennis at Different Flexion Angles of the Elbow. Mathematics, 11(16), 3524. https://doi.org/10.3390/math11163524




