Abstract
This study investigates the effect of long-term alpha neurofeedback training (NFT) in healthy adults using music stimuli. The optimal protocol for future research is presented in this study. The data from 40 healthy participants, divided into two groups (NFT group and Control group), were analyzed in the current study. We found a significantly enhanced alpha rhythm after training in the NFT group which was not observed in the control group. The immediate subsequent effects were greater in more than 80% of the sessions from the initial recordings. Stroop task and behavioral questionnaires, mini-mental state exam (MMSE), and perceived stress scale (PSS) did not reveal any training-specific changes. Within-training session effects were significant from the baseline and were more pronounced at the beginning of the session as compared to the end of the session. It is also observed that a shorter session length with multiple sessions may be more effective than a long and continuous run of a single session.
1. Introduction
Neurofeedback training (NFT), a brain-computer interface (BCI) procedure to help individuals to learn and control their brain activation, is a growing research area. This procedure is based on the fact that signals recorded from the human brain may be modulated via conditioning responses which were demonstrated using electroencephalogram (EEG) for the first time in the 1930s and 1940s [1]. In this procedure, the brain signals of a person are recorded and analyzed in real-time and feedback is provided to the person [2]. This feedback helps the person to learn to regulate their brain activity. In other words, the person tries to control their brain activity with the help of feedback. NFT has shown promising results in a clinical population such as attention-deficit/hyperactivity disorder (ADHD) [3] as well as for healthy people to improve their cognition [4].
For instance, the outcome of a study revealed that healthy participants were able to significantly enhance their short-term memory after 20 NFT sessions [5]. Another study revealed that NFT can increase the response-conflict resolution ability of healthy people [6]. This indicates that NFT can be used in two ways: (i) as a therapeutic tool in the clinical population and (ii) as a cognitive performance enhancement tool or peak-performance training tool in a healthy population [7]. From a methodological perspective, each of the mentioned uses is limited to the corresponding population, and the effect on one type of population cannot be generalized to the other. In the current study, the NFT is specific to the healthy population. Despite the positive effects of NFT reported in the healthy population in numerous studies, this procedure is criticized due to the scarcity of supporting evidence [8]. Moreover, the methodological differences among the NFT studies lead to lower replicability of results that points to a systematic methodological gap [9].
It is believed that each frequency band in the EEG signal is associated with the cognitive abilities of human beings, and the neural mechanism of EEG features depends on the oscillatory behavior of that band. That is why the success of the NFT is strongly dependent on the selection of the EEG band that is used for the feedback signal [10]. Therefore, in the NFT protocol, a specific EEG band is chosen for the feedback signal that is important for the goal of the study. According to our knowledge from the literature review, almost all EEG bands have been used in different feedback protocols in different studies [9]. However, many studies have reported the relationship between cognitive abilities, for example, memory performance [11] and attentional processes [12], and the alpha EEG band. Therefore, the alpha band is a common target frequency range for NFT in the healthy population. Stroop task is used to measure selective attention and cognitive control. In a study conducted by Rachel Atchley et al., it was observed that the participants made error on the incongruent trials of the Stroop task which can be characterized by a decrease in alpha and theta activity prior to stimulus presentation when compared to EEG prior to stimuli generating a correct response [13]. Anna M Berger reported the effect of alpha oscillations over the prefrontal cortex on behavioral measures of top-down cognitive (attentional) control using Stroop task [14]. They used NFT to manipulate the magnitude of alpha power within the same participants. An increase in frontal alpha was associated with enhanced attentional processing [14]. For the Stroop task, a between-subject ANOVA did not show any group-level effects. This was the result of the high individual variability in neural and behavioral measures which is widely reported in NFT research (see [7]). Based on the above studies, it is expected that after NFT, an enhanced alpha amplitude will result in enhanced attention and cognitive control. Hence, in this study, the Stroop task will be administered to the participants and the questionnaires for Mini Mental State Examination (MMSE) and Perceived Stress Scale (PSS) filled in to measure their psychological dimensions.
Very few studies exploring the effects of NFT, have been performed with consumer-grade devices that are easily available on the market [15]. Portable EEG devices are relatively low-cost, have wireless EEG sensors, and their corresponding software tools can process the signals in real-time [16]. However, to our knowledge, NFT studies are mostly performed with (high cost) medical grade equipment which results in fewer opportunities for common people in society to avail themselves of NFT. Therefore, one of the goals of this study is to explore the feasibility of NFT with a low-priced, consumer-grade device that can be easily made available to common people.
The use of random mental strategies chosen by the participants during NFT to control their own brain activity leads to trial-and-error learning [17]. In the dual-process theory of learning to regulate one’s own brain activity, the learning is an interaction process between the feed-forward and feed-back [18]. For a naïve user, due to the trial-and-error process, the selection of an effective mental strategy requires a high amount of attentional resources [17]. The use of an aiding tool as a strategic factor during NFT reduces this extra cognitive effort by the participants. Many research studies have explored the importance of music for human wellbeing [19,20,21,22]. Besides being a source of entrainment, the research has shown that human beings can benefit from music physically, psychologically, and socially [23]. Despite its noticeable benefits, there has been a paucity of research studies that utilize music for neurofeedback training. For example, in mindfulness studies, music is used as auditory feedback in the form of binaural beats [24]. Given the advancement of the neurofeedback training procedures and the development of the BCI [25,26,27], it is surprising that the capabilities of music listening are not combined with the training session. For example, the existing studies have utilized either neurofeedback training or music entrainment and have not explored the advantages by integrating them [28]. Due to the extra amount of attentional resources required, choosing the best mental strategy [17] solely by the feedback signal without an aiding tool (for example listening to music) is challenging during neurofeedback training [29]. To overcome this limitation, in the current study, we utilized the relaxing music in the background as an aiding tool for neurofeedback training. This structure of the neurofeedback system makes it unique among the existing systems that use either neurofeedback training or music entrainment [28]. We expect that this will enhance the implicit learning ability of the participants to try to maximize the feedback signal and produce more brain activity in the EEG band of interest.
In line with previous studies reporting increased alpha amplitude for NFT training.
Hypothesis 1 (H1).
The alpha neurofeedback has a positive effect on the alpha amplitudes (i.e., increased alpha amplitudes) while no such increase is observed in the control group.
Hypothesis 2 (H2).
The increase in alpha amplitude after the neurofeedback may result in an increase in the cognitive flexibility studied by Stroop task (i.e., a decrease in error rate and response time) and a decrease in cognitive impairment and stress, compared to the control group.
2. Materials and Methods
2.1. Participants
This study is evaluated and approved by the scientific and ethical review committee of the Universiti Tunku Abdul Rahman (UTAR), Malaysia, under the reference number U/SERC/90/2018. The experimental procedures followed the declaration of Helsinki. Fifty (50) healthy participants (18 females) were recruited via an email advertisement and were equally divided into two groups, the NFT group and the control group. The two-sample t-test showed no significant difference between the ages of the two groups [t(48) = −1.9944, p = 0.0518]. During a one-to-one interview with the researcher, the participants received a unique participant ID and were told about their group allocation. Therefore, both the researcher and participant were aware of their assigned group. The participants were briefed about the experimental procedures, and an informed consent form was obtained from each participant. One participant in the control group could not continue the experiment until the end of the study and hence was excluded from the final analysis. Furthermore, the EEG data of five participants in the NFT group and four participants in the control group were not included in the final analysis due to the excessive artifacts. Thus, the sample size in the final analyses was 20 participants in each group. The mean age of the NFT group (including 8 females) was (23.8 ± 5.02 y) and of the control group (including 6 females) was (26.35 ± 5.01 y).
2.2. Music Stimuli
A meta-analysis conducted in [30] shows that listening to music strengthens the brain frequency that corresponds to the music stimuli [30]. This suggests that a careful selection of the music stimuli for the intended purposes may bring fruitful results. Since music is a potent phenomenon for human auditory perception and cognition; it can produce entirely different results based on the characteristics and emotions present in the music. For example, it can make us happy or sad, or help us to fall asleep. Therefore, the selection of the music stimuli requires the findings drawn from neuroscience and perceptual psychology to develop a hypothesis about the desired music. This music may then be tested on a massive scale. This makes it a two-stage process (building a hypothesis followed by testing). All these considerations motivated us to consider Brain.fm as a platform for music stimuli [31,32]. Brain.fm is a collaboration among scientists, musicians, and developers to create music stimuli based on a hypothesis for different mental states (e.g., focus, relax, attention, etc.) and then test the hypothesis. Hence, the music stimuli are scientifically validated by independent research studies [33,34]. In the Brain.fm platform, the music stimuli are created and categorized according to the mental states. The time duration for listening to these music stimuli is 15 min L or 30 min. The subscribers can choose the music type and duration according to their requirements. In the current study, we selected the music stimuli from the relaxed mental state category, and the length of a single music session is 15 min (because this duration is equal to the half of the session length of our NFT design; see NFT sessions section below). This means that, in the first half of NFT session, the participants will listen to the first music for 15 min uninterrupted. Similarly, in the second half of the NFT session, the participants will listen to a different music for 15 min, making the total duration of music listening session to 30 min. As mentioned earlier, we choose the music stimuli from the relaxed category which contains dozens of relaxing music divided into further sub-categories (e.g., rain relaxing sounds, beach side relaxing sounds etc.) The two pieces of music played during the two sub-sessions of a single NFT session were always different for the same participant. Furthermore, the selection of the two music for the next session was also different from the music played in recent previous session of NFT. As the main goal of the study was to provide NFT rather than investigating the effect of a specific music stimuli. Therefore, we tried to avoid the repetition of the music during the training. Therefore, the participants do not establish a learning skill which is based on the feeling of music in the implicit memory. This means that the background music acted as a temporary aiding tool in the session. This will help to minimize the learning effect from the music and will integrate the true effect which is coming from the NFT.
2.3. Neurofeedback Training Protocol
The NFT sessions were performed with a customized NFT protocol designed in an open-source BCI software toolbox called OpenVibe [35]. The EEG signals were obtained from the Emotiv Epoc+ device, containing 14 active EEG sensors placed on the scalp at AF3, F7, F3, FC5, T7, P7, O1, O2, P8, T8, FC6, F4, F8, and AF4 locations according to the 10–20 international standard. The frontal lobe of the brain plays an important role in the higher level of cognitive functions and is responsible for immediate and sustained attention, time management, social skills, emotions, empathy, working memory, and executive planning [36]. Therefore, the signals from the frontal region (frontal sensors F7, F3, F4, and F8) were used for the feedback. The signal from these sensors was processed online in real-time via a signal processing module designed in the OpenVibe. Alpha EEG band (8–13 Hz) was extracted using Butterworth filter with 0.5 dB passband ripples deploying “Modifiable Temporal Box” in the designer window of OpenVibe. The absolute value of alpha-band power was computed using Welch’s method using a 5 s window which was updated every sec [22] and was shown to the participants as feedback. The feedback value was shown in the form of a vertical bar which was designed in another open-source software toolbox called Unity3D [37]. Both programs, OpenVibe and Unity3D, were connected with the help of a network protocol, known as Lab Streaming Layer (LSL). Data acquisition and processing were controlled from the researcher’s computer (Acer swift, 8GB ram, Windows 10 OS, 13.5-inch native display). The participants viewed the bar on a 17-inch LCD monitor as an extended display connected to the researcher’s computer. The EEG data were also recorded for offline analyses. The complete design of the NFT protocol is summarized in Figure 1.
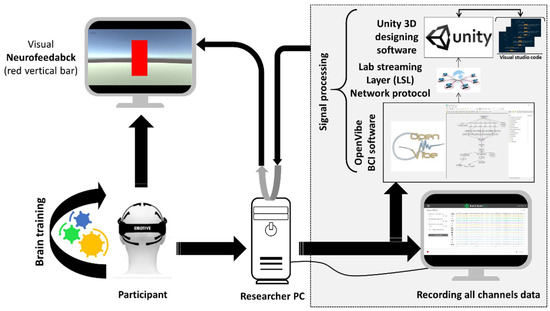
Figure 1.
Design of the neurofeedback protocol. The red vertical bar represents the absolute value of alpha-band power.
2.4. Neurofeedback Training Sessions
Participants in the NFT group underwent a long-term training of 600 min divided into 20 sessions. Each of the 20 sessions was 30 min long which was further divided into two sub-sessions (15 min long). A short break of 1–2 min was provided between the two sub-sessions. A minimum of one and a maximum of three sessions were performed per week by a single participant. To minimize the influence of circadian rhythm [38], the time slot for the training was kept consistent across all the sessions. After each NFT session, a 3 min resting state EEG (RS-EEG) data with close eyes were also recorded. There were 20 NFT sessions, and after each session there was 3 min of RS-EEG recording which resulted in twenty 3 min RS-EEG samples for each participant. Before starting the NFT session, the researcher instructed the participants to increase the height of the red vertical bar (i.e., feedback bar) by adopting their strategy. During the NFT session, the participants were seated in a chair in front of the 17-inch display monitor at a comfortable distance. As the participants had to look at the vertical bar, they were asked to keep their eyes open and minimize the blinking. The participants in the NFT group took around 2.5 months from the beginning of the first NFT session to complete 20 sessions.
The participants’ cognitive ability and behavioral responses were measured using the Stroop task and two questionnaires, mini-mental state exam (MMSE), and perceived stress scale (PSS), respectively. The Stroop task is widely used in cognitive psychology to measure selective attention and cognitive control of human participants. The participants were presented with a word written in different colors, and the participants were required to name the color of the word. When the name of the color and the presented word match, this is referred to as the congruent condition, otherwise, it is known as the incongruent condition. In the incongruent condition, due to the interference of the color and word, the reaction times are prolonged [39].
MMSE is a 30-point questionnaire that is used in research [40] to measure cognitive impairment. This questionnaire consists of simple questions related to several cognitive aspects such as arithmetic ability and language comprehension [41]. The maximum score that can be achieved in the MMSE is 30. MMSE score equal to 24 or above represents normal mental cognition, and a score lower than 9 indicates severe cognitive impairment [41]. Hence higher MMSE score indicates better cognition and vice versa.
PSS is widely used for measuring the perception of stress. The PSS score reflects the degree of stress in one’s life [42]. A higher score represents more stressful experiences and vice versa. The questions in the PSS questionnaire ask about one’s feelings and thoughts during the last month. The questionnaire consists of 10 questions, and the participants are required to answer each question on a 5-point (ranging from 0 to 4) Likert scale. The minimum score that can be achieved on the PSS is 0, and the maximum score is 40. A score ranging from 0 to 13 is considered as low stress, from 14 to 26 is considered as moderate stress, and from 27 to 40 is considered as high stress.
The Stroop task, MMSE, and PSS were administered at three stages during the training: before conducting the first session (hereinafter Baseline), after 10 sessions, and after 20 sessions. Besides the behavioral responses, a 3 min EEG was also recorded at Baseline, after 10 sessions, and after 20 sessions, first with eyes open and then with eyes close condition. The participants in the control group did not perform any training. However, their EEG recordings (with eyes closed and eyes open conditions), Stroop task, and MMSE and PSS responses were administered three times just like the NFT group. To compare the NFT effects in an unbiased manner in terms of timings, the second and third attempts at recording the data for the control group were carried out approximately after 5 and 10 weeks, respectively. The flow of NFT sessions for the NFT group is shown in Figure 2.
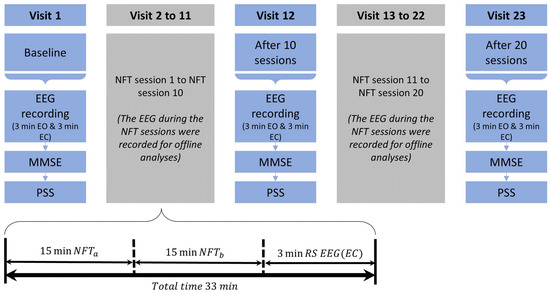
Figure 2.
The flow of sessions and recording of RS–EEG and Stroop task, MMSE, and PSS responses for the NFT group.
2.5. EEG Recordings and Processing
The baseline EEG recordings and EEG data during the NFT sessions were recorded via Emotiv Epoc+ wireless EEG device [20,21]. The Emotiv Epoc+ is very convenient to set up, and in addition, it is found to be one of the best consumer-grade EEG devices that can be used reliably for BCI applications, such as neurofeedback training [15]. The data sampling rate of this device is 128 samples/s and 0–64 Hz bandwidth range. Two built-in notch filters at 50 Hz and 60 Hz are used to eliminate the line frequencies. Emotiv Pro software is used to monitor the contact quality of the sensors with the scalp in real-time.
After recording the EEG data, the offline analyses were carried out in MATLAB with custom-written scripts and an EEGLAB toolbox [43]. Continuous EEG data were filtered via FIR filter between 0.5 and 40 Hz using Hamming window with the help of the pop_eegfiltnew function in EEGLAB. After that, an automatic method known as artifact subspace reconstruction (ASR) is used to reconstruct the artifacts portion of the data with clean data [44]. This method removes the non-stationary high variance signal and then reconstructs the missing data using a spatial mixing matrix. ASR method is implemented with clean_rawdata function in the EEGLAB. Then independent component analysis (ICA) is performed to compute the independent component time series of the EEG data using extended infomax algorithm [45]. After that, the data were segmented into 2-s short epochs and each epoch was inspected for the artifact using the pop_autorej function in EEGLAB. An epoch was selected as a bad epoch if any of the data points exceeded five standard deviations of the amplitude and was rejected from the data. This algorithm was iteratively implemented via a custom-written MATLAB script. If the number of epochs selected for rejection was greater than 5% of the data, the procedure was repeated with a more liberal threshold (increased by 0.5 SD) [9].
Separate power spectral density (PSD) analysis was performed on 3 min EEG data (i.e., RS-EEG for the NFT group only) and Baseline, after 10 sessions and after 20 sessions for both groups) and 30 min long EEG data collected during the NFT sessions to compute power in the alpha band (8–13 Hz). PSD was estimated using Welch’s averaged, modified periodogram method (512 DFT points and 75% overlap between consecutive windows) and Hann tapering window. The PSD is performed for each channel separately and then the mean power is computed across all 14 EEG channels.
2.6. Statistical Analysis
First, we investigated whether NFT effectively produced any significant changes in cognitive performance and subjective well-being by analyzing the reaction times (RT) and error rates of the Stroop task and the MMSE and PSS scores. A 2 (Group: NFT vs. Control) 3 (Session: Baseline vs. after 10 sessions vs. after 20 sessions) 2-way repeated measures ANOVA is used to compare the RT, error rates, and MMSE and PSS scores. The Group and Session were between-subject and within-subject factors respectively. A pairwise t-test is performed as a post-hoc comparison where needed.
Second, we analyzed the EEG data from the Baseline, after 10 sessions and after 20 sessions to investigate whether changes in alpha power are due to the training effect. To do so, we analyzed the effect on the alpha power in both groups. Thus, we performed between the group comparisons. Alpha power in both groups at Baseline, after 10 and 20 sessions were tested using one-way repeated measures ANOVA. The dependent variable was absolute power in the alpha band and the independent variable was the repeated measures at Baseline, after 10 and 20 sessions.
Third, we investigated 3 min RS-EEG data after each NFT session to explore the between-session effect of the training. For this purpose, we compared the alpha power at Baseline with the RS-EEG alpha power after each NFT session. We looked subsequently whether the training effect would be visible in the resting state of the individual. We performed permutation testing on the RS-EEG and compared it with the baseline separately for each session.
Finally, we analyzed the EEG data collected during the NFT sessions to investigate whether the within-session alpha power changed in response to the training. For this purpose, we compared the mean alpha power within the 1st half (i.e., first 15 min) and 2nd half (i.e., last 15 min) of the NFT session to the baseline using separate one-way repeated measures ANOVA. Additionally, we explored the within-session changes by investigating each half of the NFT session. We divided each half of the NFT session into three five minute sub-blocks (B1, B2, and B3). Then we applied a 2-way ANOVA to investigate the alpha power differences between the 1st half and 2nd half and also between B1, B2, and B3.
3. Results
3.1. Behavioral Results (RT, Error Rates, MMSE, and PSS)
Behavioral results are shown in Figure 3. The Stroop task RT of the correct trials was included in the analysis. Moreover, the Stroop trials that have RT lower than 200 ms were identified as response anticipation and were not included in the analysis. The general Stroop interference was observed in both groups by lower RT and lower error rates in the congruent trials as compared to the incongruent trials as shown in Figure 3A,B. The ANOVA results revealed that there is no significant change in the RT of Stroop trials as revealed by no significant interaction between incongruent [] and incongruent trials []. Similarly, the error rates in the Stroop task were also not different in both congruent [] and incongruent trials [] in both groups.
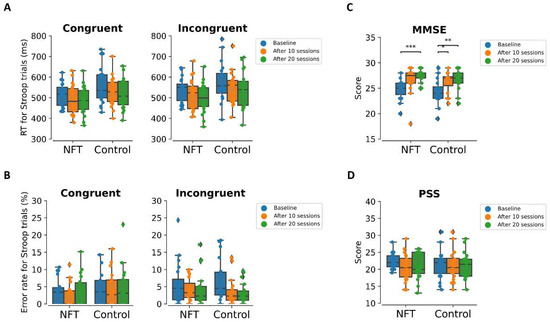
Figure 3.
Behavioral results (A) RT, (B) error rates, (C) MMSE, and (D) PSS score for NFT and control group at baseline, after 10 sessions, and after 20 sessions. * p < 0.05, ** p < 0.01, *** p < 0.001.
The MMSE score after 10 sessions and after 20 sessions was higher than the baseline in both groups as shown in Figure 3C. The ANOVA results revealed that there was no significant interaction []. However, the main effect of the Session was significant []. Similarly, the PSS score after 10 sessions and after 20 sessions was lower than the baseline as shown in Figure 3D. But the ANOVA revealed that this decrease was not significant as indicated by no significant interaction []. See Supplementary Materials for detailed results of Stroop RT and Error rate, MMSE and PSS.
3.2. EEG Results
3.2.1. Alpha Power (between the Group Analysis)
The absolute alpha power for the NFT group and control group is computed from the 3 min EEG recordings at the baseline, after 10 sessions, and after 20 sessions. The mean alpha power is shown in Figure 4A. The alpha power after 20 sessions increased from the baseline in the NFT group. Whereas the alpha power in the Control group showed no appreciable change. The power at the baseline was higher in the posterior region of the brain in both groups. The NFT group power increased in the frontal region of the brain after 20 sessions while in the Control group no change is observed in the frontal region as shown in Figure 4B. The statistical analysis revealed a significant change in the NFT group as revealed by ANOVA . Post hoc pairwise t-test indicated that the difference was present between the alpha power at the baseline and after 20 sessions and between 10 and 20 sessions . Thus, the main increase in alpha power in the NFT group took place between the 11th and 20th sessions. On the other hand, the alpha power in the control group after 10 and 20 sessions was not statistically different from the baseline . The alpha power in the control group was also non-significant between 10 and 20 sessions .
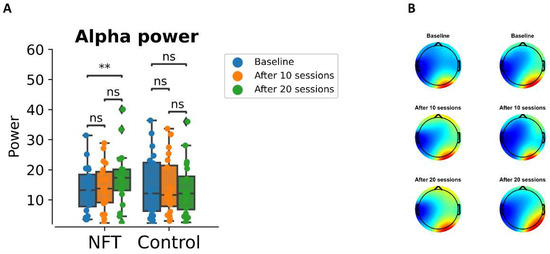
Figure 4.
(A) The plot shows the absolute alpha power at baseline, after 10 sessions and after 20 sessions for both groups. (B) the topographical plots of absolute alpha power in NFT group (left column) and Control group (right column). ** p < 0.001; ns, not significant.
3.2.2. Alpha Power between the Session Analysis
The difference of mean alpha power in RS-EEG with eyes closed from the baseline EEG with eyes closed across all sessions is shown in Figure 5. The blue dashed line in Figure 5A represents the alpha power of baseline EEG with eyes closed. It is observed that the training effect was visible in the eyes closed RS-EEG of the participant with the increased alpha power from the eyes closed baseline EEG in 80% of the sessions. However, in four sessions (sessions 8, 11, 14, and 15), non-specific training effects were that the alpha power slightly decreased from the alpha power of eyes closed baseline EEG. As shown in Figure 5B, the permutation testing revealed that the change in alpha power was not significantly different from the baseline . This is also obvious from the distribution plots under the null hypothesis for each session in Figure 5B. Every time, the observed statistic (also known as t-value) was not significantly far from the distribution mean as shown by a dotted red vertical line in Figure 5B. However, the alpha power of eyes closed RS-EEG after session 19 was marginally significant from the alpha power of eyes closed baseline EEG .
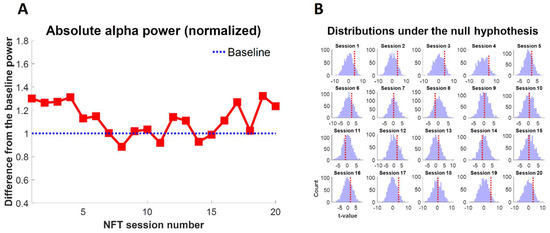
Figure 5.
(A) The difference in alpha power after each NFT session from the baseline and (B) the distribution of each session under the null hypothesis (using permutation testing). The dotted red vertical line represents the observed statistic.
3.2.3. Alpha Power within-Session Analysis
The within-session mean alpha power for the 1st half and 2nd half of the NFT session is shown in Figure 6A. One-way repeated-measures ANOVA revealed that within-session, alpha power was significantly greater than the baseline alpha power . The post hoc pairwise t-test revealed that the alpha power in both halves of the session was significantly greater than the baseline with and for 1st half and 2nd half, respectively, but the alpha power in the 1st half and 2nd half was not significantly different from each other .
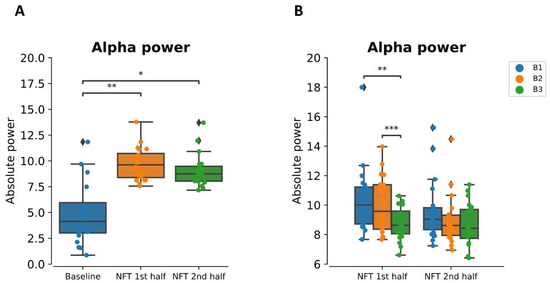
Figure 6.
(A) Absolute alpha power at baseline and within-session (1st half = first 15 min, 2nd half = last 15 min) and (B) at within-session (first half and second half) sub-blocks (B1, B2, and B3 each five minutes). * p < 0.05, ** p < 0.001, *** p < 0.0001.
Overall, alpha power within each half of the session significantly increased from the baseline, but this increase was reduced from the beginning (B1) to the end (B3) of the session as shown in Figure 6B. From the two-way ANOVA, we found that this decrease (from B1 to B3) within each half of the session was significant . The post hoc test revealed that this significant main effect was observed between B1 and B3 and B2 and B3 (). However, the difference between B1 and B2 was not significant .
4. Discussion
In the current study, we investigated whether EEG-based neurofeedback training can enhance the cognition of healthy human beings. One of the goals was to see if a low-priced EEG device can be utilized for this purpose. Hence, we used the Emotiv Epoc + EEG device, which is comparatively cheaper and commercially available in the market. We performed the training with a customized setup using OpenVibe BCI software and Unity3D tool. The participants performed cognitive and behavioral testing (Stroop task, MMSE and PSS) and EEG recordings at baseline, halfway through training, and after completing the training.
Previous studies have shown the relation of higher alpha activity to cognitive enhancement in human beings, for example, short-term memory [5], mood enhancement [46], and attention [14]. The results from these studies allowed our intuition to consider an enhancing effect of the increased alpha activity on the cognition of human beings (healthy participants). The main result of the current study revealed an increase in the alpha power throughout 20 sessions in the NFT group but not in the control group. Alpha activity between baseline, after 10 sessions and after 20 sessions, showed a significant increase in the group that performed neurofeedback training (see Figure 4). Moreover, it is observed that this significant difference was achieved in the later stages of the training (i.e., at 20 sessions). This significant increase of the alpha band towards the end of the training as compared to the baseline confirms the general effect of neurofeedback on the trainability of EEG activity [47]. On the other hand, no significant variation in the alpha activity was observed in the control group. However, in the study, the measured features from the EEG data which are used for the analysis were the same for the experimental group and control group, but the control group was not a placebo and just appeared three times for the recordings during the study. Thus, one might argue that the participants in the experimental group were more adjusted to the experimental conditions. However, it is also not guaranteed that the placebo nature of the control group would explain the produced effect [48,49] because the procedures for the training group and the control group would still be different. Thus, the control group would always receive a different protocol during the study. However, a different design, for example, the training of the control group in a different EEG band, would be one of the possible future studies. Furthermore, the inclusion of another experimental group without background music in the future studies is recommended. It would help to disentangle the effects of the background music from the learning effects which are solely achieved by NFT. We found a significant increase in the alpha power in the experimental group, but unfortunately, the current experimental design does not allow us to disentangle whether this increase was due to the NFT or the music presented during the NFT. Hence, we consider the absence of an active-control (e.g., sham-feedback) and experimental group without music as a limitation of the study, and this should be avoided in the future studies.
4.1. Between the Session Effects
It is important to understand the transfer effect of the training into situations outside the experimental conditions. Thus, variation in the EEG activity from the baseline observed in the subsequent session is crucial to justify the use of NFT outside the experimental conditions. This was examined in the current study using a 3 min RS-EEG data collected after each session. We found that alpha power was higher in this post-session RS-EEG from the baseline in most of the sessions (80% of the sessions). However, this increase was not significant. The possible interpretation of these results addresses the problem of adequate reference (or baseline) level which is highlighted in [9] and shown in [50] by identifying learning indices of training. The proper selection of the baseline can produce considerable differences in the analysis, but unfortunately, there is the inconsistency of the baseline selection in the literature that leads to the issue of adequate baseline. Some have used the baseline measure from the first session [51] while others used the initial block of the first session [52]. Despite the use of different baseline setups for the analysis, Dempster and Vernon in their study [50] found a gradual increase in both the baseline and training. In our study, the insignificant changes in the RS-EEG alpha power from the baseline could be linked to the findings in their study [50]. Another possible indication of this insignificant variation in the RS-EEG and baseline is the potential focus of the participants on the feedback bar during the session. The participants try to keep their attention on the feedback information that could produce an interference effect in the genesis of alpha activity [53,54] in the RS-EEG because the RS-EEG was recorded immediately after the training session.
Nan et al. also did not see any significant difference in the resting state EEG after training [5] and concluded that this lack of significance might be due to less intensive training (i.e., total of 60 min) and suggested longer training. However, in the current study, the training was intensive as compared to their study, but still, a non-significant effect is observed in the RS-EEG. However, interestingly, they found a significant improvement in cognitive performance (i.e., short-term memory) [5] despite non-significant changes in the RS-EEG. This provides a rationale to support the current findings and motivations for neurofeedback training.
4.2. Within Session Training Effect
As expected, the alpha power during the session increased from the baseline due to the training. A significant increase in the alpha power during the NFT session was observed as power in both halves of the session was greater than the baseline power (see Figure 6). This within-session increment corresponds to the operant contingencies of the training. This helps to analyze the learning process that takes place during the training session. It also helps to investigate the specificity of NFT and relate the brain activity improvements to electrophysiological outcomes as indicated in [55]. As in the offline analysis, we are analyzing the average alpha activity of the entire brain region. Therefore, an interesting finding of the current study is that alpha activity feedback at frontal regions facilitates increments in that activity in the rest of the brain regions as well. Despite the small decrements in the alpha activity from the start to the end of each half of the session (i.e., a small decrease from B1 to B2 and then B3 in both halves of the session; see Figure 6), the mean alpha power over 15 min (first half and second half) was significantly higher than the baseline. This suggests that in the entire long run of the session, the training does facilitate the real increment in the main targeted feedback band.
The success of the neurofeedback training is crucial without addressing the issues of efficacy, specificity, and methodological problems associated with this procedure. In a review paper, the authors have greatly contributed to the discussion on specificity [55], but still, to our knowledge, none of the studies have reported on the session length that can address the methodological problem. The single-session considerations are briefly highlighted in a review paper [56]. Despite the commonly used session length (20–40 min) [56], there are subjective differences in focusing on training that varies across individuals and groups. Nonetheless, our findings suggest that short breaks should be provided in the middle if a single session is longer than 5 min. As we can see that within-session alpha power is higher at the beginning of the session; alpha power at the B1 stage of both halves is greater than B2 and B3 (see Figure 6). This indicates the difficulties of the implementation of mental strategies of participants towards the end of the training. One of the possible reasons for this difficulty could be the frustration of the perceived feedback parameters that are highlighted for optimizing the NFT protocols in [57]. The decrease from B1 to B2 is not significant, which suggests a maximum session length of up to 10 min, but the optimal session length should be 5 min. In contrast, the decrease between B2 and B3 was significant, which strongly suggests avoiding a session length of more than 10 min without break. These findings suggest short time breaks within a long NFT run, which have also shown enhanced training effects in the previous studies [58].
4.3. Behavioral Effect
The current study revealed positive effects of the training with enhanced electrophysiological alpha activity in the NFT group only. The alpha activity in the NFT group presented significantly higher power after the training which was not seen in the control group. Although the participants were able to respond to the stimulus more quickly and accurately in the post-NF session as shown by reduced response time and lower error rates of the Stroop task, respectively, this effect was statistically non-significant. Similarly, the PSS scores were less in the post-NF session, but again, the effect was statistically non-significant.
The general Stroop interference was observed in both groups by lower RT and lower error rates in the congruent trials as compared to the incongruent. This may result because of the high individual variability in neural and behavioral measures which is widely reported in NFT research (see [59]).
The MMSE score after 10 sessions and after 20 sessions was higher than the baseline in both groups. A significant change in the MMSE score is observed from the baseline to after 10 sessions and after 20 sessions. However, this change may reflect a time effect because this significant increase was observed in both groups, NFT and control. A similar effect in the MMSE score was observed in [60], where both groups had similar performance before and after neurofeedback training. This may also be attributed to the fact that the MMSE is a screening tool for cognitive impairment, especially dementia, not a test for general cognitive ability of healthy adults [61]. Hence MMSE scores may not change significantly in healthy adults over the span of 2–3 months due to any sort of cognitive or relaxation training, apart from the learning effects found, which only indicate that people score higher when they do the MMSE repeatedly.
PSS demonstrated normal scores in both groups in all three recordings. The mean PSS score after 10 sessions and after 20 sessions was much lower than the baseline in the NFT group as compared to the Control group (NFT group: Baseline = 22.25, after 10 sessions = 20.40, after 20 sessions = 20.60; Control group: Baseline = 21.40, after 10 sessions = 21.10, after 20 sessions = 21.10). No significant change is observed in the PSS scores in both groups.
5. Conclusions
The current study investigated the effects of neurofeedback training with the low-priced device. The study revealed positive effects in terms of enhanced electrophysiological alpha activity in the NFT group only. The alpha activity in the NFT group presented significantly higher power after the training which was not seen in the control group. The behavioral results of the Stroop task and the change in the PSS score which represents the perception of stress level indicative of effects of alpha activity training failed to reach statistical significance. The MMSE score did not reflect any training related changes and hence was found to be of no use with healthy participants in future studies. The current study also led to several possible protocol suggestions that could benefit the enhanced training gained from NFT. This includes the optimal number of NFT sessions and the session length of a single run for the training. This will help future NFT research with designing better protocols. We hope that the effectiveness of the current study will inspire systems and applications exploiting BCI for the well-being of healthy people.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/math10071095/s1.
Author Contributions
Conceptualization, R.N. and H.N.; methodology, R.N. and H.N.; software, R.N.; validation, R.N. and H.N.; formal analysis, R.N.; investigation, R.N.; resources, H.N., V.V.Y. and C.-Y.T.; data curation, R.N.; writing—original draft preparation, R.N.; writing revision, R.N., H.N., V.V.Y. and C.-Y.T.; visualization, R.N.; supervision, H.N. and V.V.Y.; project administration, R.N., H.N. and V.V.Y.; funding acquisition, H.N., V.V.Y. and C.-Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Universiti Tunku Abdul Rahman Research Fund (UTARRF) (Grant No. IPSR/RMC/UTARRF/2020-C2/H01), (Grant No. IPSR/RMC/UTARRF/2021-C2/H03), and Excellent Research Center Award Fund, Centre for Healthcare Science and Technology, UTAR, Malaysia.
Institutional Review Board Statement
All techniques used in studies involving human subjects complied with the institutional and/or national research committee’s ethical requirements as well as the 1964 Helsinki Declaration and its subsequent modifications or similar ethical standards. The university’s Research Ethics Committee gave its approval to this study.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study. The participants have given their permission for the study to be published in a journal.
Data Availability Statement
All the data included in this study are available upon request by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franchi, J.-A.M.; Jeunet, C.; Lotte, F. Neurofeedback: A challenge for integrative clinical neurophysiological studies. Neurophysiol. Clin. Neurophysiol. 2020, 50, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.; Kober, S.E.; Neuper, C.; Wood, G. How much do strategy reports tell about the outcomes of neurofeedback training? A study on the voluntary up-regulation of the sensorimotor rhythm. Front. Hum. Neurosci. 2020, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Friel, P.N. EEG biofeedback in the treatment of attention deficit/hyperactivity disorder. Altern. Med. Rev. 2007, 12, 146. [Google Scholar] [PubMed]
- Mennella, R.; Patron, E.; Palomba, D. Frontal alpha asymmetry neurofeedback for the reduction of negative affect and anxiety. Behav. Res. Ther. 2017, 92, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Rodrigues, J.P.; Ma, J.; Qu, X.; Wan, F.; Mak, P.I.; Mak, P.U.; Vai, M.I.; Rosa, A. Individual alpha neurofeedback training effect on short term memory. Int. J. Psychophysiol. 2012, 86, 83–87. [Google Scholar] [CrossRef]
- Nawaz, R.; Nisar, H.; Voon, Y.V. Changes in spectral power and functional connectivity of response-conflict task after neurofeedback training. IEEE Access 2020, 8, 139444–139459. [Google Scholar] [CrossRef]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef]
- Rogala, J.; Jurewicz, K.; Paluch, K.; Kublik, E.; Cetnarski, R.; Wróbel, A. The do’s and don’ts of neurofeedback training: A review of the controlled studies using healthy adults. Front. Hum. Neurosci. 2016, 10, 301. [Google Scholar] [CrossRef]
- Jurewicz, K.; Paluch, K.; Kublik, E.; Rogala, J.; Mikicin, M.; Wróbel, A. EEG-neurofeedback training of beta band (12–22 Hz) affects alpha and beta frequencies—A controlled study of a healthy population. Neuropsychologia 2018, 108, 13–24. [Google Scholar] [CrossRef]
- Smetanin, N.; Volkova, K.; Zabodaev, S.; Lebedev, M.A.; Ossadtchi, A. NFBLab-A Versatile Software for Neurofeedback and Brain-Computer Interface Research. Front. Neuroinform. 2018, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W.; Doppelmayr, M.; Hanslmayr, S. Upper alpha ERD and absolute power: Their meaning for memory performance. Prog. Brain Res. 2006, 159, 151–165. [Google Scholar] [PubMed]
- Doppelmayr, M.; Klimesch, W.; Stadler, W.; Pöllhuber, D.; Heine, C. EEG alpha power and intelligence. Intelligence 2002, 30, 289–302. [Google Scholar] [CrossRef]
- Atchley, R.; Klee, D.; Oken, B. EEG frequency changes prior to making errors in an easy stroop task. Front. Hum. Neurosci. 2017, 11, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A.M.; Davelaar, E.J. Frontal alpha oscillations and attentional control: A virtual reality neurofeedback study. Neuroscience 2018, 378, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Naas, A.; Rodrigues, J.; Knirsch, J.-P.; Sonderegger, A. Neurofeedback training with a low-priced EEG device leads to faster alpha enhancement but shows no effect on cognitive performance: A single-blind, sham-feedback study. PLoS ONE 2019, 14, e0211668. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sourina, O.; Hou, X. Neurofeedback games to improve cognitive abilities. In Proceedings of the 2014 International Conference on Cyberworlds, Santander, Spain, 6–8 October 2014; pp. 161–168. [Google Scholar]
- Kober, S.E.; Witte, M.; Ninaus, M.; Neuper, C.; Wood, G. Learning to modulate one’s own brain activity: The effect of spontaneous mental strategies. Front. Hum. Neurosci. 2013, 7, 695. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, J.M. Mechanisms of biofeedback control. In Consciousness and Self-Regulation; Springer: Berlin/Heidelberg, Germany, 1986; pp. 137–162. [Google Scholar]
- Ramirez, R.; Palencia-Lefler, M.; Giraldo, S.; Vamvakousis, Z. Musical neurofeedback for treating depression in elderly people. Front. Neurosci. 2015, 9, 354. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, R.; Nisar, H.; Yap, V.V. Recognition of Useful Music for Emotion Enhancement Based on Dimensional Model. In Proceedings of the 2018 2nd International Conference on BioSignal Analysis, Processing and Systems (ICBAPS), Kuching, Malaysia, 24–26 July 2018; pp. 176–180. [Google Scholar]
- Nawaz, R.; Ng, J.T.; Nisar, H.; Voon, Y.V. Can background music help to relieve stress? An EEG analysis. In Proceedings of the 2019 IEEE International Conference on Signal and Image Processing Applications (ICSIPA), Kuala Lumpur, Malaysia, 17–19 September 2019; pp. 68–72. [Google Scholar]
- Nawaz, R.; Nisar, H.; Voon, Y.V. The effect of music on human brain; Frequency domain and time series analysis using electroencephalogram. IEEE Access 2018, 6, 45191–45205. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Marx, M.S.; Thein, K.; Dakheel-Ali, M. The impact of stimuli on affect in persons with dementia. J. Clin. Psychiatry 2011, 72, 480. [Google Scholar] [CrossRef] [Green Version]
- Sas, C.; Chopra, R. MeditAid: A wearable adaptive neurofeedback-based system for training mindfulness state. Pers. Ubiquitous Comput. 2015, 19, 1169–1182. [Google Scholar] [CrossRef] [Green Version]
- Hinterberger, T. The sensorium: A multimodal neurofeedback environment. Adv. Hum.-Comput. Interact. 2011, 2011, 724204. [Google Scholar] [CrossRef] [Green Version]
- Nacke, L.E.; Kalyn, M.; Lough, C.; Mandryk, R.L. Biofeedback game design: Using direct and indirect physiological control to enhance game interaction. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 103–112. [Google Scholar]
- Stinson, B.; Arthur, D. A novel EEG for alpha brain state training, neurobiofeedback and behavior change. Complement. Ther. Clin. Pract. 2013, 19, 114–118. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, G.J.M.; Denissen, A.J.M.; Jäger, M.; Vernon, D.; Dekker, M.K.J.; Mihajlović, V.; Sitskoorn, M.M. A novel self-guided approach to alpha activity training. Int. J. Psychophysiol. 2012, 83, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Jang, H.S.; Jeong, S.-H.; Jang, I.-S.; Choi, B.-J.; Lee, M.-G.T. Alpha neurofeedback improves the maintaining ability of alpha activity. Neuroreport 2008, 19, 315–317. [Google Scholar] [CrossRef]
- Pigott, E.; Alter, G.; Marikis, D. Frequency-Based Light & Sound Neurotherapy (LSN) Research: A Review of the Research. 2015. Available online: https://www.semanticscholar.org/paper/Frequency-Based-Light-%26-Sound-Neurotherapy-(-LSN-)-Pigott-Alter/396df55b4e3ce3ef9197007df4af723cae8ad61b (accessed on 5 January 2021).
- Brain.fm. Music to Improve Focus, Meditation & Sleep. Available online: https://www.brain.fm/science (accessed on 5 January 2021).
- Brain.fm. Theory & Process. Available online: https://www.brain.fm/assets/pdfs/white-paper.pdf (accessed on 5 January 2021).
- Loui, P. Algorithmic Music Modulates Oscillatory Markers of Sustained Attention. Available online: https://www.brain.fm/assets/pdfs/performance-pilot.pdf (accessed on 5 January 2021).
- Hewett, A.; Santostasi, G. EEG Analysis on Brain.fm. Available online: https://www.brain.fm/assets/pdfs/sleep-study.pdf (accessed on 5 January 2021).
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. Openvibe: An open-source software platform to design, test, and use brain—Computer interfaces in real and virtual environments. Presence Teleoperators Virtual Environ. 2010, 19, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016, 7, 143. [Google Scholar]
- Kim, S.L.; Suk, H.J.; Kang, J.H.; Jung, J.M.; Laine, T.H.; Westlin, J. Using Unity 3D to facilitate mobile augmented reality game development. In Proceedings of the 2014 IEEE World Forum on Internet of Things (WF-IoT), Seoul, Korea, 6–8 March 2014; pp. 21–26. [Google Scholar]
- Khan, S.; Nobili, L.; Khatami, R.; Loddenkemper, T.; Cajochen, C.; Dijk, D.-J.; Eriksson, S.H. Circadian rhythm and epilepsy. Lancet Neurol. 2018, 17, 1098–1108. [Google Scholar] [CrossRef]
- Ghimire, N.; Paudel, B.H.; Khadka, R.; Singh, P.N. Reaction time in Stroop test in Nepalese medical students. J. Clin. Diagn. Res. 2014, 8, BC14. [Google Scholar] [CrossRef]
- Pangman, V.C.; Sloan, J.; Guse, L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: Implications for clinical practice. Appl. Nurs. Res. 2000, 13, 209–213. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Cohen, S. Perceived stress in a probability sample of the United States. In The Social Psychology of Health; Sage Publications, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Trans. Biomed. Eng. 2019, 67, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Girolami, M.; Sejnowski, T.J. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 1999, 11, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Phneah, S.W.; Nisar, H. EEG-based alpha neurofeedback training for mood enhancement. Australas. Phys. Eng. Sci. Med. 2017, 40, 325–336. [Google Scholar] [CrossRef]
- Zoefel, B.; Huster, R.J.; Herrmann, C.S. Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage 2011, 54, 1427–1431. [Google Scholar] [CrossRef]
- Gevensleben, H.; Holl, B.; Albrecht, B.; Vogel, C.; Schlamp, D.; Kratz, O.; Studer, P.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry 2009, 50, 780–789. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Dempster, T.; Vernon, D. Identifying indices of learning for alpha neurofeedback training. Appl. Psychophysiol. Biofeedback 2009, 34, 309–318. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Huster, R.J.; Scharfenort, R.; Mokom, Z.N.; Zimmermann, J.; Herrmann, C.S. Modulation of frontal-midline theta by neurofeedback. Biol. Psychol. 2014, 95, 59–69. [Google Scholar] [CrossRef]
- Escolano, C.; Aguilar, M.; Minguez, J. EEG-based upper alpha neurofeedback training improves working memory performance. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2327–2330. [Google Scholar]
- Aftanas, L.I.; Golocheikine, S.A. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci. Lett. 2001, 310, 57–60. [Google Scholar] [CrossRef]
- Cooper, N.R.; Croft, R.J.; Dominey, S.J.J.; Burgess, A.P.; Gruzelier, J.H. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int. J. Psychophysiol. 2003, 47, 65–74. [Google Scholar] [CrossRef]
- Zuberer, A.; Brandeis, D.; Drechsler, R. Are treatment effects of neurofeedback training in children with ADHD related to the successful regulation of brain activity? A review on the learning of regulation of brain activity and a contribution to the discussion on specificity. Front. Hum. Neurosci. 2015, 9, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriquez-Geppert, S.; Huster, R.J.; Herrmann, C.S. EEG-neurofeedback as a tool to modulate cognition and behavior: A review tutorial. Front. Hum. Neurosci. 2017, 11, 51–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandmeyer, T.; Delorme, A. Closed-Loop Frontal Midlineθ Neurofeedback: A Novel Approach for Training Focused-Attention Meditation. Front. Hum. Neurosci. 2020, 14, 246. [Google Scholar] [CrossRef]
- Ebbinghaus, H. Memory: A Contribution to Experimental Psychology; Dover: Long Island, NY, USA, 1964. [Google Scholar]
- Gruzelier, J.H. EEG-neurofeedback for optimising performance. III: A review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 2014, 44, 159–182. [Google Scholar] [CrossRef]
- Hsueh, J.-J.; Chen, T.-S.; Chen, J.-J.; Shaw, F.-Z. Neurofeedback training of EEG alpha rhythm enhances episodic and working memory. Hum. Brain Mapp. 2016, 37, 2662–2675. [Google Scholar] [CrossRef] [Green Version]
- Franco-Marina, F.; Garcia-González, J.J.; Wagner-Echeagaray, F.; Gallo, J.; Ugalde, O.; Sánchez-Garcia, S.; Espinel-Bermúdez, C.; Juárez-Cedillo, T.; Rodriguez, M.Á.V.; Garcia-Peña, C. The Mini-mental State Examination revisited: Ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 2010, 22, 72–81. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).