Identifying the Association of Time-Averaged Serum Albumin Levels with Clinical Factors among Patients on Hemodialysis Using Whale Optimization Algorithm
Abstract
:1. Introduction
2. Methods
2.1. Datasets
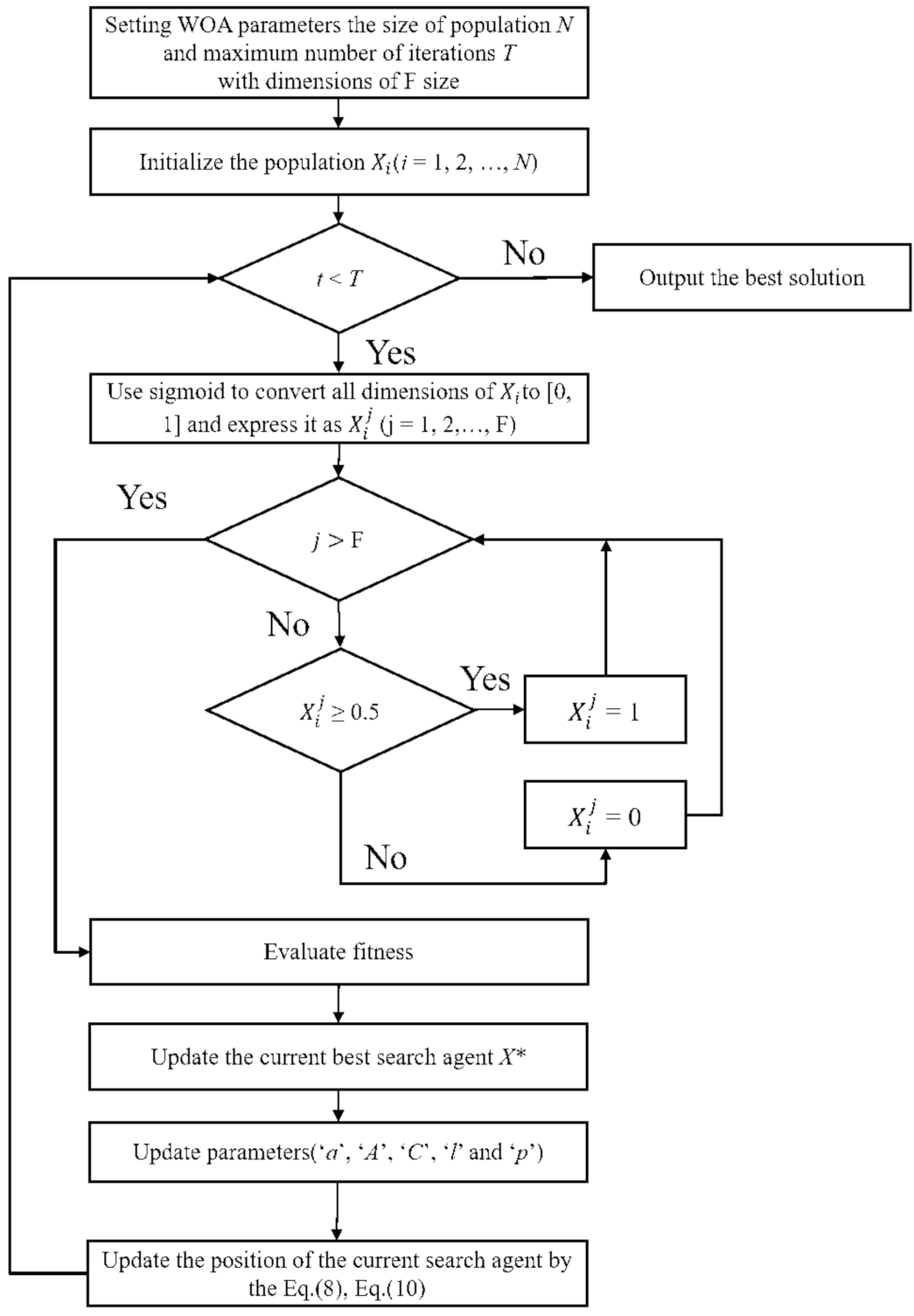
2.2. Whale Optimization-Based Feature Selection Model
2.3. Statistical Models
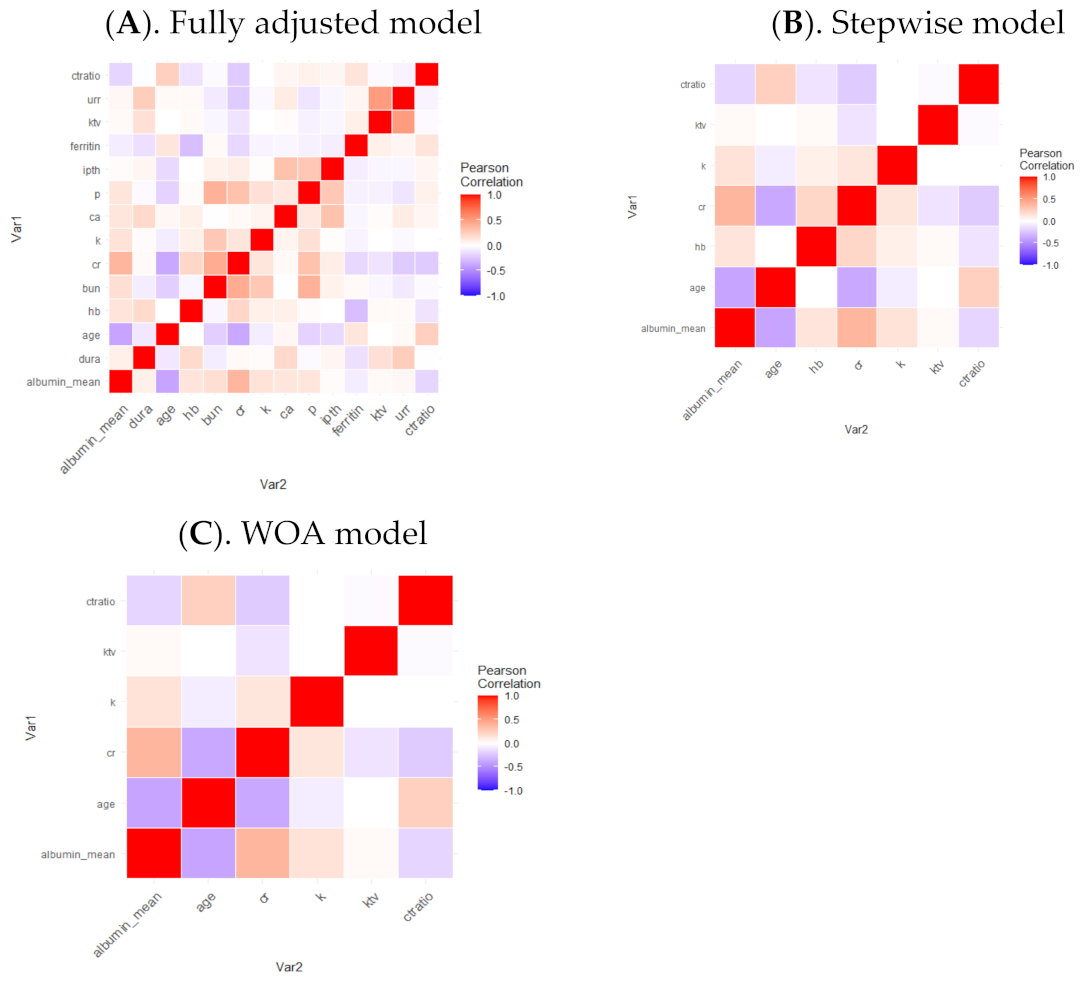
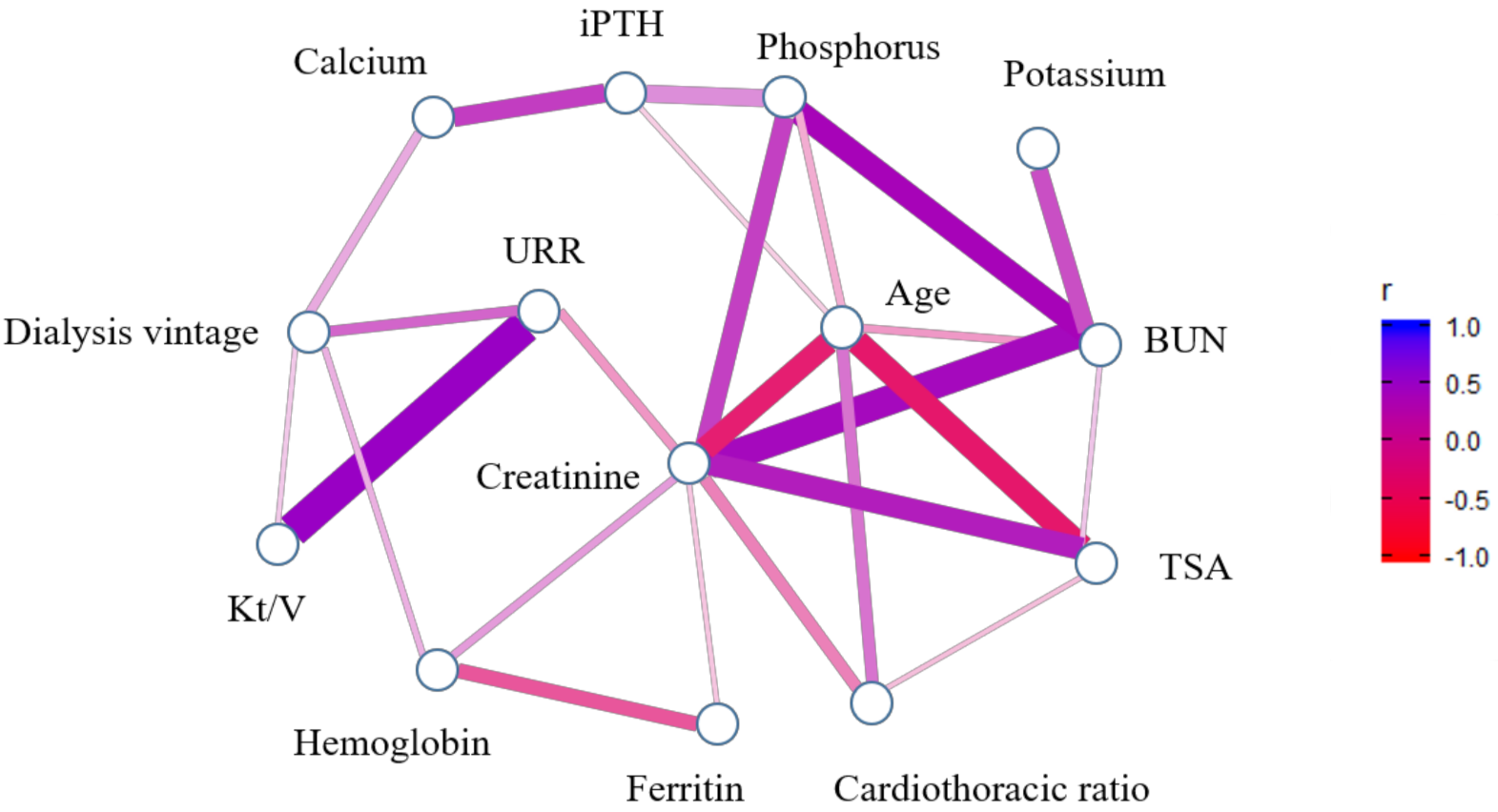
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.B.; Cheng, B.C.; Liu, W.H.; Liao, S.C.; Fu, M.M.; Moi, S.H.; Yang, C.H. Longitudinal analysis of cardiac structure and function in incident-automated peritoneal dialysis: Comparison between icodextrin solution and glucose-based solution. BMC Nephrol. 2018, 19, 109. [Google Scholar] [CrossRef]
- Beberashvili, I.; Erlich, A.; Azar, A.; Sinuani, I.; Feldman, L.; Gorelik, O.; Stav, K.; Efrati, S. Longitudinal study of serum uric acid, nutritional status, and mortality in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1015–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-B.; Cheng, B.-C.; Yang, C.-H.; Hua, M.-S. An association between time-varying serum albumin level and the mortality rate in maintenance haemodialysis patients: A five-year clinical cohort study. BMC Nephrol. 2016, 17, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaedal, S.; Qureshi, A.R.; Lund, S.H.; Germanis, G.; Hylander, B.; Heimbürger, O.; Carrero, J.J.; Stenvinkel, P.; Bárány, P. Dialysis modality and nutritional status are associated with variability of inflammatory markers. Nephrol. Dial. Transpl. 2016, 31, 1320–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.B.; Lee, W.C.; Cheng, B.C.; Moi, S.H.; Yang, C.H.; Lin, Y.D. Impact of risk factors on functional status in maintenance hemodialysis patients. Eur. J. Med. Res. 2017, 31, 22. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Chang, J.W.; Park, Y. Nutritional status predicts 10-year mortality in patients with end-stage renal disease on hemodialysis. Nutrients 2017, 9, 399. [Google Scholar] [CrossRef]
- Giglio, J.; Kamimura, M.A.; Lamarca, F.; Rodrigues, J.; Santin, F.; Avesani, C.M. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis. J. Ren. Nutr. 2018, 28, 197–207. [Google Scholar] [CrossRef]
- Eriguchi, R.; Obi, Y.; Streja, E.; Tortorici, A.R.; Rhee, C.M.; Soohoo, M.; Kim, T.; Kovesdy, C.P.; Kalantar-Zadeh, K. Longitudinal associations among renal urea clearance–corrected normalized protein catabolic rate, serum albumin, and mortality in patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1109–1117. [Google Scholar] [CrossRef]
- Wang, J.; Streja, E.; Soohoo, M.; Chen, J.L.; Rhee, C.M.; Kim, T.; Molnar, M.Z.; Kovesdy, C.P.; Mehrotra, R.; Kalantar-Zadeh, K. Concurrence of serum creatinine and albumin with lower risk for death in twice-weekly hemodialysis patients. J. Ren. Nutr. 2017, 27, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Nakazato, Y.; Kurane, R.; Hirose, S.; Watanabe, A.; Shimoyama, H. Aging and death-associated changes in serum albumin variability over the course of chronic hemodialysis treatment. PLoS ONE 2017, 12, e0185216. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, T.; Matsuzawa, R.; Yoneki, K.; Harada, M.; Watanabe, T.; Yoshida, A.; Takeuchi, Y.; Matsunaga, A. Combined contribution of reduced functional mobility, muscle weakness, and low serum albumin in prediction of all-cause mortality in hemodialysis patients: A retrospective cohort study. J. Ren. Nutr. 2018, 28, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tsur, N.; Menashe, I.; Haviv, Y.S. Risk Factors Before Dialysis Predominate as Mortality Predictors in Diabetic Maintenance Dialysis patients. Sci. Rep. 2019, 9, 10633. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, D.; Koide, S.; Takahashi, K.; Hayashi, H.; Hasegawa, M.; Yuzawa, Y. Relationship between serum calcium level at dialysis initiation and subsequent prognosis. Ren. Replace. Ther. 2017, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Noppakun, K.; Kasemset, T.; Wongsawad, U.; Ruengorn, C.; Thavorn, K.; Sood, M.M.; Nochaiwong, S. Changes in serum albumin concentrations during transition to dialysis and subsequent risk of peritonitis after peritoneal dialysis initiation: A retrospective cohort study. J. Nephrol. 2020, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Cheng, B.C.; Yang, H.T.; Wu, C.H.; Lei, Y.Y.; Chao, M.C.; Wang, P.Y.; Kuo, L.C.; Moi, S.H.; Yang, C.H.; et al. Time-varying serum albumin levels and all-cause mortality in prevalent peritoneal dialysis patients: A 5-year observational study. BMC Nephrol. 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Ren, N.; Hou, X. An improved logistic regression model based on a spatially weighted technique (ILRBSWT v1. 0) and its application to mineral prospectivity mapping. Geosci. Model Dev. 2018, 11, 2525. [Google Scholar] [CrossRef] [Green Version]
- Rigoni, M.; Torri, E.; Nollo, G.; Zarantonello, D.; Laudon, A.; Sottini, L.; Guarrera, G.M.; Brunori, G. Survival and time-to-transplantation of peritoneal dialysis versus hemodialysis for end-stage renal disease patients: Competing-risks regression model in a single Italian center experience. J. Nephrol. 2017, 30, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.N.; Fregonesi, B.M.; Machado, C.S.; Zagui, G.S.; Kusumota, L.; Suzuki, A.K.; da Costa, J.A.C.; Llopart, J.S.; Nadal, M.; Domingo, J.L. Hemodialysis Water Parameters as Predisposing Factors for Anemia in Patients in Dialytic Treatment: Application of Mixed Regression Models. Biol. Trace Elem. Res. 2019, 190, 30–37. [Google Scholar] [CrossRef]
- Yang, C.H.; Chuang, L.Y.; Lin, Y.D. An improved fuzzy set-based multifactor dimensionality reduction for detecting epistasis. Artif. Intell. Med. 2020, 102, 30–37. [Google Scholar] [CrossRef]
- Yang, C.H.; Lin, Y.D.; Chuang, L.Y. Multiple-Criteria Decision Analysis-Based Multifactor Dimensionality Reduction for Detecting Gene-Gene Interactions. IEEE J. Biomed. Health 2019, 23, 416–426. [Google Scholar] [CrossRef]
- Yang, C.-H.; Chuang, L.-Y.; Lin, Y.-D. CMDR based differential evolution identifies the epistatic interaction in genome-wide association studies. Bioinformatics 2017, 33, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.D.; Van Steen, K. The search for gene-gene interactions in genome-wide association studies: Challenges in abundance of methods, practical considerations, and biological interpretation. Ann. Transl. Med. 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Kao, Y.K.; Chuang, L.Y.; Lin, Y.D. Catfish Taguchi-Based Binary Differential Evolution Algorithm for Analyzing Single Nucleotide Polymorphism Interactions in Chronic Dialysis. IEEE Trans. NanoBiosci. 2018, 17, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.Y.; Moi, S.H.; Lin, Y.D.; Yang, C.H. A comparative analysis of chaotic particle swarm optimizations for detecting single nucleotide polymorphism barcodes. Artif. Intell. Med. 2016, 73, 23–33. [Google Scholar] [CrossRef]
- Yang, C.H.; Lin, Y.D.; Chuang, L.Y.; Chang, H.W. Double-Bottom Chaotic Map Particle Swarm Optimization Based on Chi-Square Test to Determine Gene-Gene Interactions. Biomed. Res. Int. 2014, 2014, 172049. [Google Scholar] [CrossRef]
- Mirjalili, S.; Lewis, A. The whale optimization algorithm. Adv. Eng. Soft. 2016, 95, 51–67. [Google Scholar] [CrossRef]
- Mohammed, H.M.; Umar, S.U.; Rashid, T.A. A Systematic and Meta-Analysis Survey of Whale Optimization Algorithm. Comput. Intell. Neurosci. 2019, 2019, 8718571. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Nie, H.H. An adaptive enhancement method for breast X-ray images based on the nonsubsampled contourlet transform domain and whale optimization algorithm. Med. Biol. Eng. Comput. 2019, 57, 2245–2263. [Google Scholar] [CrossRef]
- Tawhid, M.A.; Ibrahim, A.M. Feature selection based on rough set approach, wrapper approach, and binary whale optimization algorithm. Int. J. Mach. Learn. Cyb. 2020, 11, 573–602. [Google Scholar] [CrossRef]
- Tubishat, M.; Abushariah, M.A.M.; Idris, N.; Aljarah, I. Improved whale optimization algorithm for feature selection in Arabic sentiment analysis. Appl. Intell. 2019, 49, 1688–1707. [Google Scholar] [CrossRef]
- Mafarja, M.M.; Mirjalili, S. Hybrid Whale Optimization Algorithm with simulated annealing for feature selection. Neurocomputing 2017, 260, 302–312. [Google Scholar] [CrossRef]
- Lu, K.; Ma, Z. A modified whale optimization algorithm for parameter estimation of software reliability growth models. J. Algorithms Comput. Technol. 2021, 15, 17483026211034442. [Google Scholar] [CrossRef]
- Serigne, G.; Meryam, A.; Souad, D.; Clement, K.; Illiassou, S.; Etienne, G. Nutritional assessment of hemodialysis patients aged over 65 years: Outcome of a cross-sectional survey conducted in the well-equipped hemodialysis center of the Cahors hospital, France. J. Nephrol. Ther. 2018, 8, 305. [Google Scholar] [CrossRef]
- Katkov, A.; Sinuani, I.; Azar, A.; Shapiro, G.; Efrati, S.; Beberashvili, I. Age modifies the association between serum obestatin, appetite and nutritional status in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2018, 72, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Iseki, K.; Kawazoe, N.; Fukiyama, K. Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int. 1993, 44, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Kilpatrick, R.D.; Kuwae, N.; McAllister, C.J.; Alcorn, H., Jr.; Kopple, J.D.; Greenland, S. Revisiting mortality predictability of serum albumin in the dialysis population: Time dependency, longitudinal changes and population-attributable fraction. Nephrol. Dial. Transplant. 2005, 20, 1880–1888. [Google Scholar] [CrossRef] [Green Version]
- Amaral, S.; Hwang, W.; Fivush, B.; Neu, A.; Frankenfield, D.; Furth, S. Serum albumin level and risk for mortality and hospitalization in adolescents on hemodialysis. Clin. J. Am. Soc. Nephrol. 2008, 3, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Mutsert, R.; Grootendorst, D.C.; Indemans, F.; Boeschoten, E.W.; Krediet, R.T.; Dekker, F.W. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 2009, 19, 127–135. [Google Scholar] [CrossRef]
- Owen, W.F., Jr.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar] [CrossRef]
- Combe, C.; Chauveau, P.; Laville, M.; Fouque, D.; Azar, R.; Cano, N.; Canaud, B.; Roth, H.; Leverve, X.; Aparicio, M. Influence of nutritional factors and hemodialysis adequacy on the survival of 1610 French patients. Am. J. Kidney Dis. 2001, 37, S81–S88. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A. Biological basis of hypoalbuminemia in ESRD. J. Am. Soc. Nephrol. 1998, 9, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
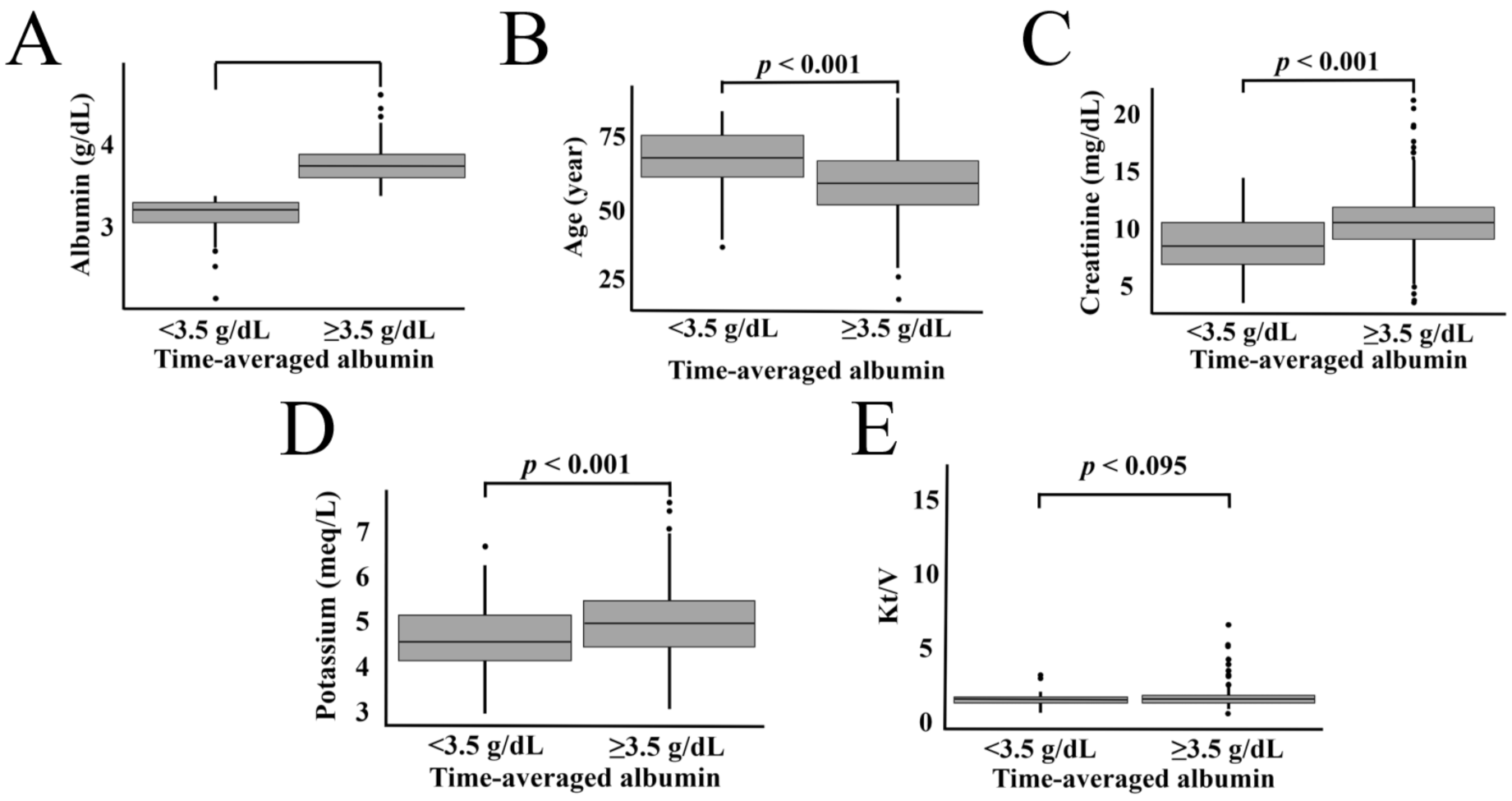
| Characteristics | High-TSA Category (≥3.5 g/dL) | Low-TSA Category (<3.5 g/dL) | p |
|---|---|---|---|
| Dialysis vintage (years) a | 5.74 (2.85–10.31) | 4.49 (2.17–9.20) | 0.176 |
| Age | 58.47 ± 12.04 | 67.20 ± 11.38 | <0.001 |
| Gender (n, %) | 0.116 | ||
| Male | 341 (46.39) | 35 (37.23) | |
| Female | 394 (53.61) | 59 (62.77) | |
| DM (n, %) | 0.013 | ||
| No | 554 (75.37) | 59 (62.77) | |
| Yes | 181 (24.63) | 35 (37.23) | |
| Laboratory measurements | |||
| Hemoglobin (g/dL) | 10.69 ± 1.28 | 10.24 ± 1.71 | 0.014 |
| Albumin (g/dL) | 3.93 ± 0.27 | 3.38 ± 0.33 | <0.001 |
| BUN (mg/dL) | 69.70 ± 16.56 | 62.70 ± 18.09 | 0.001 |
| Creatinine (mg/dL) | 10.75 ± 2.34 | 8.72 ± 2.32 | <0.001 |
| Potassium (meq/L) | 4.99 ± 0.72 | 4.68 ± 0.77 | <0.001 |
| Calcium (mg/dL) | 9.24 ± 0.85 | 9.04 ± 0.90 | 0.039 |
| Phosphorus (mg/dL) | 4.89 ± 1.42 | 4.48 ± 1.43 | 0.010 |
| iPTH (pg/mL) a | 206.70 (78.20–490.35) | 202.20 (98.50–422.18) | 0.597 |
| Ferritin (ng/mL) a | 404.50 (233.05–615.85) | 420.75 (287.05–719.38) | 0.087 |
| Kt/V (n, %) | |||
| ≥1.7 | 331 (45.04) | 37 (39.36) | 0.351 |
| <1.7 | 404 (54.97) | 57 (60.64) | |
| URR (n, %) | |||
| ≥0.65 | 699 (95.10) | 87 (92.55) | 0.422 |
| <0.65 | 36 (4.90) | 7 (7.45) | |
| Cardiothoracic ratio (n, %) | |||
| ≥0.5 | 369 (50.20) | 68 (72.34) | <0.001 |
| <0.5 | 366 (49.80) | 26 (27.66) |
| Characteristics | Comparison | Unadjusted | ||
|---|---|---|---|---|
| OR | 95% CI | p | ||
| Dialysis vintage | years | 0.97 | 0.93–1.01 | 0.173 |
| Age | years | 1.07 | 1.05–1.09 | <0.001 |
| Gender | Female vs. Male | 1.46 | 0.94–2.29 | 0.094 |
| DM | Yes vs. No | 1.82 | 1.15–2.84 | 0.009 |
| Blood analysis | ||||
| Hemoglobin | g/dL | 0.78 | 0.66–0.91 | 0.002 |
| BUN | mg/dL | 0.97 | 0.96–0.99 | <0.001 |
| Creatinine | mg/dL | 0.66 | 0.59–0.73 | <0.001 |
| Potassium | meq/L | 0.54 | 0.39–0.74 | <0.001 |
| Calcium | mg/dL | 0.75 | 0.58–0.97 | 0.029 |
| Phosphorus | mg/dL | 0.81 | 0.69–0.95 | 0.009 |
| iPTH | pg/mL | 0.9999 | 0.9994–1.0003 | 0.630 |
| Ferritin | ng/mL | 1.0005 | 0.99996–1.00095 | 0.059 |
| Kt/V | ≥1.70 vs. <1.70 | 0.79 | 0.51–1.22 | 0.298 |
| URR | ≥0.65 vs. <0.65 | 0.64 | 0.29–1.61 | 0.298 |
| Cardiothoracic ratio | ≥0.50 vs. <0.50 | 2.59 | 1.63–4.24 | <0.001 |
| Characteristics | Comparison | Fully Adjusted Model | Stepwise Model | WOFS Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Dialysis vintage | years | 1.03 | 0.97–1.08 | 0.322 | ||||||
| Age | years | 1.05 | 1.02–1.07 | <0.001 | 1.04 | 1.02–1.07 | <0.001 | 1.04 | 1.02–1.06 | 0.001 |
| Gender | Female vs. Male | 0.94 | 0.54–1.66 | 0.832 | ||||||
| DM | Yes vs. No | 1.11 | 0.64–1.89 | 0.708 | ||||||
| Blood analysis | ||||||||||
| Hemoglobin | g/dL | 0.87 | 0.72–1.06 | 0.169 | 0.88 | 0.73–1.06 | 0.172 | |||
| BUN | mg/dL | 1.00 | 0.98–1.02 | 0.877 | ||||||
| Creatinine | mg/dL | 0.72 | 0.62–0.83 | <0.001 | 0.72 | 0.64–0.82 | <0.001 | 0.71 | 0.63–0.80 | <0.001 |
| Potassium | meq/L | 0.69 | 0.49–0.97 | 0.033 | 0.70 | 0.50–0.96 | 0.027 | 0.68 | 0.49–0.93 | 0.016 |
| Calcium | mg/dL | 0.78 | 0.57–1.05 | 0.103 | ||||||
| Phosphorus | mg/dL | 1.04 | 0.85–1.27 | 0.685 | ||||||
| iPTH | pg/mL | 1.00 | 0.99–1.00 | 0.371 | ||||||
| Ferritin | ng/mL | 0.99 | 0.99–1.00 | 0.645 | ||||||
| Kt/V | ≥1.70 vs. <1.70 | 0.63 | 0.39–1.09 | 0.101 | 0.58 | 0.35–0.94 | 0.029 | 0.57 | 0.35–0.91 | 0.021 |
| URR | ≥0.65 vs. <0.65 | 0.55 | 0.22–1.56 | 0.232 | ||||||
| Cardiothoracic ratio | ≥0.50 vs. <0.50 | 1.61 | 0.94–2.80 | 0.086 | 1.60 | 0.96–2.72 | 0.079 | 1.67 | 1.01–2.84 | 0.051 |
| Optimal AIC | 511.96 | 499.25 | 499.14 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Chen, Y.-S.; Moi, S.-H.; Chen, J.-B.; Chuang, L.-Y. Identifying the Association of Time-Averaged Serum Albumin Levels with Clinical Factors among Patients on Hemodialysis Using Whale Optimization Algorithm. Mathematics 2022, 10, 1030. https://doi.org/10.3390/math10071030
Yang C-H, Chen Y-S, Moi S-H, Chen J-B, Chuang L-Y. Identifying the Association of Time-Averaged Serum Albumin Levels with Clinical Factors among Patients on Hemodialysis Using Whale Optimization Algorithm. Mathematics. 2022; 10(7):1030. https://doi.org/10.3390/math10071030
Chicago/Turabian StyleYang, Cheng-Hong, Yin-Syuan Chen, Sin-Hua Moi, Jin-Bor Chen, and Li-Yeh Chuang. 2022. "Identifying the Association of Time-Averaged Serum Albumin Levels with Clinical Factors among Patients on Hemodialysis Using Whale Optimization Algorithm" Mathematics 10, no. 7: 1030. https://doi.org/10.3390/math10071030
APA StyleYang, C.-H., Chen, Y.-S., Moi, S.-H., Chen, J.-B., & Chuang, L.-Y. (2022). Identifying the Association of Time-Averaged Serum Albumin Levels with Clinical Factors among Patients on Hemodialysis Using Whale Optimization Algorithm. Mathematics, 10(7), 1030. https://doi.org/10.3390/math10071030








