Stochastic Model of Virus–Endosome Fusion and Endosomal Escape of pH-Responsive Nanoparticles
Abstract
:1. Introduction
2. Stochastic Model for Rab5 to Rab7 Conversion
3. Endosomal Escape
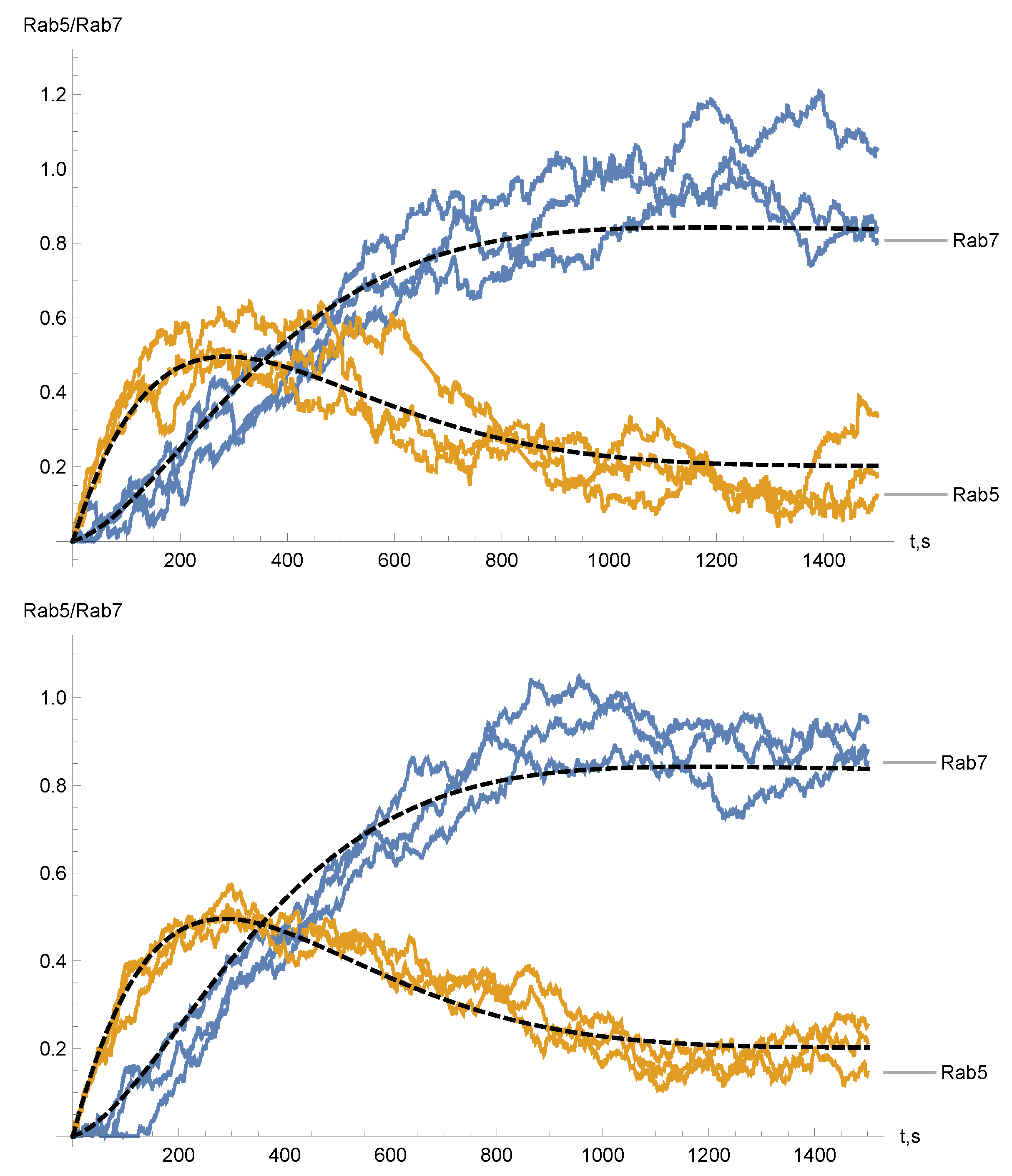
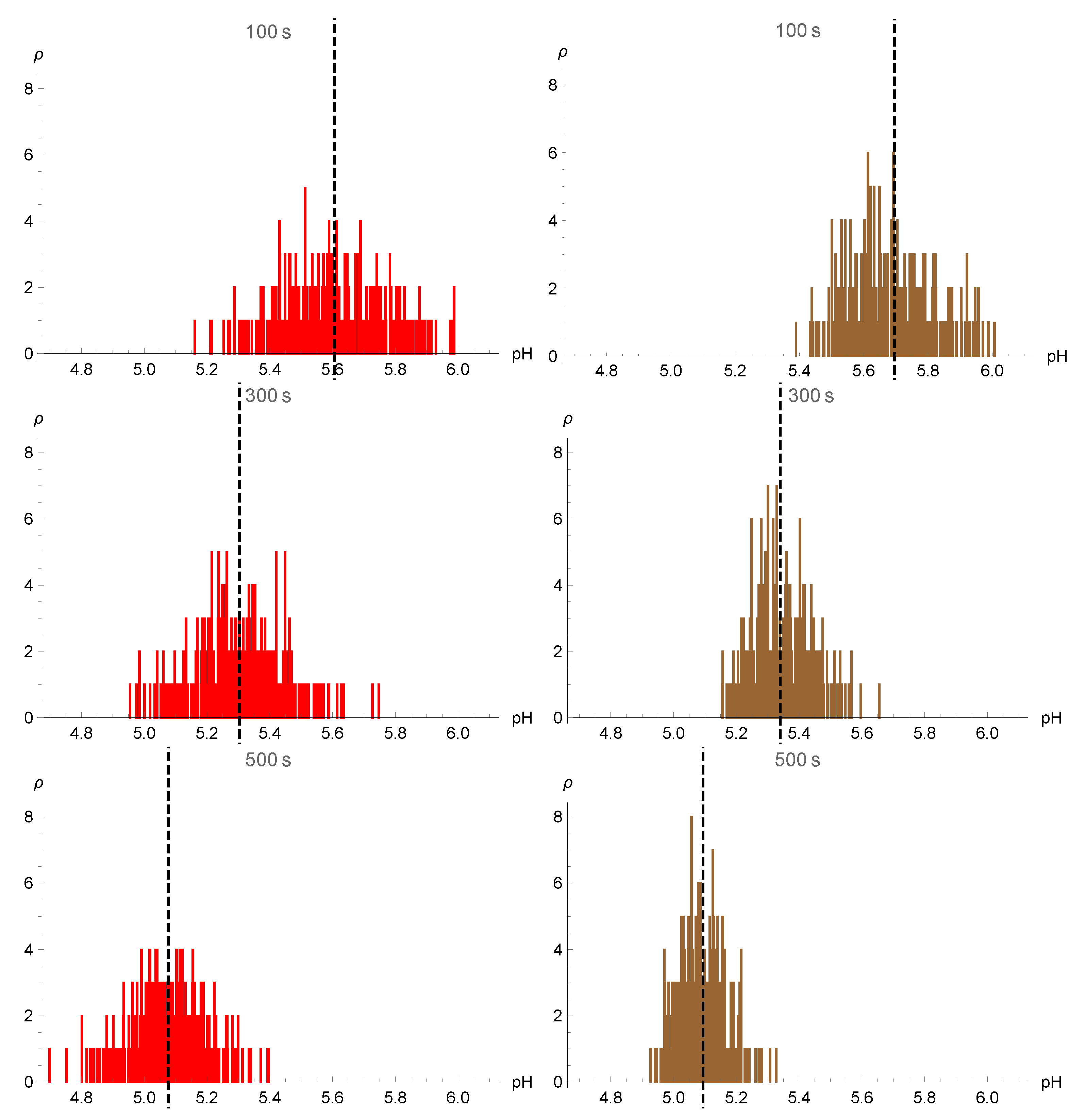
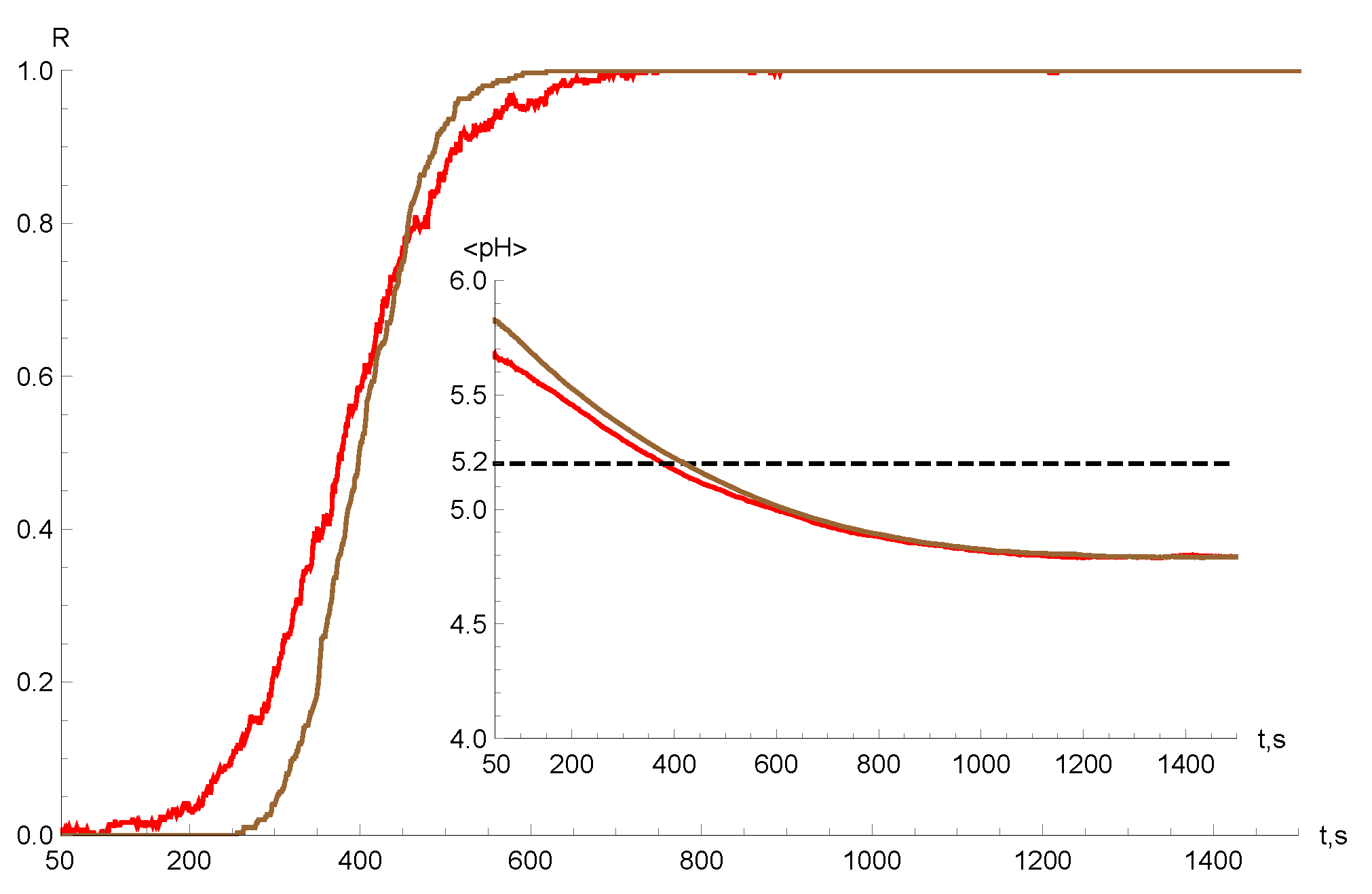
4. Numerical Simulations and Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staring, J.; Raaben, M.; Brummelkamp, T.R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 2018, 131, jcs216259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagache, T.; Danos, O.; Holcman, D. Modeling the step of endosomal escape during cell infection by a nonenveloped virus. Biophys. J. 2012, 102, 980–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.M.; Whittaker, G.R. Fusion of enveloped viruses in endosomes. Traffic 2016, 17, 593–614. [Google Scholar] [CrossRef] [Green Version]
- Lagache, T.; Sieben, C.; Meyer, T.; Herrmann, A.; Holcman, D. Stochastic model of acidification, activation of hemagglutinin and escape of influenza viruses from an endosome. Front. Phys. 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Rink, J.; Ghigo, E.; Kalaidzidis, Y.; Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122, 735–749. [Google Scholar] [CrossRef] [Green Version]
- Poteryaev, D.; Datta, S.; Ackema, K.; Zerial, M.; Spang, A. Identification of the switch in early-to-late endosome transition. Cell 2010, 141, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Zeigerer, A.; Gilleron, J.; Bogorad, R.L.; Marsico, G.; Nonaka, H.; Seifert, S.; Epstein-Barash, H.; Kuchimanchi, S.; Peng, C.G.; Ruda, V.M.; et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 2012, 485, 465–470. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Sahay, G.; Querbes, W.; Alabi, C.; Eltoukhy, A.; Sarkar, S.; Zurenko, C.; Karagiannis, E.; Love, K.; Chen, D.; Zoncu, R.; et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013, 31, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Y.; Huang, X.; Luby-Phelps, K.; Sumer, B.D.; Gao, J. Tunable, Ultrasensitive pH-Responsive Nanoparticles Targeting Specific Endocytic Organelles in Living Cells. Angew. Chem. Int. Ed. 2011, 50, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1452. [Google Scholar] [CrossRef] [PubMed]
- Kongkatigumjorn, N.; Smith, S.A.; Chen, M.; Fang, K.; Yang, S.; Gillies, E.R.; Johnston, A.P.; Such, G.K. Controlling endosomal escape using pH-responsive nanoparticles with tunable disassembly. ACS Appl. Nano Mater. 2018, 1, 3164–3173. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.; Such, G.K. pH-responsive polymer nanoparticles for drug delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [Green Version]
- Andrian, T.; Riera, R.; Pujals, S.; Albertazzi, L. Nanoscopy for endosomal escape quantification. Nanoscale Adv. 2021, 3, 10–23. [Google Scholar] [CrossRef]
- Del Conte-Zerial, P.; Brusch, L.; Rink, J.C.; Collinet, C.; Kalaidzidis, Y.; Zerial, M.; Deutsch, A. Membrane identity and GTPase cascades regulated by toggle and cut-out switches. Mol. Syst. Biol. 2008, 4, 206. [Google Scholar] [CrossRef]
- Castro, M.; Lythe, G.; Smit, J.; Molina-París, C. Fusion and fission events regulate endosome maturation and viral escape. Sci. Rep. 2021, 11, 7845. [Google Scholar] [CrossRef]
- Katsnelson, B.; Privalova, L.; Sutunkova, M.; Khodos, M.; Shur, V.Y.; Shishkina, E.; Tulakina, L.; Pichugova, S.; Beikin, J. Uptake of some metallic nanoparticles by, and their impact on pulmonary macrophages in vivo as viewed by optical, atomic force, and transmission electron microscopy. J. Nanomed. Nanotechnol. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Rees, P.; Wills, J.W.; Brown, M.R.; Barnes, C.M.; Summers, H.D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 2019, 10, 2341. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.; Piattelli, V.; Montizaan, D.; Salvati, A. Sources of variability in nanoparticle uptake by cells. Nanoscale 2021, 13, 17530–17546. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008, 4, e1000244. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, C.W. Handbook of Stochastic Methods; Springer: Berlin, Germany, 1985; Volume 3. [Google Scholar]
- Horsthemke, W.; Lefever, R. Noise-induced transitions in physics, chemistry, and biology. In Noise-Induced Transitions: Theory and Applications in Physics, Chemistry, and Biology; Springer: Berlin/Heidelberg, Germany, 1984; pp. 164–200. [Google Scholar]
- Bressloff, P.C.; Newby, J.M. Stochastic models of intracellular transport. Rev. Mod. Phys. 2013, 85, 135. [Google Scholar] [CrossRef] [Green Version]
- Fedotov, S.; Korabel, N.; Waigh, T.A.; Han, D.; Allan, V.J. Memory effects and Lévy walk dynamics in intracellular transport of cargoes. Phys. Rev. E 2018, 98, 042136. [Google Scholar] [CrossRef] [Green Version]
- Korabel, N.; Han, D.; Taloni, A.; Pagnini, G.; Fedotov, S.; Allan, V.; Waigh, T.A. Local analysis of heterogeneous intracellular transport: Slow and fast moving endosomes. Entropy 2021, 23, 958. [Google Scholar] [CrossRef]
- Han, D.; Alexandrov, D.V.; Gavrilova, A.; Fedotov, S. Anomalous Stochastic Transport of Particles with Self-Reinforcement and Mittag–Leffler Distributed Rest Times. Fractal Fract. 2021, 5, 221. [Google Scholar] [CrossRef]
- Simpson, C.; Yamauchi, Y. Microtubules in influenza virus entry and egress. Viruses 2020, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Ando, D.; Korabel, N.; Huang, K.C.; Gopinathan, A. Cytoskeletal network morphology regulates intracellular transport dynamics. Biophys. J. 2015, 109, 1574–1582. [Google Scholar] [CrossRef] [Green Version]
- Wannasarit, S.; Wang, S.; Figueiredo, P.; Trujillo, C.; Eburnea, F.; Simón-Gracia, L.; Correia, A.; Ding, Y.; Teesalu, T.; Liu, D.; et al. A virus-mimicking pH-responsive Acetalated dextran-based membrane-active polymeric nanoparticle for intracellular delivery of antitumor therapeutics. Adv. Funct. Mater. 2019, 29, 1905352. [Google Scholar] [CrossRef]
- Braga, C.B.; Kido, L.A.; Lima, E.N.; Lamas, C.A.; Cagnon, V.H.; Ornelas, C.; Pilli, R.A. Enhancing the Anticancer Activity and Selectivity of Goniothalamin Using pH-Sensitive Acetalated Dextran (Ac-Dex) Nanoparticles: A Promising Platform for Delivery of Natural Compounds. ACS Biomater. Sci. Eng. 2020, 6, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.M.; Fleischmann, D.; Goepferich, A. Biomedical nanoparticle design: What we can learn from viruses. J. Control. Release 2021, 329, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Hill, B.D.; Hoang, T.; Wen, F. Virus-inspired Strategies for Cancer Therapy. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Units | Parameter | Value | Units |
|---|---|---|---|---|---|
| s | s | ||||
| s | s | ||||
| s | s | ||||
| s | 6 | - | |||
| s | - | ||||
| s | 0.01 | s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotov, S.; Alexandrov, D.; Starodumov, I.; Korabel, N. Stochastic Model of Virus–Endosome Fusion and Endosomal Escape of pH-Responsive Nanoparticles. Mathematics 2022, 10, 375. https://doi.org/10.3390/math10030375
Fedotov S, Alexandrov D, Starodumov I, Korabel N. Stochastic Model of Virus–Endosome Fusion and Endosomal Escape of pH-Responsive Nanoparticles. Mathematics. 2022; 10(3):375. https://doi.org/10.3390/math10030375
Chicago/Turabian StyleFedotov, Sergei, Dmitri Alexandrov, Ilya Starodumov, and Nickolay Korabel. 2022. "Stochastic Model of Virus–Endosome Fusion and Endosomal Escape of pH-Responsive Nanoparticles" Mathematics 10, no. 3: 375. https://doi.org/10.3390/math10030375
APA StyleFedotov, S., Alexandrov, D., Starodumov, I., & Korabel, N. (2022). Stochastic Model of Virus–Endosome Fusion and Endosomal Escape of pH-Responsive Nanoparticles. Mathematics, 10(3), 375. https://doi.org/10.3390/math10030375









