Extracellular Vesicle Integrins Distinguish Unique Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Extracellular Vesicle Enrichment and Protein Quantification
2.3. SDS-PAGE and In-Gel Digestion
2.4. Mass Spectrometry
2.5. Protein Enrichment Analyses
2.6. Statistical Analyses
3. Results
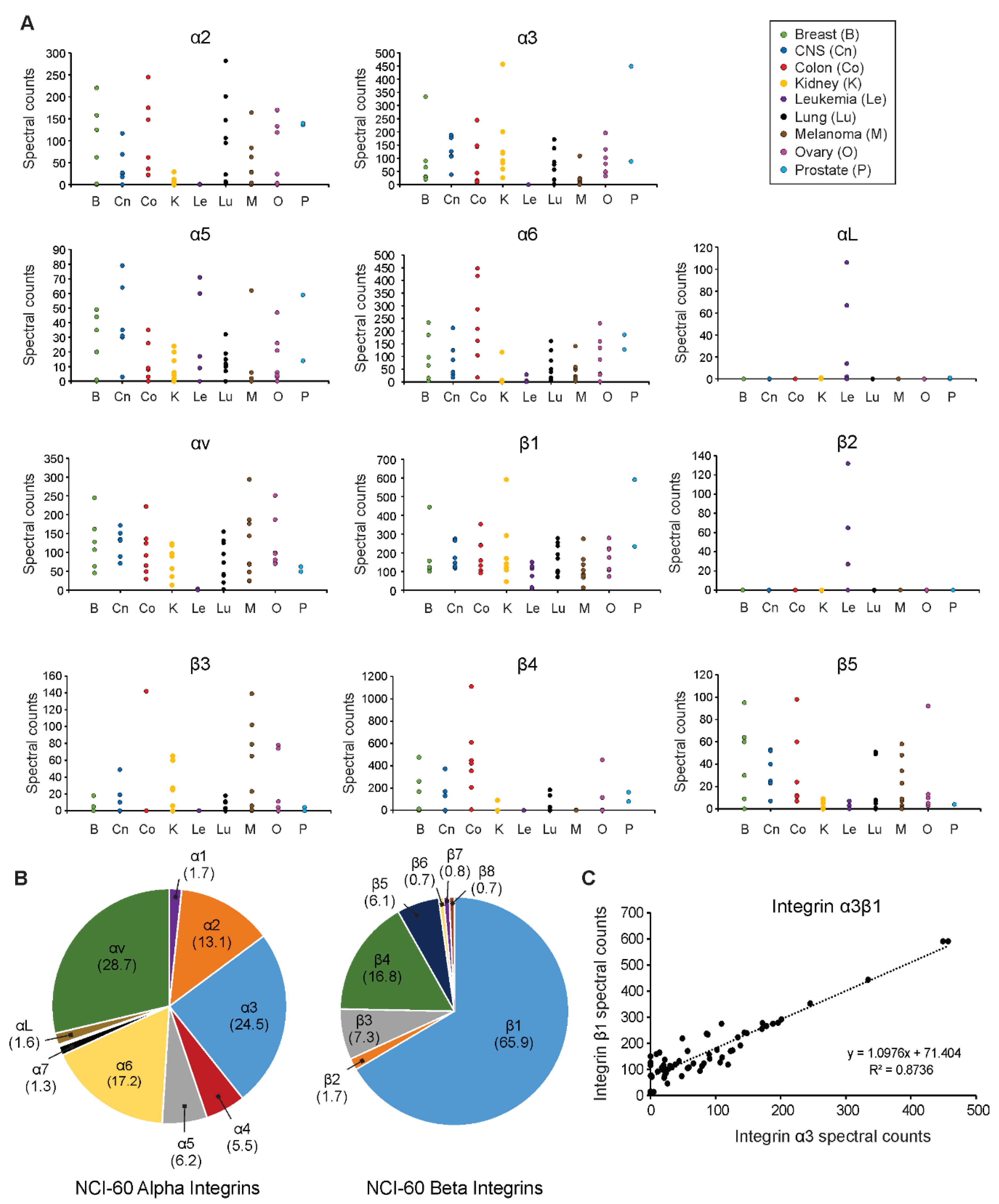
3.1. Extracellular Vesicle Integrin Profiling across the NCI-60 Panel
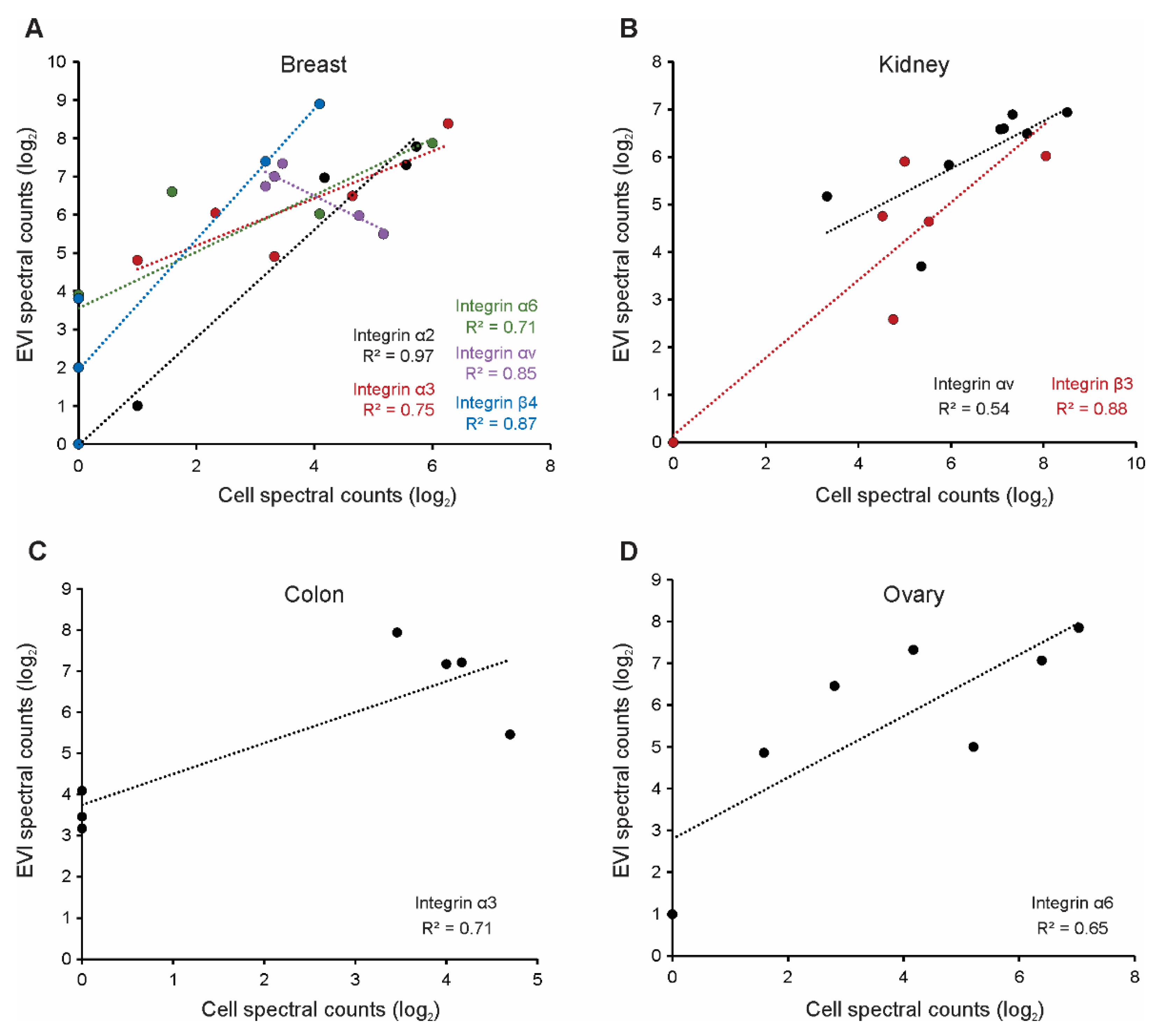
3.2. Selected Vesicle Integrin Proteins Reflect Progenitor Cell Expression
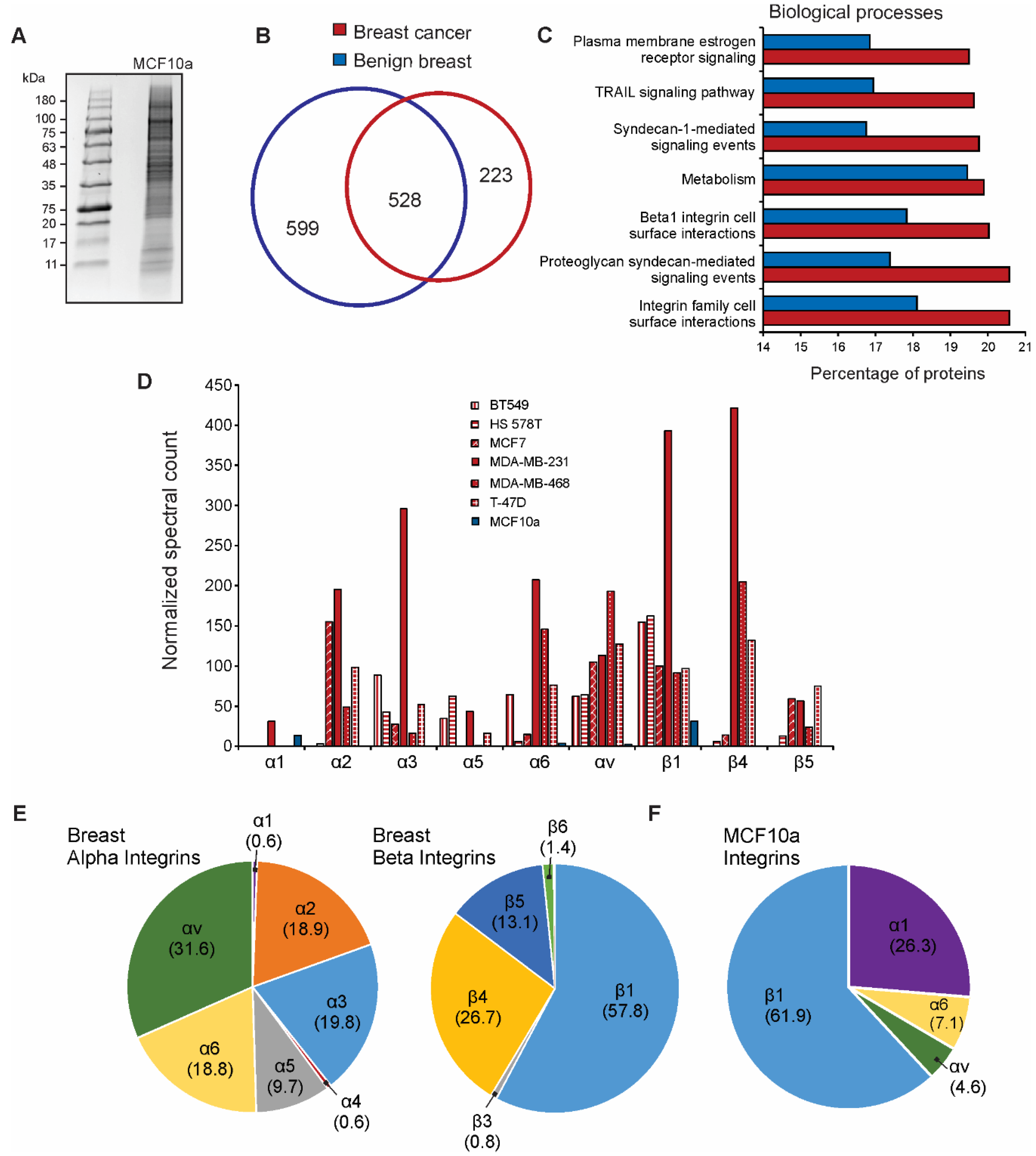
3.3. Integrin Expression Differs in Cancer Cell-Derived EVs Compared to Benign EVs
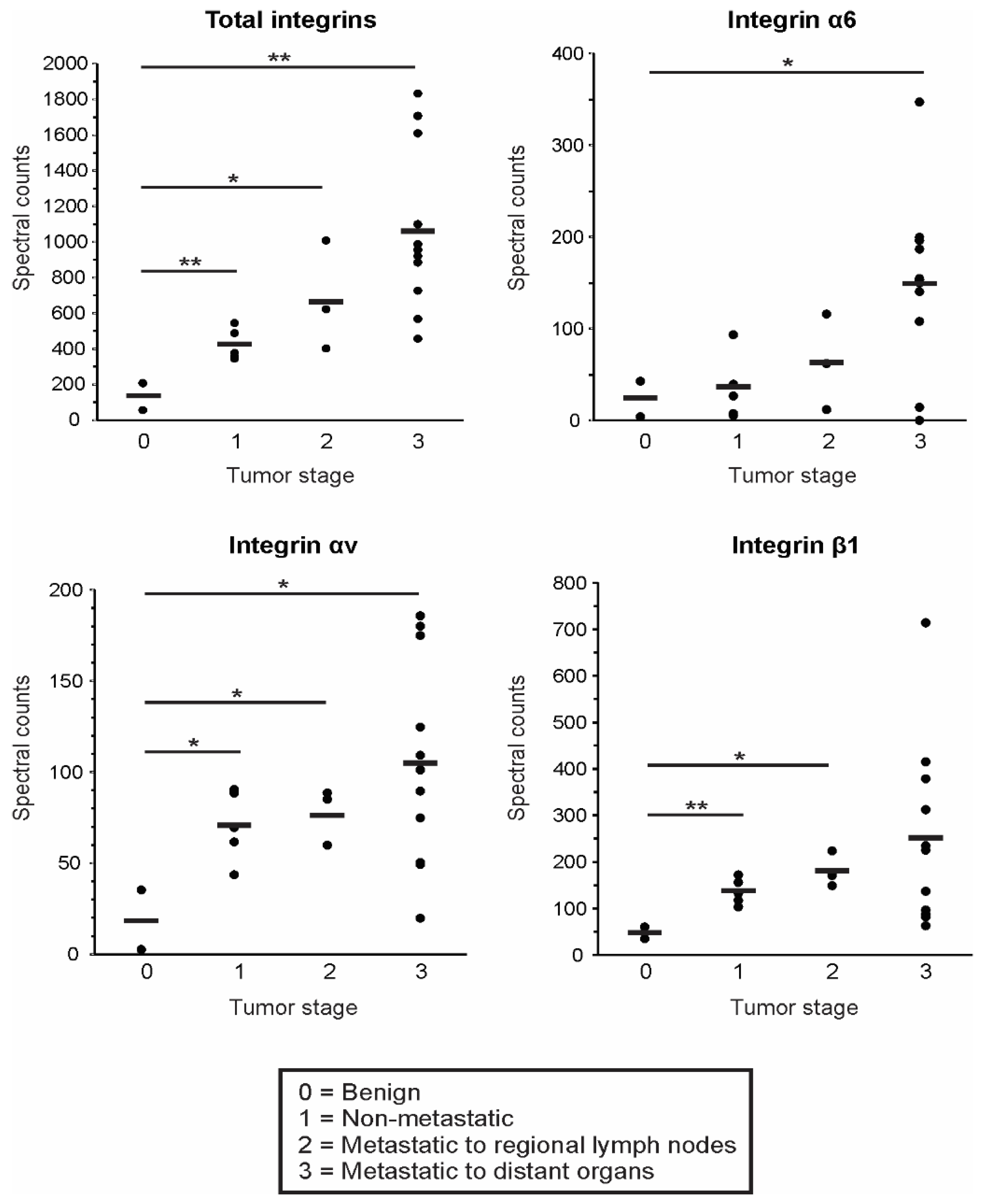
3.4. EV Integrin Levels Predict Cancer Stage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamkun, J.W.; DeSimone, D.W.; Fonda, D.; Patel, R.S.; Buck, C.; Horwitz, A.F.; Hynes, R.O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell 1986, 46, 271–282. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrin heterodimer and receptor complexity in avian and mammalian cells. J. Cell Biol. 1989, 109, 409–420. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genom. Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Hynes, R. Integrins: A family of cell surface receptors. Cell 1987, 48, 549–554. [Google Scholar] [CrossRef]
- Hynes, R. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Hynes, R.O. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004, 23, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A.; Wang, J.-H. The Three-Dimensional Structure of Integrins and their Ligands, and Conformational Regulation of Cell Adhesion. Adv.Protein Chem. 2004, 68, 29–63. [Google Scholar] [PubMed]
- Askari, J.A.; Buckley, P.A.; Mould, A.P.; Humphries, M.J. Linking integrin conformation to function. J. Cell Sci. 2009, 122, 165–170. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Arnaout, M.; Mahalingam, B.; Xiong, J.-P. Integrin Structure, Allostery, and Bidirectional Signaling. Annu. Rev. Cell Dev. Biol. 2005, 21, 381–410. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Giancotti, F.G. Complexity and specificity of integrin signalling. Nature 2000, 2, E13–E14. [Google Scholar] [CrossRef]
- Giancotti, F.G. A Structural View of Integrin Activation and Signaling. Dev. Cell 2003, 4, 149–151. [Google Scholar] [CrossRef]
- Gahmberg, C.G.; Fagerholm, S.C.; Nurmi, S.M.; Chavakis, T.; Marchesan, S.; Grönholm, M. Regulation of integrin activity and signalling. BBA Bioenerg. 2009, 1790, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Layseca, P.; Streuli, C.H. Signalling pathways linking integrins with cell cycle progression. Matrix Biol. 2014, 34, 144–153. [Google Scholar] [CrossRef]
- Huttenlocher, A.; Horwitz, A.R. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011, 3, a005074. [Google Scholar] [CrossRef]
- Shen, B.; Delaney, M.K.; Du, X. Inside-out, outside-in, and inside–outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. Curr. Opin. Cell Biol. 2012, 24, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef]
- Alghisi, G.C.; Ruegg, C. Vascular Integrins in Tumor Angiogenesis: Mediators and Therapeutic Targets. Endothelium 2006, 13, 113–135. [Google Scholar] [CrossRef]
- Bornstein, S.; Schmidt, M.; Choonoo, G.; Levin, T.; Gray, J.; Thomas, C.R.; Wong, M.; McWeeney, S. IL-10 and integrin signaling pathways are associated with head and neck cancer progression. BMC Genom. 2016, 17, 139. [Google Scholar] [CrossRef]
- Stallmach, A.; Von Lampe, B.; Matthes, H.; Bornhöft, G.; O Riecken, E. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut 1992, 33, 342–346. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Mainiero, F. Integrin-mediated adhesion and signaling in tumorigenesis. BBA Bioenerg. 1994, 1198, 47–64. [Google Scholar] [CrossRef]
- Lu, X.; Lu, D.; Scully, M.; Kakkar, V. The Role of Integrins in Cancer and the Development of Anti-Integrin Therapeutic Agents for Cancer Therapy. Perspect. Med. Chem. 2008, 2, 57–73. [Google Scholar] [CrossRef]
- Ubramani, D.; Alahari, S.K. Integrin-mediated function of Rab GTPases in cancer progression. Mol. Cancer 2010, 9, 312. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Erratum: Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. 2010, 10, 890. [Google Scholar] [CrossRef]
- Xiong, J.; Balcioglu, H.E.; Danen, E.H. Integrin signaling in control of tumor growth and progression. Int. J. Biochem. Cell Biol. 2013, 45, 1012–1015. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Wu, Y.J.; Pagel, M.A.; Muldoon, L.L.; Fu, R.; Neuwelt, E.A. High αv Integrin Level of Cancer Cells Is Associated with Development of Brain Metastasis in Athymic Rats. Anticancer. Res. 2017, 37, 4029–4040. [Google Scholar]
- De Franceschi, N.; Hamidi, H.; Alanko, J.; Sahgal, P.; Ivaska, J. Integrin traffic—The update. J. Cell Sci. 2015, 128, 839–852. [Google Scholar] [CrossRef]
- Alanko, J.; Ivaska, J. Endosomes: Emerging Platforms for Integrin-Mediated FAK Signalling. Cell Biol. 2016, 26, 391–398. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Kawakami, K.; Fujita, Y.; Kato, T.; Mizutani, K.; Kameyama, K.; Tsumoto, H.; Miura, Y.; Deguchi, T.; Ito, M. Integrin β4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int. J. Oncol. 2015, 47, 384–390. [Google Scholar] [CrossRef]
- 35 Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated Transfer of αvβ3 Integrin from Tumorigenic to Non-Tumorigenic Cells Promotes a Migratory Phenotype. Mol. Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef]
- Krishn, S.R.; Singh, A.; Bowler, N.; Duffy, A.N.; Friedman, A.; Fedele, C.; Kurtoglu, S.; Tripathi, S.K.; Wang, K.; Hawkins, A.; et al. Prostate cancer sheds the αvβ3 integrin in vivo through exosomes. Matrix Biol. 2018. [Google Scholar] [CrossRef]
- Fedele, C.; Singh, A.; Zerlanko, B.J.; Iozzo, R.V.; Languino, L.R. The αvβ6 Integrin Is Transferred Intercellularly via Exosomes*. J. Biol. Chem. 2015, 290, 4545–4551. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Meckes, D.G.; Gunawardena, H.P.; DeKroon, R.M.; Heaton, P.R.; Edwards, R.H.; Ozgur, S.; Griffith, J.D.; Damania, B.; Raab-Traub, N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc. Natl. Acad. Sci. 2013, 110, E2925–E2933. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef]
- Paolillo, M.; Schinelli, S.; Sheldrake, H.M. Integrins and Exosomes, a Dangerous Liaison in Cancer Progression. Adv. Cancer Res. 2017, 9, 95. [Google Scholar]
- Li, K.; Chen, Y.; Li, A.; Tan, C.; Liu, X. Exosomes play roles in sequential processes of tumor metastasis. Int. J. 2018, 144, 1486–1495. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Conlon, M.M.; Rider, M.A.; Brownstein, N.C.; Meckes, D.G. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 2016, 5, 15. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J. Virol. 2017. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Meckes, D.G. An Adaptable Polyethylene Glycol-Based Workflow for Proteomic Analysis of Extracellar Vesicles. Methods Mol. Biol. 2017, 1660, 303–317. [Google Scholar]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G. Tetraspanin CD63 bridges autophagic and endosomal processes to regulate exosomal secretion and intracellular signaling of Epstein-Barr virus LMP1. J. Virol. 2017. [Google Scholar] [CrossRef]
- Meckes, D.G. Affinity Purification Combined with Mass Spectrometry to Identify Herpes Simplex Virus Protein–Protein Interactions. Methods Mol. Biol. 2014, 1144, 209–222. [Google Scholar]
- Gholami, A.M.; Hahne, H.; Wu, Z.; Auer, F.J.; Meng, C.; Wilhelm, M.; Küster, B. Global Proteome Analysis of the NCI-60 Cell Line Panel. Cell Rep. 2013, 4, 609–620. [Google Scholar] [CrossRef]
- Worst, T.S.; Von Hardenberg, J.; Gross, J.C.; Erben, P.; Schnölzer, M.; Hausser, I.; Bugert, P.; Michel, M.S.; Boutros, M. Database-augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker. Mol. Cell. Proteom. 2017, 16, 998–1008. [Google Scholar] [CrossRef]
- Benito-Martin, A.; Peinado, H.; Benito-Martin, A. FunRich proteomics software analysis, let the fun begin! Proteomics 2015, 15, 2555–2556. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.E.; Chung, A.; A McDonald, J. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J. Cell Sci. 1995, 108, 2511–2523. [Google Scholar]
- Kreidberg, J.A. Functions of alpha3beta1 integrin. Curr. Opin. Cell Biol. 2000, 12, 548–553. [Google Scholar] [CrossRef]
- Adachi, M.; Taki, T.; Huang, C.; Higashiyama, M.; Doi, O.; Tsuji, T.; Miyake, M. Reduced integrin alpha3 expression as a factor of poor prognosis of patients with adenocarcinoma of the lung. J. Clin. Oncol. 1998, 16, 1060–1067. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Kaczmarek, J.; Nicoló, G.; Risso, A.M.; Tarone, G.; Rossino, P.; Defilippi, P.; Castellani, P. Localization of the alpha 3 beta 1 integrin in some common epithelial tumors of the ovary and in normal equivalents. Anticancer. Res. 1993, 13, 1–11. [Google Scholar]
- Pignatelli, M.; Hanby, A.M.; Stamp, G.W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J. Pathol. 1991, 165, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Damjanovich, L.; Fülöp, B.; Adány, R.; Nemes, Z. Integrin expression on normal and neoplastic human breast epithelium. Chir. Hung. 1997, 36, 69–71. [Google Scholar]
- Sordat, I.; Bosman, F.T.; Dorta, G.; Rousselle, P.; Aberdam, D.; Blum, A.L.; Sordat, B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J. Pathol. 1998, 185, 44–52. [Google Scholar] [CrossRef]
- Koukoulis, G.K.; Virtanen, I.; Moll, R.; Quaranta, V.; E Gould, V. Immunolocalization of integrins in the normal and neoplastic colonic epithelium. Virchows Arch. B Cell Pathol. 1993, 63, 373–383. [Google Scholar] [CrossRef]
- Barr, L.F.; E Campbell, S.; Bochner, B.S.; Dang, C.V. Association of the decreased expression of alpha3beta1 integrin with the altered cell: environmental interactions and enhanced soft agar cloning ability of c-myc-overexpressing small cell lung cancer cells. Cancer Res. 1998, 58, 5537–5545. [Google Scholar] [PubMed]
- Judware, R.; A Culp, L. N-myc over-expression downregulates alpha3beta1 integrin expression in human Saos-2 osteosarcoma cells. Clin. Exp. Metastasis 1997, 15, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Judware, R.; Culp, L.A. Concomitant down-regulation of expression of integrin subunits by N-myc in human neuroblastoma cells: differential regulation of alpha2, alpha3 and beta1. Oncogene 1997, 14, 1341–1350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berditchevski, F.; Tolias, K.F.; Wong, K.; Carpenter, C.L.; Hemler, M.E. A Novel Link between Integrins, Transmembrane-4 Superfamily Proteins (CD63 and CD81), and Phosphatidylinositol 4-Kinase. J. Biol. Chem. 1997, 272, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Bavi, P.; Wang, J.Y.; Roehrl, M.H. Immuno-proteomic discovery of tumor tissue autoantigens identifies olfactomedin 4, CD11b, and integrin alpha-2 as markers of colorectal cancer with liver metastases. J. Proteom. 2017, 168, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Marthick, J.R.; Dickinson, J.L. Emerging Putative Biomarkers: The Role of Alpha 2 and 6 Integrins in Susceptibility, Treatment, and Prognosis. Prostate Cancer 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature 2015, 17, 816–826. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurwitz, S.N.; Meckes, D.G., Jr. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes 2019, 7, 14. https://doi.org/10.3390/proteomes7020014
Hurwitz SN, Meckes DG Jr. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes. 2019; 7(2):14. https://doi.org/10.3390/proteomes7020014
Chicago/Turabian StyleHurwitz, Stephanie N., and David G. Meckes, Jr. 2019. "Extracellular Vesicle Integrins Distinguish Unique Cancers" Proteomes 7, no. 2: 14. https://doi.org/10.3390/proteomes7020014
APA StyleHurwitz, S. N., & Meckes, D. G., Jr. (2019). Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes, 7(2), 14. https://doi.org/10.3390/proteomes7020014




