Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing, Experimental Treatments, and ROS Induction Assays
2.2. Extraction, Solubilization, and Digestion of Proteins
2.3. Online 2D LC-MS/MS Analysis
2.4. Database Searching and Label-Free Quantification Analysis
2.5. qRT-PCR
2.6. Statistical Analyses
3. Results
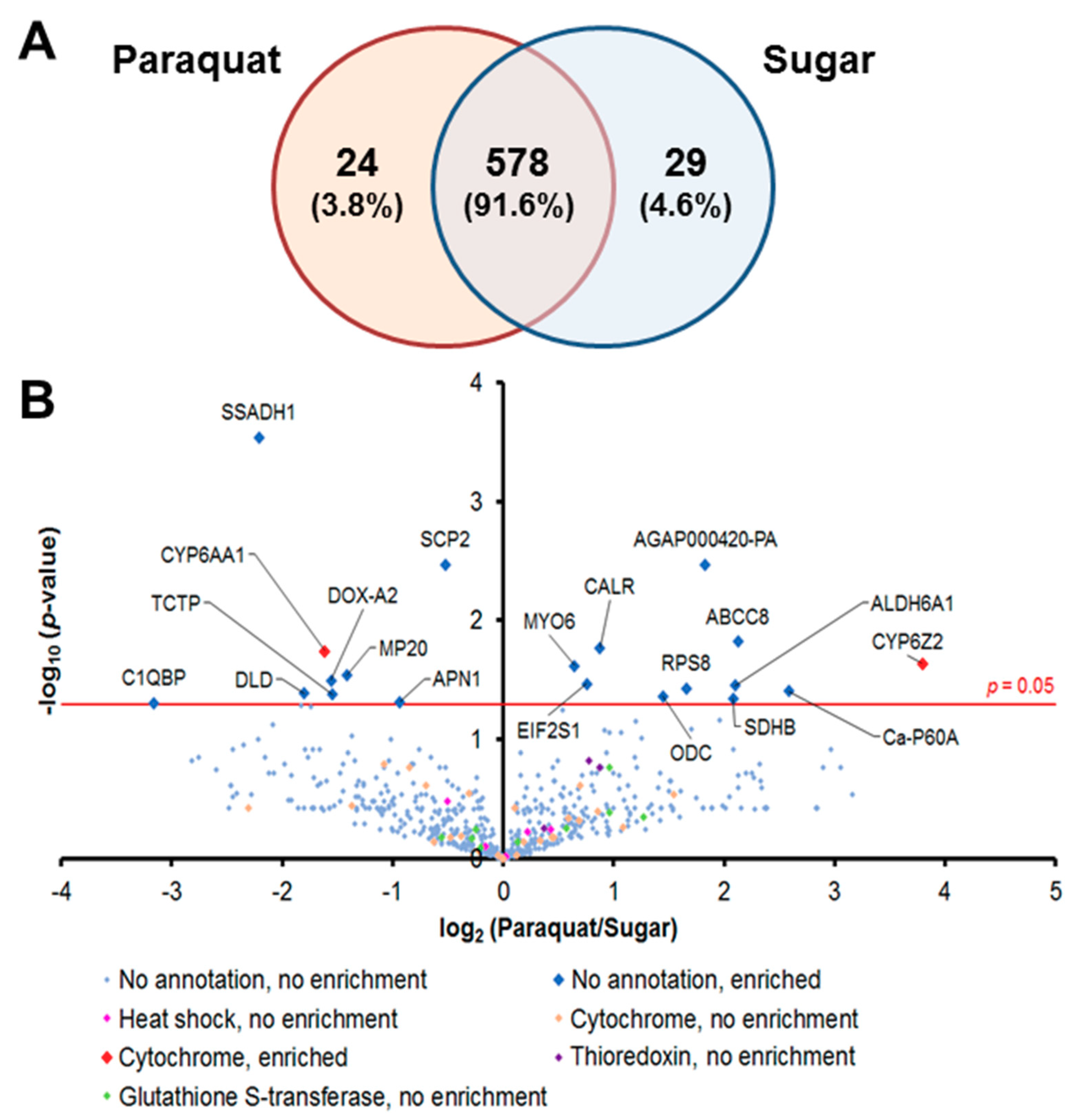
3.1. Global Proteomic Profiles of Midgut Epithelial Cells under Pqt-Induced Oxidative Stress Are Largely Conserved
3.2. Antioxidant Proteins Are Not Involved in the Regulation of Pqt-Induced Oxidative Stress in An. Gambiae Midguts
3.3. Evidence of an Endoplasmic Reticulum (ER) Stress Response in Pqt-Treated Midguts
3.4. Proteins Involved in the Detoxification Process Are Enriched in Pqt-Treated Midguts
3.5. P. Falciparum Ookinete Invasion of An. Gambiae Midguts Does Not Upregulate Trx and GSH-Dependent Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baton, L.A.; Ranford-Cartwright, L.C. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 2005, 21, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lensen, A.H.W.; Bolmer-Van de Vegte, M.; van Gemert, G.J.; Eling, W.M.; Sauerwein, R.W. Leukocytes in a Plasmodium falciparum-infected blood meal reduce transmission of malaria to anopheles mosquitoes. Infect. Immun. 1997, 65, 3834–3837. [Google Scholar] [PubMed]
- Naotunne, T.S.; Karunaweera, N.D.; Mendis, K.N.; Carter, R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 1993, 78, 555–562. [Google Scholar] [PubMed]
- Peterson, T.M.L.; Gow, A.J.; Luckhart, S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic. Biol. Med. 2007, 42, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Graça-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.C.; Paes, M.C.; Sorgine, M.H.F.; Oliveira, M.F.; Oliveira, P.L. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Thompson, J.; Kafatos, F.C.; Barillas-Mury, C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: The time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000, 19, 6030–6040. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Barillas-Mury, C. Implications of time bomb model of ookinete invasion of midgut cells. Insect Biochem. Mol. Biol. 2002, 32, 1311–1316. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, L.; Han, Y.S.; Barillas-Mury, C. Inducible peroxidases mediate nitration of Anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J. Biol. Chem. 2004, 279, 53475–53482. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rodríguez, J.; Franke-Fayard, B.; Dinglasan, R.R.; Janse, C.J.; Pastrana-Mena, R.; Waters, A.P.; Coppens, I.; Rodríguez-Orengo, J.F.; Srinivasan, P.; Jacobs-Lorena, M.; et al. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog. 2009, 5, e1000302. [Google Scholar] [CrossRef]
- Pastrana-Mena, R.; Dinglasan, R.R.; Franke-Fayard, B.; Vega-Rodríguez, J.; Fuentes-Caraballo, M.; Baerga-Ortiz, A.; Coppens, I.; Jacobs-Lorena, M.; Janse, C.J.; Serrano, A.E. Glutathione reductase-null malaria parasites have normal blood stage growth but arrest during development in the mosquito. J. Biol. Chem. 2010, 285, 27045–27056. [Google Scholar] [CrossRef] [PubMed]
- Kanzok, S.M.; Fechner, A.; Bauer, H.; Ulschmid, J.K.; Müller, H.M.; Botella-Munoz, J.; Schneuwly, S.; Schirmer, R.; Becker, K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 2001, 291, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.M.L.; Luckhart, S. A Mosquito 2-cys peroxiredoxin protects against nitrosative and oxidative stresses associated with malaria parasite infection. Free Radic. Biol. Med. 2006, 40, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Cutierrez, G.; Barillas-Mury, C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Turturice, B.A.; Lamm, M.A.; Tasch, J.J.; Zalewski, A.; Kooistra, R.; Schroeter, E.H.; Sharma, S.; Kawazu, S.-I.; Kanzok, S.M. Expression of cytosolic peroxiredoxins in Plasmodium berghei ookinetes is regulated by environmental factors in the mosquito bloodmeal. PLoS Pathog. 2013, 9, e1003136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Molina-cruz, A.; Gupta, L.; Gupta, J.; Barillas-Mury, C. A Peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 2010, 327, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.L.S.; Oliveira, J.H.M.; Oliveira, G.A.; Andersen, J.F.; Oliveira, M.F.; Oliveira, P.L.; Barillas-Mury, C. Mitochondrial reactive oxygen species modulate mosquito susceptibility to Plasmodium infection. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Bahia, A.C.; Oliveira, J.H.M.; Kubota, M.S.; Araújo, H.R.C.; Lima, J.B.P.; Ríos-Velásquez, C.M.; Lacerda, M.V.G.; Oliveira, P.L.; Traub-Csekö, Y.M.; Pimenta, P.F.P. The role of reactive oxygen species in Anopheles aquasalis response to Plasmodium vivax infection. PLoS ONE 2013, 8, e57014. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, L.A.; Oo, M.M.; Jacobs-Lorena, M. Peritrophic matrix proteins of Anopheles gambiae and Aedes aegypti. Insect Mol. Biol. 1996, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Galun, R.; Avi-Dor, Y.; Bar-Zeev, M. Feeding response in Aedes aegypti: Stimulation by adenosine triphosphate. Science 1963, 142, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, P.F.; Rudin, W. The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J. Parasitol. 1992, 78, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Drexler, A.L.; Pietri, J.E.; Pakpour, N.; Hauck, E.; Wang, B.; Glennon, E.K.K.; Georgis, M.; Riehle, M.A.; Luckhart, S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014, 10, e1004231. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; King, J.G.; Tweedell, R.E.; Jost, P.J.; Boddey, J.A.; Dinglasan, R.R. The acute transcriptomic and proteomic response of HC-04 hepatoma cells to hepatocyte growth factor and its implications for Plasmodium falciparum sporozoite invasion. Mol. Cell. Proteom. 2014, 13, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Tweedell, R.; Tao, D.; Dinglasan, R.R. The cellular and proteomic response of primary and immortalized murine kupffer cells following immune stimulation diverges from that of monocyte-derived macrophages. Proteomics 2015, 15, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Ubaida-Mohien, C.; Mathias, D.K.; King, J.G.; Pastrana-Mena, R.; Tripathi, A.; Goldowitz, I.; Graham, D.R.; Moss, E.; Marti, M.; et al. Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol. Cell. Proteom. 2014, 13, 2705–2724. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.F.; Paton, N.W.; Lilley, K.S.; Binz, P.-A.; Jr, R.K.J.; Jones, A.R. The minimum information about a proteomics experiment (MIAPE). Nat. Biotechnol. 2007, 25, 887–893. [Google Scholar] [PubMed]
- Vizcaíno, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Nkondjio, C.; Poupardin, R.; Tene, B.F.; Kopya, E.; Costantini, C.; Awono-Ambene, P.; Wondji, C.S. Investigation of mechanisms of bendiocarb resistance in Anopheles gambiae populations from the city of Yaoundé, Cameroon. Malar. J. 2016, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E.; Bissinger, B.W.; Egekwu, N.; Donohue, K.V.; Khalil, S.M.; Roe, R.M. First transcriptome of the testis-vas deferens-male accessory gland and proteome of the spermatophore from Dermacentor variabilis (Acari: Ixodidae). PLoS ONE 2011, 6, e24711. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Kono, T.; Evans-Molina, C. Nitric oxide stress and activation of AMP-activated protein kinase impair β-cell sarcoendoplasmic reticulum calcium ATPase 2b activity and protein stability. Cell Death Dis. 2015, 6, e1790. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Evangelista, A.; Cohen, R.A. Targeting the redox regulation of SERCA in vascular physiology and disease. Curr. Opin. Pharmacol. 2010, 10, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Zhou, Y.; Lee, J.; Lee, J.; Ozcan, U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc. Natl. Acad. Sci. USA 2010, 107, 19320–19325. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, M.A.; Stevens, J.L.; Lawrence, J.W. Xenobiotic perturbation of ER stress and the unfolded protein response. Toxicol. Pathol. 2013, 41, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Kageyama, K.; Kondo, T. Overexpression of calreticulin sensitizes SERCA2a to oxidative stress. Biochem. Biophys. Res. Commun. 2005, 329, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bowes, R.C.; Van De Water, B.; Sillence, C.; Nagelkerke, J.F.; Stevens, J.L. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J. Biol. Chem. 1997, 272, 21751–21759. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, L.W.; Molinari, M. N-glycan processing in ER quality control. J. Cell Sci. 2006, 119, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative stress-induced calreticulin expression and translocation: New insights into the destruction of melanocytes. J. Investig. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, J.H.J.; Rodland, G.E.; Boe, C.A.; Haland, T.W.; Sunnerhagen, P.; Grallert, B.; Boye, E. Stress-induced inhibition of translation independently of EIF2 phosphorylation. J. Cell Sci. 2015, 128, 4420–4427. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Scheuner, D.; Han, J.; Song, B.; Ribick, M.; Wang, J.; Gildersleeve, R.D.; Pennathur, S.; Kaufman, R.J. Translation attenuation through EIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metab. 2009, 10, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Seaman, J.A.; Alout, H.; Meyers, J.I.; Stenglein, M.D.; Dabiré, R.K.; Lozano-Fuentes, S.; Burton, T.A.; Kuklinski, W.S.; Black, W.C.; Foy, B.D. Age and prior blood feeding of Anopheles gambiae influences their susceptibility and gene expression patterns to ivermectin-containing blood meals. BMC Genom. 2015, 16, 797. [Google Scholar] [CrossRef] [PubMed]
- ter Beek, J.; Guskov, A.; Slotboom, D.J. Structural diversity of ABC transporters. J. Gen. Physiol. 2014, 143, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.M.; George, A.M. The ABC Transporter structure and mechanism: Perspectives on recent Research. Cell. Mol. Life Sci. 2004, 61, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Ackrell, B.A. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett 2000, 466, 1–5. [Google Scholar] [CrossRef]
- Oyedotun, K.S.; Lemire, B.D. The Quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. homology modeling, cofactor docking, and molecular dynamics simulation studies. J. Biol. Chem. 2004, 279, 9424–9431. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Kanzok, S.M.; Schirmer, R.H. Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: Isolation and characterization of A second thioredoxin in D. melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J. Biol. Chem. 2002, 277, 17457–17463. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Gromer, S.; Urbani, A.; Schnolzer, M.; Schirmer, R.H.; Muller, H.-M. Thioredoxin reductase from the malaria mosquito Anopheles gambiae. Eur. J. Biochem. 2003, 270, 4272–4281. [Google Scholar] [CrossRef] [PubMed]
- Prapanthadara, L.A.; Hemingway, J.; Ketterman, A.J. Partial purification and characterization of glutathione S-transferases involved in DDT resistance from the mosquito Anopheles gambiae. Pestic. Biochem. Physiol. 1993, 47, 119–133. [Google Scholar] [CrossRef]
- Ranson, H.; Rossiter, L.; Ortelli, F.; Jensen, B.; Wang, X.; Roth, C.W.; Collins, F.H.; Hemingway, J. Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem. J. 2001, 359, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Peluso, I.; Rendina, R.; Digilio, A.; Furia, M. The clot gene of Drosophila melanogaster encodes a conserved member of the thioredoxin-like protein superfamily. Mol. Genet. Genomics 2003, 268, 692–697. [Google Scholar] [PubMed]
- Mercer, S.W.; Burke, R. Evidence for a role for the putative drosophila HGRX1 orthologue in copper homeostasis. BioMetals 2016, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, R.; Muralidhara. Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Arch. Insect Biochem. Physiol. 2013, 83, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan, R.R.; Devenport, M.; Florens, L.; Johnson, J.R.; Mchugh, C.A.; Carucci, D.J.; Yates, J.R.; Jacobs-Lorena, M. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem. Mol. Biol. 2009, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Brüne, B.; von Knethen, A.; Sandau, K.B. Nitric oxide and its role in apoptosis. Eur. J. Pharmacol. 1998, 351, 261–272. [Google Scholar] [CrossRef]
- Gouagna, L.C.; Mulder, B.; Noubissi, E.; Tchuinkam, T.; Verhave, J.P.; Boudin, C. The early sporogonic cycle of Plasmodium falciparum in laboratory-infected Anopheles gambiae: An estimation of parasite efficacy. Trop. Med. Int. Health 1998, 3, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sinden, R.E.; Billingsley, P.F. Plasmodium invasion of mosquito cells: Hawk or dove? Trends Parasitol. 2001, 17, 209–212. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Shiao, S.H.; Levashina, E.A. Mosquito midguts and malaria: Cell biology, compartmentalization and immunology. Parasite Immunol. 2006, 28, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Vega-Rodríguez, J.; Jacobs-Lorena, M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem. Inst. Oswaldo Cruz 2014, 1–18. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, J.J.M.; Brenner, M.B.; Thomas, D.Y.; Williams, D.B. Calnexin: A membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 1994, 19, 124–128. [Google Scholar] [CrossRef]
- Helenius, A.; Trombetta, E.S.; Hebert, D.N.; Simons, J.F. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997, 7, 193–200. [Google Scholar]
- Miyawaki, A.; Llopis, J.; Heim, R.; JM, M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Rice, W.J.; Green, N.M. The mechanism of Ca2+ transport by sarco(endo) plasmic reticulum Ca2+-ATPases. J. Biol. Chem. 1997, 272, 28815–28818. [Google Scholar] [CrossRef] [PubMed]
- Pozzan, T.; Rizzuto, R.; Volpe, P.; Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 1994, 74, 595–636. [Google Scholar] [CrossRef] [PubMed]
- Ostwald, T.J.; MacLennan, D.H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 1974, 249, 974–979. [Google Scholar] [PubMed]
- Baksh, S.; Michalak, M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 1991, 266, 21458–21465. [Google Scholar] [PubMed]
- Marva, E.; Chevion, M.; Golenser, J. The effect of free radicals induced by paraquat and copper on the in vitro development of Plasmodium falciparum. Free Radic. Res. Commun. 1991, 12–13, 137–146. [Google Scholar] [CrossRef]
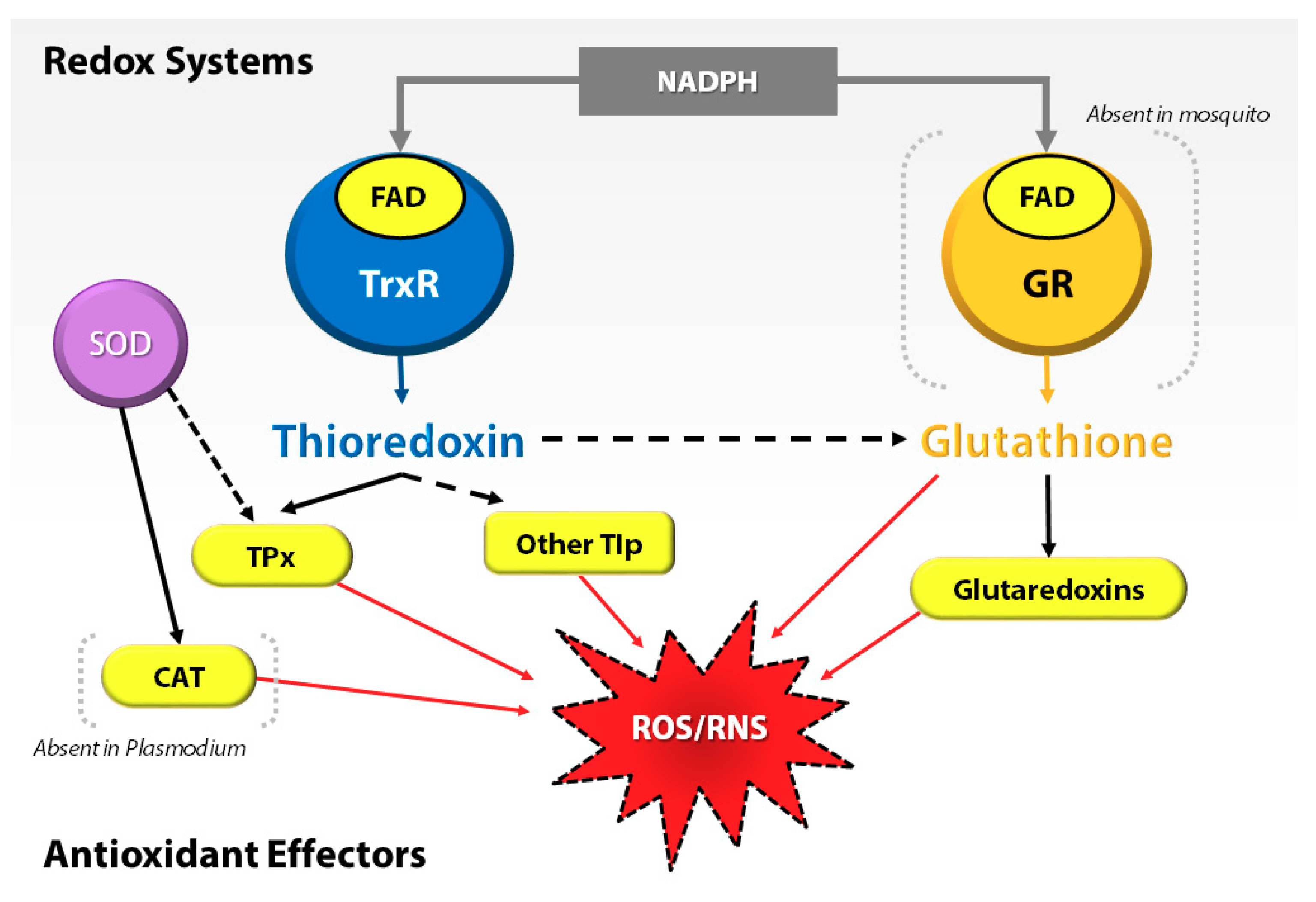
| Protein Description | Fold Change | p-Value | Function |
|---|---|---|---|
| ABCC8 (AGAP008437) ATP-binding cassette transporter (ABC transporter) family C member 8 | 4.37 | 0.015 | Upregulated in bendiocarb resistance Anopheles gambiae, a detoxification gene [31] |
| ALDH6A1 (AGAP002499) Methylmalonate-semialdehyde dehydrogenase (acylating), mitochondrial | 4.28 | 0.035 | Classified as environmental and oxidative stress proteins [32] |
| Ca-P60A (AGAP006186) Calcium-transporting ATPase sarcoplasmic/endoplasmic reticulum type | 5.99 | 0.039 | Function impaired by oxidative stress [33,34,35,36] |
| CRT (AGAP004212) Calreticulin | 1.64 | 0.017 | Ca2+ homeostasis [37,38,39] and pro-apoptotic protein [40] |
| EIF2S1 (AGAP011190) Eukaryotic translation initiation factor 2 subunit alpha | 1.69 | 0.034 | Conserved in eukaryotes, the phosphorylation form of this protein serves as a signal of cell survival by attenuating the translation of mRNA [41,42,43] |
| ODC (AGAP011806) Ornithine decarboxylase | 2.72 | 0.043 | Upregulated after ivermectin-containing blood meals [44] |
| SDHB (AGAP007309) Succinate dehydrogenase (ubiquinone) iron-sulfur subunit | 4.23 | 0.045 | Ferredoxin balance system |
| Transcript/Accession ID | Function/Annotation | Response to P. Falciparum Blood Meal Ingestion. |
|---|---|---|
| Thioredoxin-1 (Trx-1; AGAP009584) | Dithiol–disulfide exchange reaction with GSSG to produce GSH [12] | None (p-value = 0.3088) |
| Thioredoxin-2 (Trx-2; AGAP007201) | Antioxidative function as electron donor to TPx [49] | None (p-value = 0.7309) |
| Thioredoxin reductase (TrxR; AGAP000565) | Key enzyme of the Trx system responsible for replenishing Trx-1 [50] | None (p-value = 0.8806) |
| Thioredoxin peroxidase-1 (TPx-1; AGAP000396) | Antioxidant enzyme that catalyzes peroxides [49] | None (p-value = 0.7976) |
| Atypical 2-Cys peroxiredoxin (Peroxiredoxin V; PrxV; AGAP001325) | Antioxidant enzyme that protects against ROS/RNS [13] | None (p-value = 0.8736) |
| Glutathione synthase (GS; AGAP000534) | Involved in the GSH biosynthesis pathway | None (p-value = 0.8515) |
| Glutathione peroxidase (GPx; AGAP004247) | Antioxidant enzyme that catalyzes peroxides [14] | None (p-value = 0.8998) |
| Glutathione S-transferase delta class 1 (GSTD1; AGAP004164) | Implicated in insecticide resistance and detoxifies xenobiotic compounds [51,52] | None (p-value = 0.9195) |
| Glutaredoxin-1 (Grx-1; AGAP011107) | Essential component of the GSH system [53,54] | None (p-value = 0.4838) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarimo, B.B.; Law, H.C.H.; Tao, D.; Pastrana-Mena, R.; Kanzok, S.M.; Buza, J.J.; Dinglasan, R.R. Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response. Proteomes 2018, 6, 47. https://doi.org/10.3390/proteomes6040047
Tarimo BB, Law HCH, Tao D, Pastrana-Mena R, Kanzok SM, Buza JJ, Dinglasan RR. Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response. Proteomes. 2018; 6(4):47. https://doi.org/10.3390/proteomes6040047
Chicago/Turabian StyleTarimo, Brian B., Henry Chun Hin Law, Dingyin Tao, Rebecca Pastrana-Mena, Stefan M. Kanzok, Joram J. Buza, and Rhoel R. Dinglasan. 2018. "Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response" Proteomes 6, no. 4: 47. https://doi.org/10.3390/proteomes6040047
APA StyleTarimo, B. B., Law, H. C. H., Tao, D., Pastrana-Mena, R., Kanzok, S. M., Buza, J. J., & Dinglasan, R. R. (2018). Paraquat-Mediated Oxidative Stress in Anopheles gambiae Mosquitoes Is Regulated by An Endoplasmic Reticulum (ER) Stress Response. Proteomes, 6(4), 47. https://doi.org/10.3390/proteomes6040047








