Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms
Abstract
1. Introduction
2. Proteomic Characterization of AD Amyloid Plaques and Neurofibrillary Tangles by Laser Capture Microdissection
3. Deep Analysis of Aggregated Proteome in AD by Differential Extraction
4. Implication of Disease Mechanisms by Aggregated Proteins in AD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- James, B.D.; Leurgans, S.E.; Hebert, L.E.; Scherr, P.A.; Yaffe, K.; Bennett, D.A. Contribution of alzheimer disease to mortality in the united states. Neurology 2014, 82, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; Bonito-Oliva, A.; Sakmar, T.P. Update on alzheimer’s disease therapy and prevention strategies. Annu. Rev. Med. 2017, 68, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E. The genetics of alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Bras, J.; Hardy, J. Snapshot: Genetics of alzheimer’s disease. Cell 2013, 155, 968. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.; Stefansson, H.; Jonsson, T.; Johannsdottir, H.; Ingason, A.; Helgason, H.; Sulem, P.; Magnusson, O.T.; Gudjonsson, S.A.; Unnsteinsdottir, U.; et al. Loss-of-function variants in abca7 confer risk of alzheimer’s disease. Nat. Genet. 2015, 47, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; van der Lee, S.J.; Naj, A.C.; Bellenguez, C.; Badarinarayan, N.; Jakobsdottir, J.; Kunkle, B.W.; Boland, A.; Raybould, R.; Bis, J.C.; et al. Rare coding variants in plcg2, abi3, and trem2 implicate microglial-mediated innate immunity in alzheimer’s disease. Nat. Genet. 2017, 49, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Wang, Y. Trem2 variants: New keys to decipher alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wetzel-Smith, M.K.; Hunkapiller, J.; Bhangale, T.R.; Srinivasan, K.; Maloney, J.A.; Atwal, J.K.; Sa, S.M.; Yaylaoglu, M.B.; Foreman, O.; Ortmann, W.; et al. A rare mutation in unc5c predisposes to late-onset alzheimer’s disease and increases neuronal cell death. Nat. Med. 2014, 20, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Eimer, W.A.; Kumar, D.K.V.; Shanmugam, N.K.N.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; Gyorgy, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s disease-associated beta-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018, 99, 56–97. [Google Scholar] [CrossRef] [PubMed]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale analysis of independent alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018, 99, 64–82.e67. [Google Scholar] [CrossRef] [PubMed]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and parkinson diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National institute on aging-alzheimer’s association guidelines for the neuropathologic assessment of alzheimer’s disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Ashe, K.H.; Zahs, K.R. Probing the biology of alzheimer’s disease in mice. Neuron 2010, 66, 631–645. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N. Animal models of alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement. (N Y) 2018, 4, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Volloch, V.; Rits, S. Results of beta secretase-inhibitor clinical trials support amyloid precursor protein-independent generation of beta amyloid in sporadic alzheimer’s disease. Med. Sci. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of solanezumab for mild dementia due to alzheimer’s disease. N. Eng. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Troncoso, J. Asymptomatic alzheimer’s disease: A prodrome or a state of resilience? Curr. Alzheimer Res. 2011, 8, 330–335. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The cellular phase of alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-synuclein in filamentous inclusions of lewy bodies from parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated tdp-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, T.; Renier, N.; Bettayeb, K.; Greengard, P.; Tessier-Lavigne, M.; Flajolet, M. Three-dimensional study of alzheimer’s disease hallmarks using the idisco clearing method. Cell Rep. 2016, 16, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Emmert-Buck, M.R.; Bonner, R.F.; Smith, P.D.; Chuaqui, R.F.; Zhuang, Z.; Goldstein, S.R.; Weiss, R.A.; Liotta, L.A. Laser capture microdissection. Science 1996, 274, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gozal, Y.M.; Cheng, D.; Duong, D.M.; Lah, J.J.; Levey, A.I.; Peng, J. Merger of laser capture microdissection and mass spectrometry: A window into the amyloid plaque proteome. Methods Enzymol. 2006, 412, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, D.A.T.; Stingl, C.; Luider, T.M. Laser capture microdissection of fluorescently labeled amyloid plaques from alzheimer’s disease brain tissue for mass spectrometric analysis. Methods Mol. Biol. 2015, 1243, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Woltjer, R.L.; Cimino, P.J.; Pan, C.; Montine, K.S.; Zhang, J.; Montine, T.J. Proteomic analysis of neurofibrillary tangles in alzheimer disease identifies gapdh as a detergent-insoluble paired helical filament tau binding protein. FASEB J. 2005, 19, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Nayak, S.; Pires, G.; Ueberheide, B.; Wisniewski, T. Isolation of amyloid plaques and neurofibrillary tangles from archived alzheimer’s disease tissue using laser-capture microdissection for downstream proteomics. Methods Mol. Biol. 2018, 1723, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Hardy, J.; Fischbeck, K.H. Toxic proteins in neurodegenerative disease. Science 2002, 296, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Guo, J.L.; Narasimhan, S.; Changolkar, L.; He, Z.; Stieber, A.; Zhang, B.; Gathagan, R.J.; Iba, M.; McBride, J.D.; Trojanowski, J.Q.; et al. Unique pathological tau conformers from alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016, 213, 2635–2654. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Hales, C.M.; Chen, P.C.; Gozal, Y.; Dammer, E.B.; Fritz, J.J.; Wang, X.; Xia, Q.; Duong, D.M.; Street, C.; et al. U1 small nuclear ribonucleoprotein complex and rna splicing alterations in alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16562–16567. [Google Scholar] [CrossRef] [PubMed]
- Gozal, Y.M.; Duong, D.M.; Gearing, M.; Cheng, D.; Hanfelt, J.J.; Funderburk, C.; Peng, J.; Lah, J.J.; Levey, A.I. Proteomics analysis reveals novel components in the detergent-insoluble subproteome in alzheimer’s disease. J. Proteome Res. 2009, 8, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, W.J.; Roses, A.D. Apolipoprotein e and alzheimer disease. Proc. Natl. Acad. Sci. USA 1995, 92, 4725–4727. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, A.; Lashley, T.; Ng, D.; Meyerson, J.; Braendgaard, H.; Plant, G.; Bojsen-Moller, M.; Holton, J.; Frangione, B.; Revesz, T.; et al. Preferential association of serum amyloid p component with fibrillar deposits in familial british and danish dementias: Similarities with alzheimer’s disease. J. Neurol. Sci. 2007, 257, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L.; Frenkel, D. Immunology and immunotherapy of alzheimer’s disease. Nat. Rev. Immunol. 2006, 6, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.P.; Guthrie, C. Mechanical devices of the spliceosome: Motors, clocks, springs, and things. Cell 1998, 92, 315–326. [Google Scholar] [CrossRef]
- Hales, C.M.; Seyfried, N.T.; Dammer, E.B.; Duong, D.; Yi, H.; Gearing, M.; Troncoso, J.C.; Mufson, E.J.; Thambisetty, M.; Levey, A.I.; et al. U1 small nuclear ribonucleoproteins (snrnps) aggregate in alzheimer’s disease due to autosomal dominant genetic mutations and trisomy 21. Mol. Neurodegener. 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Dammer, E.B.; Deng, Q.; Duong, D.M.; Gearing, M.; Troncoso, J.C.; Thambisetty, M.; Lah, J.J.; Shulman, J.M.; Levey, A.I.; et al. Changes in the detergent-insoluble brain proteome linked to amyloid and tau in alzheimer’s disease progression. Proteomics 2016, 16, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.M.; Ahmoudi, J. Amyloid-beta: A crucial factor in alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Zhang, K.; Brodbeck, J.; Daub, A.C.; Sharma, P.; Finkbeiner, S.; Cui, B.; Mucke, L. Tau reduction prevents abeta-induced defects in axonal transport. Science 2010, 330, 198. [Google Scholar] [CrossRef] [PubMed]
- Zempel, H.; Luedtke, J.; Kumar, Y.; Biernat, J.; Dawson, H.; Mandelkow, E.; Mandelkow, E.M. Amyloid-beta oligomers induce synaptic damage via tau-dependent microtubule severing by ttll6 and spastin. EMBO J. 2013, 32, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Ebneth, A.; Godemann, R.; Stamer, K.; Illenberger, S.; Trinczek, B.; Mandelkow, E.-M.; Mandelkow, E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for alzheimer’s disease. J. Cell Biol. 1998, 143, 777–794. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.; Lorenzi, T.; Marzetti, E.; Landi, F.; Vetrano, D.L.; Settanni, S.; Antocicco, M.; Bonassi, S.; Valdiglesias, V.; Bernabei, R.; et al. Association of frailty with the serine protease htra1 in older adults. Exp. Gerontol. 2016, 81, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.W. The spliceosome: The most complex macromolecular machine in the cell? BioEssays 2003, 25, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Dammer, E.B.; Diner, I.; Yi, H.; Seyfried, N.T.; Gearing, M.; Glass, J.D.; Montine, T.J.; Levey, A.I.; Lah, J.J. Aggregates of small nuclear ribonucleic acids (snrnas) in alzheimer’s disease. Brain Pathol. 2014, 24, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Diner, I.; Hales, C.M.; Bishof, I.; Rabenold, L.; Duong, D.M.; Yi, H.; Laur, O.; Gearing, M.; Troncoso, J.; Thambisetty, M.; et al. Aggregation properties of the small nuclear ribonucleoprotein u1-70k in alzheimer disease. J. Biol. Chem. 2014, 289, 35296–35313. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of rna granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Kato, M.; Xiang, S.; Wu, L.; Theodoropoulos, P.; Mirzaei, H.; Han, T.; Xie, S.; Corden, J.L.; McKnight, S.L. Phosphorylation-regulated binding of rna polymerase ii to fibrous polymers of low-complexity domains. Cell 2013, 155, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Chen, P.C.; Hales, C.M.; Wu, Z.; Pagala, V.; High, A.A.; Levey, A.I.; Lah, J.J.; Peng, J. Integrated approaches for analyzing u1-70k cleavage in alzheimer’s disease. J. Proteome Res. 2014, 13, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.S.; Nunes, A.M.; Baum, J.; Brodsky, B. A peptide study of the relationship between the collagen triple-helix and amyloid. Biopolymers 2012, 97, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.V.; Ruseva, M.M.; Harris, C.L.; Morgan, B.P.; Donev, R.M. Implication of complement system and its regulators in alzheimer’s disease. Curr. Neuropharmacol. 2009, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bonham, L.W.; Desikan, R.S.; Yokoyama, J.S. The relationship between complement factor c3, apoe epsilon4, amyloid and tau in alzheimer’s disease. Acta Neuropathol. Commun. 2016, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chatterjee, M.; Baguley, T.D.; Brouillette, J.; Kurup, P.; Ghosh, D.; Kanyo, J.; Zhang, Y.; Seyb, K.; Ononenyi, C.; et al. Inhibitor of the tyrosine phosphatase step reverses cognitive deficits in a mouse model of alzheimer’s disease. PLoS Biol. 2014, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.B.; Berman, D.E.; Myaeng, J.; Staniszewski, A.; Arancio, O.; Di Paolo, G.; Kim, T.W. Reduction of synaptojanin 1 ameliorates synaptic and behavioral impairments in a mouse model of alzheimer’s disease. J. Neurosci. 2012, 32, 15271–15276. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-Cortés, H.J.; Nogueras-Ortiz, C.J.; Gearing, M.; Arnold, S.E.; Vega, I.E. Amphiphysin-1 protein level changes associated with tau-mediated neurodegeneration. Neuroreport 2012, 23, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Zimbrón, L.F.; Rivas-Arancibia, S. Syntaxin 5 overexpression and β-amyloid 1-42 accumulation in endoplasmic reticulum of hippocampal cells in rat brain induced by ozone exposure. BioMed. Res. Inter. 2016, 2016, 2125643. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Roy, D. Structural insight into grip1-pdz6 in alzheimer’s disease: Study from protein expression data to molecular dynamics simulations. J. Biomol. Struct. Dyn. 2017, 35, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Levault, K.R.; Brewer, G.J. Relative importance of redox buffers gsh and nad(p)h in age-related neurodegeneration and alzheimer disease-like mouse neurons. Aging Cell 2014, 13, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Shaerzadeh, F.; Motamedi, F.; Minai-Tehrani, D.; Khodagholi, F. Monitoring of neuronal loss in the hippocampus of abeta-injected rat: Autophagy, mitophagy, and mitochondrial biogenesis stand against apoptosis. Neuromol. Med. 2014, 16, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.V.; Fuhrmann, N. Dominant optic atrophy, opa1, and mitochondrial quality control: Understanding mitochondrial network dynamics. Mol. Neurodegener. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Bishof, I.; Dammer, E.B.; Duong, D.M.; Kundinger, S.R.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Rna-binding proteins with basic-acidic dipeptide (bad) domains self-assemble and aggregate in alzheimer’s disease. J. Biol. Chem. 2018, 293, 11047–11066. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Wada, T.; Nagai, M.; Kojima, F.; Harada, S.; Takeuchi, T.; Takahashi, H.; Hirokawa, K.; Tsumita, T. Increased gamma-aminobutyrate aminotransferase activity in brain of patients with alzheimer’s disease. Chem. Pharm. Bull. 1990, 38, 1748–1749. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Xiong, D.D.; Patel, T.; Sasaki, Y.; Wang, Y.; Bauer, A.Q.; Singh, R.; Finn, S.L.; Culver, J.P.; Milbrandt, J.; et al. Nmnat1 protects neuronal function without altering phospho-tau pathology in a mouse model of tauopathy. Ann. Clin. Transl. Neurol. 2016, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, R.; Viht, K.; Balaspiri, L.; Bogdanovic, N.; Saar, K.; Soomets, U.; Land, T.; Zilmer, M.; Karelson, E.; Langel, U. Amyloid precursor protein carboxy-terminal fragments modulate g-proteins and adenylate cyclase activity in alzheimer’s disease brain. Mol. Brain Res. 2003, 117, 73–82. [Google Scholar] [CrossRef]
- Tong, Y.; Sun, Y.; Tian, X.; Zhou, T.; Wang, H.; Zhang, T.; Zhan, R.; Zhao, L.; Kuerban, B.; Li, Z.; et al. Phospholipid transfer protein (pltp) deficiency accelerates memory dysfunction through altering amyloid precursor protein (app) processing in a mouse model of alzheimer’s disease. Hum. Mol. Genet. 2015, 24, 5388–5403. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Chouliaras, L.; Grover, A.; Liang, W.S.; Hauns, K.; Rogers, J.; Coleman, P.D. Reduced ran expression and disrupted transport between cytoplasm and nucleus; a key event in alzheimer’s disease pathophysiology. PLoS ONE 2013, 8, e53349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Su, M.; Lucast, L.; Liu, L.; Netzer, W.J.; Gandy, S.E.; Cai, D. Dynamin 1 regulates amyloid generation through modulation of bace-1. PLoS ONE 2012, 7, e45033. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Arima, K.; Mizusawa, H.; Satoh, J. Close association of water channel aqp1 with amyloid-beta deposition in alzheimer disease brains. Acta Neuropathol. 2008, 116, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Li, Y.I.; Wong, G.; Humphrey, J.; Wang, M.; Ramdhani, S.; Wang, Y.C.; Ng, B.; Gupta, I.; Haroutunian, V.; et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in alzheimer’s disease susceptibility. Nat. Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
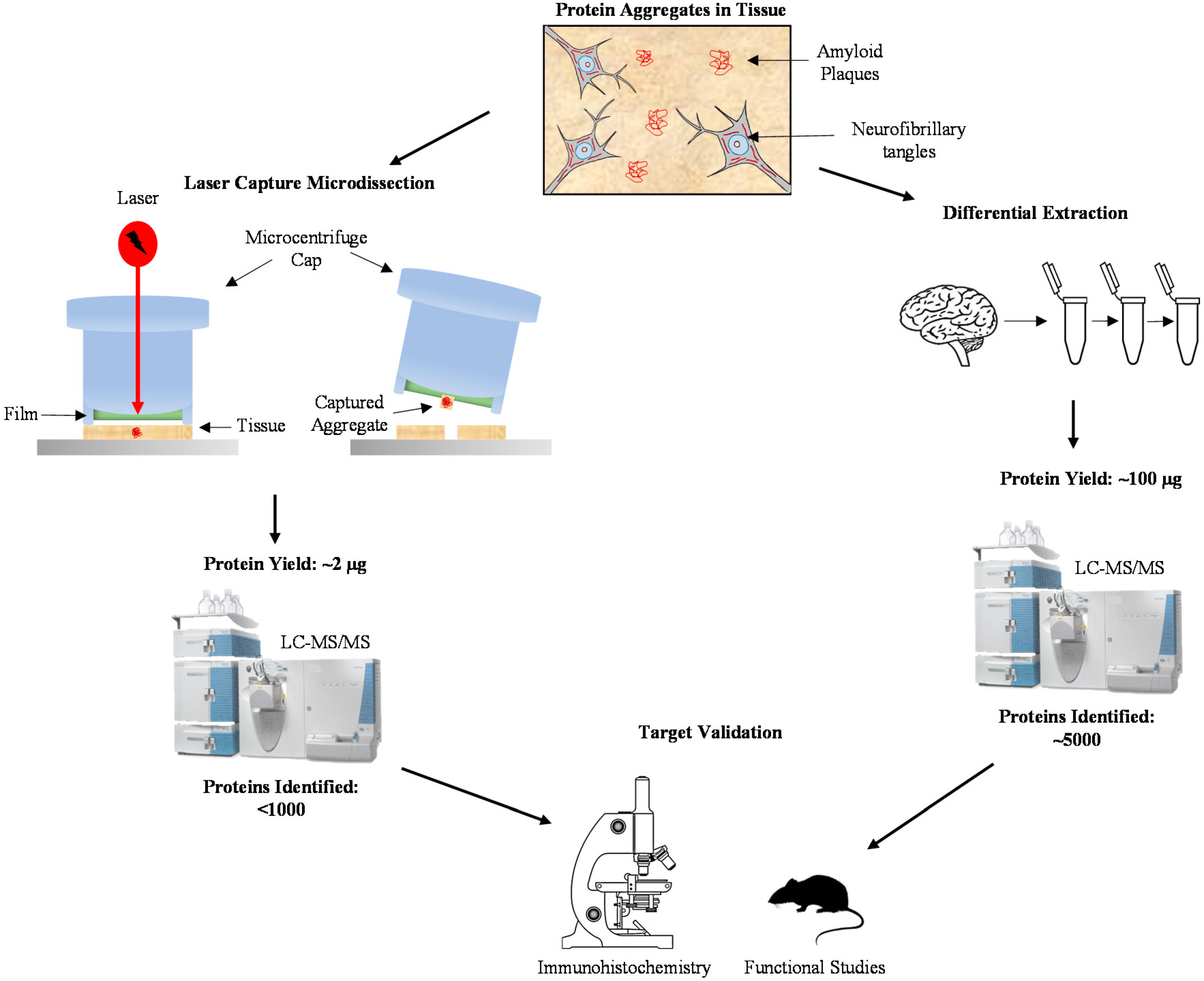
| Technique | Protein Yield | Instruments Required | Number of Proteins Identified * | Advantages | Disadvantages |
|---|---|---|---|---|---|
| LCM | ~2 µg from 1000 plaques | Fluorescent Microscope with Laser Capture capability LC-MS/MS | 155–900 [33,34,35] | (1) Precise collection of cellular components (2) Conservation of tissue integrity (3) Cellular region comparison within the same tissue | (1) Small amount of protein recovery (2) Extensive time required for LCM |
| Differential fractionation | 1% of total protein input (e.g., 100 µg from 10 mg of tissue) | Centrifuge LC-MS/MS | 512–4216 [39,40,49] | (1) A sufficient amount of protein can be extracted from individual samples (2) Flexible extraction methods using different combinations of detergents | (1) Detergent soluble aggregate proteins may not be included in the MS analysis (2) Contamination of the aggregated proteome by other detergent insoluble components |
| Protein | GeneBank™ Accession Number | Association with AD |
|---|---|---|
| Identified by Bai, B., et al., PNAS, 2013 [39] | ||
| Collagen Type XXV, alpha 1 isoform 2 | NP_000032.1 | [63] |
| Cellular retinoic acid binding protein | NP_004369.1 | [48] |
| Dystrobrevin alpha | NP_009224.2 | [48] |
| Complement component 4a preproprotein | NP_116757.2 | [64] |
| Complement component 3 | NP_000055.2 | [65] |
| Cyclin G-associated kinase | NP_005246.2 | Not Found |
| Protein tyrosine phosphatase, zeta1 | NP_002842.2 | [66] |
| T-cell activation protein phosphatase 2C | NP_644812.1 | Not Found |
| Synaptojanin 1 | NP_982271.1 | [67] |
| Amphiphysin | NP_001626.1 | [68] |
| Syntaxin binding protein 5 | NP_640337.3 | [69] |
| Regulating synaptic membrane exocytosis 1 | NP_055804.2 | Not Found |
| Neuroblastoma-amplified protein (with a Sec39 domain) | NP_056993.2 | Not Found |
| Glutamate receptor interacting protein 1 | NP_066973.1 | [70] |
| Mitochondrial nicotinamide nucleotide transhydrogenase | NP_892022.2 | [71] |
| Mitochondrial NFS1 nitrogen fixation 1 | NP_066923.3 | Not Found |
| Mitochondrial fumarate hydratase | NP_000134.2 | [72] |
| Optic atrophy 1 | NP_570847.1 | [73] |
| Mitochondrial processing peptidase | NP_004270.2 | Not Found |
| U1 small nuclear ribonucleoprotein 70 kDa | NP_003080.2 | [74] |
| U1 small nuclear ribonucleoprotein A | NP_004587.1 | [39] |
| ATP-dependent RNA helicase DDX46, Prp5 | NP_055644.2 | Not Found |
| 4-Aminobutyrate aminotransferase | NP_001120920.1 | [75] |
| 10-Formyltetrahydrofolate dehydrogenase | NP_036322.2 | Not Found |
| Phytanoyl-CoA dioxygenase domain containing protein 1 | NP_001094346.1 | Not Found |
| Nicotinamide nucleotide adenylyltransferase 3 | NP_835471.1 | [76] |
| Asparagine-linked glycosylation 2 | NP_149078.1 | Not Found |
| GTPase activating protein and VPS9 domains 1 | NP_056450.2 | [77] |
| Phosphatidylinositol-dependent Rac exchanger 1 | NP_065871.2 | Not Found |
| Aminophospholipid transporter | NP_006086.1 | [78] |
| RAN binding protein 16 (exportin 7) | NP_055839.3 | [79] |
| ALFY, involved in macroautophagy | NP_055806.2 | Not Found |
| Identified by Gozal, Y., et al., J. Proteome Res., 2009 [40] | ||
| serum amyloid P component precursor | NP_001630.1 | [42] |
| serine protease 15 | NP_004784.2 | Not Found |
| 14-3-3, eta polypeptide | NP_003396.1 | Not Found |
| 14-3-3, zeta polypeptide | NP_663723.1 | Not Found |
| ankyrin B | NP_066187.2 | Not Found |
| dynamin 1 | NP_004399.2 | [80] |
| aquaporin 1 | NP_000376.1 | [81] |
| Identified in both studies | ||
| Apolipoprotein E | NP_000032.1 | [41] |
| Microtubule-associated protein tau | NP_058519.2 | [16] |
| Amyloid β peptide | NP_000475.1 | [82] |
| Complement component 4b | NP_001002029.3 | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutz, B.M.; Peng, J. Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms. Proteomes 2018, 6, 46. https://doi.org/10.3390/proteomes6040046
Lutz BM, Peng J. Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms. Proteomes. 2018; 6(4):46. https://doi.org/10.3390/proteomes6040046
Chicago/Turabian StyleLutz, Brianna M., and Junmin Peng. 2018. "Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms" Proteomes 6, no. 4: 46. https://doi.org/10.3390/proteomes6040046
APA StyleLutz, B. M., & Peng, J. (2018). Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms. Proteomes, 6(4), 46. https://doi.org/10.3390/proteomes6040046





