Abstract
Polyploid crops such as wheat, Brassica, and cotton are critical in the global agricultural and economic system. However, their productivity is threatened increasingly by biotic stresses such as disease, and abiotic stresses such as heat, both exacerbated by climate change. Understanding the molecular basis of stress responses in these crops is crucial but remains challenging due to their complex genetic makeup—characterized by large sizes, multiple genomes, and limited annotation resources. Proteomics is a powerful approach to elucidate molecular mechanisms, enabling the identification of stress-responsive proteins; cellular localization; physiological, biochemical, and metabolic pathways; protein–protein interaction; and post-translational modifications. It also sheds light on the evolutionary consequences of genome duplication and hybridization. Breeders can improve stress tolerance and yield traits by characterizing the proteome of polyploid crops. Functional and subcellular proteomics, and identification and introgression of stress-responsive protein biomarkers, are promising for crop improvement. Nevertheless, several challenges remain, including inefficient protein extraction methods, limited organelle-specific data, insufficient protein annotations, low proteoform coverage, reproducibility, and a lack of target-specific antibodies. This review explores the genomic complexity of three key allopolyploid crops (wheat, oilseed Brassica, and cotton), summarizes recent proteomic insights into heat stress and pathogen response, and discusses current challenges and future directions for advancing proteomics in polyploid crop improvement through proteomics.
1. Introduction
Polyploid crops—with multiple sets of chromosomes—play an important role in global agriculture. They are the key drivers of crop evolution and contribute to global food security [1]. Polyploids exhibit greater vigor and yield potential than their diploid relatives. Whether natural or induced, polyploidization enhances heterozygosity, reduces inbreeding depression and mitigates deleterious mutations. Polyploids are classified into two major types: autoploids, with duplicated identical genomes [2], and alloploids formed by hybridization of more than two copies of divergent genomes [3]. Potato (Solanum tuberosum; 2n = 4x = 48 [4]) and sugarcane (Saccharum spp.; 2n = 62, 80, 96, 112, or 128 [5]) are the examples of autoploid crops, while wheat (Triticum aestivum; AABBDD; 2n = 6x = 42 [6]), Brassica species (Brassica spp.; 2n = 2x = 34 (BBCC), 36 (AABB) and 38 (AACC) [7]), and cotton (Gossypium hirsitum; AADD; 2n = 2x = 52 [8]) are allopolyploids. Other notable alloploids include oat (Avena sativa; AACCDD; 2n = 6x = 42), different millets (e.g., finger millet, porso millete, fonio millet, and Indian and Japanese barnyard millet), and quinoa (Chenopodium quinoa; AABB; 2n = 4x = 36) [8]. These polyploids contribute to our diet, industry, and economy. This review focuses on three globally significant allopolyploid crops—wheat, oilseed Brassica, and cotton.
Wheat (Triticum aestivum) is the most widely grown cereal crop, critical for food, animal feed and trade. In 2023–2024, global wheat production was estimated at 788 million tons (MT), with 209.6 MT traded [9]. However, production has declined while demand continues to rise, highlighting the urgent need for yield improvement.
Oilseed Brassica, a member of the Brassicaceae family, includes 4000 species, many of which are cultivated for edible oil [10]. Among them, B. napus (rapeseed/canola), B. campestris, and B. juncea are the most important [11]. However, B. napus is widely grown globally due to its low erucic acid and glucosinolate levels and high oleic and linolenic acid content. In contrast, B. campestris and B. juncea, which have higher erucic acid, are more regionally cultivated—B. campestris in temperate countries such as Australia, Germany, Sweden, France, and Canada, and B. juncea in Asia [12]. Global canola production reached 89.39 MT in 2023–2024, with a projected decline for 2024–2025 [13].
Cotton has industrial value in textiles, paper, oil, chipboard and livestock feed industries [14]. It has a value of around USD 600 billion in the global textile industry economy [15], with an estimated production of 24.12 MT in 2023–2024 [16].
The demand for these crops is rising due to global population growth—projected to increase by 25% by 2050 [17]. Wheat production must double by 2050 [18,19], cotton production must reach 28.1 MT by 2032 [20], and canola production in Canada is projected to increase by 3 MT by 2030 [21]. However, achieving these targets is hampered by biotic (e.g., pathogens and pests) and abiotic (e.g., heat, drought and salinity) stresses. These stresses disrupt plant phenotypes, physiology, biochemical, and metabolism, ultimately reducing yield and product quality. Among abiotic stresses, climate change–induced heat stress (HS) has become a major constraint to crop production, often causes drought by increasing evapotranspiration [22]. A 1 °C increase above 15 °C can reduce global wheat yields by 6% [23], and a similar increase above 30 °C can reduce canola and cotton yields by 7% [24] and 6.1% [25], respectively. An individual HS and combined HS and drought stresses reduce canola yield and oil concentration significantly [26], and impair cotton ball development [27]. In wheat, HS at the reproductive stage can cause pollen malformation and male sterility, [28,29,30] and a combined HS and drought stresses can cause mitotic and meiotic arrest, ovule abortion, and reduce stigma receptivity [31], drastically reducing grain number and quality. Besides abiotic stresses, biotic stresses are equally harmful. The presence of powdery mildew in wheat causes up to 33% yield loss [32], and clubroot disease in canola [33] and cotton leafroll dwarf virus [34] can result in up to 100% yield loss in severe cases.
Understanding the molecular basis of stress responses is essential for combatting these threats. During stress, the plant nucleus receives signals to alter gene expressions—exportation of mRNA from nucleus to cytoplasm and biosynthesis of stress-induced novel proteins [35,36]. To acclimatize to stress, the plant phenotype changes due to the biological function of the novel proteins. As biotic and abiotic stress responses, the biological function of a protein is governed by proteoforms—the basic molecular forms of proteins produced from a single gene including genetic variants, alternative splice variants, and post-translational modifications (PTMs) [37]—their cellular localization, and protein–protein and protein–other non-protein compound’s interactions [35,36]. Therefore, while genomics, transcriptomics, and phenomics have been useful to study changes in DNA, RNA [38], and phenotypes [39], respectively, proteomics provides more direct insights into stress adaptation mechanisms [40], while transcriptomics may not correlate with actual protein levels [41]. For example, transcriptomics techniques such as microarray and RNA-seq cover 48–57% of phenotypic variation and have moderate correlation with mRNA and protein (identified by label-free proteomics) expression [42]. Proteomics can identify the proteins and proteoforms actively involved in stress-related physiological, biochemical, and metabolic processes [43], and protein–protein interactions [44]. Proteomics is also a valuable tool for understanding the consequences of polyploidization [45].
Several proteomic tools are commonly used in polyploid crops to obtain stress-responsive molecular insights (Figure 1). Mass spectrometry (MS) has been the most extensively used tool in proteomics since its discovery in 1913 [46]. Techniques such as two-dimensional polyacrylamide gel electrophoresis–MS (2-DE-PAGE/MS) and its improved version, 2D-DIGE (2-dimensional difference gel electrophoresis), have evolved into advanced methods such as quadrupole (Q), time-of-flight (TOF), orbitrap, matrix-assisted laser desorption/ionization couple with TOF (MALDI-TOF), and shotgun proteomics [40,45]. Shotgun methods such as the label-based method, isobaric tags for relative and absolute quantitation (iTRAQ), and label-free quantitative proteomics are widely used. While label-free approaches offer precise quantification of all peptides of a sample, they are data-dependent acquisition (DDA) methods and therefore often fail to identify extensive low-abundant proteins or peptides [47]. To overcome this challenge, sequential window acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH-MS), a data-independent acquisition (DIA) method, is being applied in crops-, including wheat [48,49] and cotton [50].
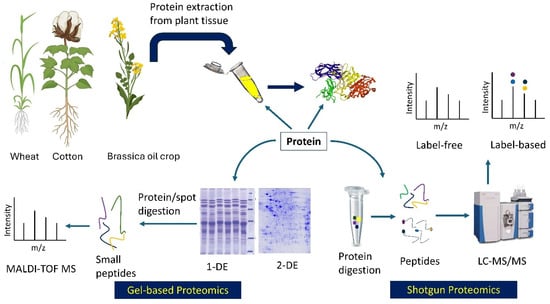
Figure 1.
Common proteomic approaches used in allopolyploid crops: Gel-based (bottom left) and shotgun (bottom right). The gel photos were modified from Gris and Baldoni (2013) [51] and Nadeem et al. (2016) [52]. Crops were generated from BioRender (https://BioRender.com/, accessed on 3 October 2025). Stable isotope labels for label-based method were represented by different colors; 1-DE and 2-DE = one- and two-dimensional gel electrophoresis; MALDI-TOF = matrix-assisted laser desorption ionization–time-of-flight; MS = mass spectrophotometry; LC = liquid chromatography.
Despite these advances, proteomic research in polyploid crops remains challenging due to genome complexity, inefficient protein extraction methods, lack of organelle-specific databases, insufficient protein annotations, and limited protein validation [40,53,54,55]. The genomic complexity of allopolyploids arises from the genomic shocks due to hybridization causing genome rearrangement and altered gene expression compared to their diploid parents [56]. Due to multiple genomic interactions between progenitor genomes, chromosomal rearrangements, genetic and well as epigenetic modifications, and subgenome biasness occur, which lead to significant differences in their gene, protein, and metabolite expression [57,58]. Additionally, cytonuclear co-evolution commonly occurs among polyploids, leading to cytonuclear incompatibility causing gene expression and protein product differentiation between the nucleus and cytoplasm [59,60]. However, genomic plasticity allows the allopolyploids to adapt with these significant changes—alteration in chromosome number and structure (insertion, deletion, and translocation), genome sizes, and epigenetics—and reconstruct the genes and proteins for different phenotypes for their adaptability [58]. New phenotypes of the polyploids compared to their parents are the result of the genomic complexity of their transcriptome, proteome, and metabolome, while orthologous protein interactions determine the parental gene expression in the allopolyploids [61]. Therefore, genome sequence or transcriptome analyses are insufficient to understand the adaptability mechanisms of polyploids due to polyploidization and environmental changes [62,63]. Compared to diploids, the proteomic data interpretation in the polyploids can be challenged by “homeologs collapse”—the wrong assembly of two homeologs into a single gene [64]—and “homeologe expression bias”—the unequal expressions between homeologs caused by the duplicated gene actions [65]. For the first time, identification of “homeologe expression bias” in the protein data between the A and B subgenomes in Arachis hypogaea provides insights into the biological processes involved in homeologous and paralogous protein expression [66].
However, recent advancements in genome sequencing, functional and subcellular proteomics, improved extraction methods, and the identification of stress-related protein markers highlight the growing potential of proteomics for enhancing stress resistance in polyploid crops. Though proteomics identifies the gene response for environmental stimuli, multi-omics—the integration of other omics such as genomics, transcriptomics, epigenomics and metabolomics with proteomics—could provide a more comprehensive understanding of stress tolerance in polyploids than the single approach [67].
This review highlights the genomic complexity of polyploid crops—wheat, oilseed Brassica, and cotton—and the progress of their proteomics for HS and biotic stress (disease), focusing on comparative proteomics, challenges of proteomics in identifying functional proteins and proteoforms of polyploids, and the prospects of proteomics, particularly subcellular proteomics, in polyploid breeding.
2. Genome Complexity of Polyploidy
2.1. Wheat
Bread wheat (T. aestivum; AABBDD; 2n = 6x = 42), a hexaploidy species in the Poaceae family, is one of the world’s most important staple cereal crops. It evolved naturally through two successive hybridization events. First, a diploid wheat (T. urartu; AA; 2n = 2x = 14) crossed with a wild goat grass (Aegilops speltoides; BB; 2n = 2x = 14), producing a tetraploid wild emmer wheat (T. turgidum spp. diccoides; AABB; 2n = 4x = 28). This tetraploid then hybridized with another goat grass (A. tauschii; DD; 2n = 2x = 14), resulting in modern hexaploidy bread wheat (Figure 2a) [6,68]. The estimated genome sizes of hexaploid, tetraploid, and diploid wheat are approximately 17 Gb [69], 10.1 Gb [70], and 4.94 Gb [71], respectively.
2.2. Oilseed Brassica Crops
The genus Brassica, within the Brassicaceae family, includes economically important species cultivated for oilseeds, vegetables, and condiments [72]. The Brassica genus has three diploid species—B. nigra (BB; 2n = 16), B. oleracea (CC; 2n = 18), and B. rapa (AA; 2n = 20). They evolved, by intercrossing into three amphidiploid species: B. carinata (BBCC; 2n = 34), B. juncea (AABB; 2n = 36), and B. napus (AACC; 2n = 38) (Figure 2b) [7]. Among the three diploids, B. rapa is the oldest and most widely distributed [73], with a genome size ranging from ~389.2 to 424.59 Mb [74,75]. The genome sizes of B. oleracea and B. nigra are estimated at ~587.7–630.7 Mb [76,77] and ~446.5–534.2 Mb [78,79], respectively. The genome sizes of the amhidiploids are approximately 922–961.72 Mb for B. juncea [80,81] and 921.5–975.8 Mb for B. carinata [82].
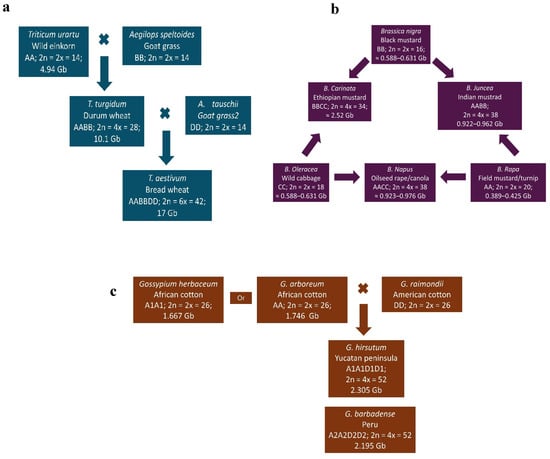
Figure 2.
Evolution and genome structure of three major allopolyploid crops: (a) wheat (Triticum aestivum L.), (b) oilseed Brassica crops, and (c) cotton (Gossypium hirsutum and G. barbadense); adapted from [83].
2.3. Cotton
Cotton, belonging to the genus Gossypium and Malvaceae family, has been cultivated for more than 7000 years [84] for fibers, medicine, and animal feed [85]. Although there are around 50 species, only four are widely cultivated: two American species—G. hirsutum (A1A1D1D1; 2n = 4x = 52) and G. barbadense (A2A2D2D2; 2n = 4x = 52)—and two Asian and African species—G. arboreum (AA; 2n = 2x = 26) and G. herbaceum (A1A1; 2n = 2x = 26) (Figure 2c) [83,85,86,87]. Forty-three Gossypium species are diploid (n = 13), classified into genome groups A–G and K, while seven are tetraploid (AADD) [88,89]. The A-genome originated in Africa and the D-genome in Mexico [88]. Allotetraploid or amphidiploid cottons species—G. hirsutum (2305.24 Mbp), G. barbadense (2195.8 Mbp), G. tomentosum (2193.56 Mbp), G. mustelinum (2315.09 Mbp), G. darwinii (2182.96 Mbp) [90], G. ekmanianum (2341.87 Mbp), and G. stephensii (2291.84 Mbp) [91]—are the result of polyploidization between G. arboreum (A genome; 1746 Mb; [92] or G. herbaceum (1667 Mb; [93]) and G. raimondii (D genome; 885 Mb; [94,95,96]). Among these cotton species, G. hirsutum is the most widely cultivated species globally [97].
3. Proteomic Studies on Heat Stress Tolerance in Polyploid Crops
Heat stress significantly impairs crop growth, development, yield and grain, and seed, or fiber quality. Although HS can adversely affect all of a plant’s developmental stages (germination, seedling, vegetative and reproductive phases, and maturity), the anthesis and post-anthesis phases are particularly sensitive in crops like wheat, oilseed brassica, and cotton. Proteins—functional gene expression products—play key roles in a plant’s HS response by regulating various metabolic pathways. For example, HS increases biomolecule kinetics, resulting in protein degradation or misfolding risk; protective proteins or proteoforms accumulate to reduce the harmful effect of HS on the cells [35]. Different HS-induced proteins, particularly heat shock proteins (HSPs), and proteoforms are strongly associated with HS tolerance across different developmental stages. This section discusses how HS affects crops, and the molecular mechanisms of the HS response revealed by proteomics approaches.
3.1. Wheat
In wheat, HS adversely impacts every stage, from seedling emergence to maturity, ultimately reducing grain yield and quality [98]. Heat stress during tillering disrupts pollen viability, spike formation, seed set, and embryo development, while terminal HS (at reproductive stage) is detrimental to yield [99]. Post-anthesis HS inhibits photosynthates translocation and starch accumulation into grains and reduces of carbohydrate content in the stem. It also leads to increased reactive oxygen species (ROS), osmolyte accumulation, decreased RuBisCo activity, and degradation of seed proteins [100]. However, the accumulation of HSPs, antioxidant enzymes (e.g., catalase (CAT), peroxidase (H2O2), superoxide dismutase (SOD) and glutathione (GSH)) and proline, along with the suppression of anabolic and catabolic processes, are critical for HS tolerance [40,99]. Numerous proteomic studies in recent years have examined HS responses at different growth stages in wheat (Table 1). Our previous review summarized recent progress in wheat proteomics under HS [40].

Table 1.
Summary of proteomic studies on heat stress tolerance in wheat, cotton, and oilseed Brassica during the last five years (2019–2025).
Proteomic studies identified that proteins associated with photosynthesis and PTMs play important roles in HS tolerance in wheat seedlings. Cytoplasmic proteins such as 2-cysteine peroxiredoxin (Ta2CP) in wheat contribute to HS tolerance and chlorophyl metabolism in seedling stage [117]. Arabidopsis with overexpressed Ta2CP had normal green leaves with high chlorophyll a and b content and low H2O2 and ROS, leading to thermotolerance. The application of 2-DE and MALDI TOF/TOF proteomics identified that the upregulation of photosynthesis-associated proteins, including RuBisCO activase A and PEP carboxylase, and the signal transduction pathway in wheat contributes to HS tolerance by increasing CO2 assimilation [118]. A small HSP gene, TaHSP23.9 (a member of the HSP20/ACD family), was significantly upregulated in wheat leaves and developed grain under HS at both the transcription (mRNA) and translation levels, making it a promising marker for HS tolerance [106]. Phosphorylation, a major PTM, also plays an important role in HS tolerance. Due to the amino acid substitution in the TaSG-D1 allele, the TaSG-D1E286K gene undergoes phosphorylation and stabilizes the expression of TraesCS5B02G380200 (TaPIF4), resulting in improved HS tolerance in the seedling and mature stages of T. sphaerococcum [119]. In rice, phosphoproteins contribute to heat-stress signaling by decreasing RuBisCo and ATP synthesis, stabilization of HS transcripts, and transduction of cellular-signaling due to dephosphorylation [120].
Proteins associated with photosynthesis and male sterility improve grain yield and quality in wheat. Sowing time affects photosynthesis and plays a crucial role in mitigating HS effects. For example, delayed sowing lead to higher photosynthetic rates in flag leaves, which resulted in 11.16% and 10.10% increases in grain weight and yield in wheat, respectively [102]. This improvement was linked to increased energy flow into the electron transport system and the upregulation of differentially abundant proteins (DAPs) involved in photosynthetic electron transport (e.g., PsbH and PsbR), the Calvin cycle, and chlorophyll biosynthesis. Conversely, terminal HS under standard sowing dates significantly reduces photosynthesis and starch accumulation, diminishing grain yield and quality, respectively. Combined 2D-PAGE and transcriptomic analyses revealed that mitogen-activated protein kinase (MAPK) contributes to terminal HS tolerance in wheat by promoting proline, starch, and H2O2 accumulation, enhancing granule integrity, positively regulating stress-related gene expression and amylolytic activities, while suppressing photosynthesis-related genes [104]. Moreover, MAPK improves oxidative stress tolerance through its positive correlations with SOD and HSP17. The role of MAPK in HS tolerance in other crops including rice, corn, cotton, potato, and tomato has been reviewed earlier [121]. MAPK signaling is also involved in HS-induced male sterility in wheat. The downregulation of MAPK-related genes (e.g., TraesCS3A02G149800.1) under HS negatively affects ROS scavenging and pollen development [28]. Furthermore, DAPs related to phenylpropanoid biosynthesis accumulates excess ROS in anthers and inhibit starch and sucrose synthesis through phenylpropanoid biosynthesis, resulting in anther indehiscence. Upregulation of the HS pathway thermosensitive male sterile 1 gene, TraesCS4B02G193500.2, causes male sterility under HS.
ABA biosynthesis and proline accumulation improve HS tolerance in the spike differentiation stage in wheat [48]. A comparative transcriptomic and proteomic study across three spike differentiation stages identified 210 HS-associated transcripts/proteins. Downregulation of 9-cis-epoxy carotenoid dioxygenases and upregulation of zeaxanthin epoxidase and ascorbate oxidation modulate ABA biosynthesis. Similarly, ornithine aminotransferase and pyrroline-5-carboxylate reductase enhance proline accumulation via ornithine and glutamate pathways. Phosphorylation regulates reproductive organ development and photosynthetic apparatus in leaves in wheat under HS [122]. A DDA LC-MS/MS identified 14 phosphoproteins under HS, which activated phosphatases and kinases and generated their phosphoforms. A protein kinase, TraesCS6B01G377500.3, was associated with upregulated S711 and S762 phosphosites. Serine or threonine dominated phosphosites—S3236 and T3238—maintained protein functions by preserving the phosphorylation pool under HS. However, identification of proteins and proteoforms is recommended to understand the co-regulation of these phosphosites to combat HS.
Heat stress affects wheat grain weight and quality. A 2-DE and MALDI-TOF showed that HS reduced grain weight by decreasing starch synthesis proteins such as glucose-1-phosphate adenyl transferase, although catalase protected cells from H2O2 [123]. Heat stress also weakens dough properties due to reductions in glutenin and gliadins, and an increase in HSPs, although sHSPs improve thermotolerance during grain filling stage [124].
Increased proline and H2O2 levels in flag leaves leads to 9% more grain weight lost under combined day–night temperature HS conditions than high daytime temperatures alone [103]. Day–night temperatures reduce thermotolerance, grain yield, and quality by restricting the expression of transcription factors (TFs), genes associated with signaling pathway and abiotic stress, and the function of protein folding machinery. Using 2D-PAGE and MALDI-TOF, 153 and 95 DAPs were identified in wheat grain under day and day–night HS conditions, respectively. Proteins associated with starch biosynthesis, cell wall synthesis, defense, and storage were altered, with the particularly noteworthy downregulation of starch and cell wall-related proteins, resulting in poor grain development and premature senescence in both conditions.
Heat stress also affects flour quality and dough quality by altering protein abundance in wheat grain [108] and by modulating the abundance of storage proteins [99], respectively. A decrease in starch accumulation and an increase in gluten, gliadin, globulin, and albumin content was shown to result in a reduced thousand grain-yield in wheat [108]. The downregulation of HSP90, low-molecular-weight glutenin subunits (LMW-GSs), and starch branching enzyme IIb reduced both grain yield and quality. Five DAPs—elicitor-responsive gene 3, brassinosteroid-insensitive 1, histone cell cycle regulator, chaperone protein, and splicing arginine serine-rich 7—were also linked to protein–protein interactions affecting grain yield and quality. Heat stress affects dough quality in wheat grain by affecting high-molecular-weight glutenin subunits, LMW-GSs, α- and γ-gliadins, and avenin-like proteins [101].
Moreover, peroxidase, RuBisCo activase, sHSPs, and photosynthetic electron transport are crucial for thermotolerance during the seedling stage, while proteins related to antioxidants, MAPK, proline and H2O2 accumulation, and phosphofroms are vital during the grain filling stage. Globulin, gliadin, and albumin are essential in determining grain quality under HS.
3.2. Oilseed Brassica Crops
Heat stress affects all growth stages of the oilseed Brassica, but the flowering stage is the most sensitive [125]. It reduces gametophyte fertility, pollen viability, germination, and causes seedless pod resulting in a decreased thousand grain weight, reduced yield, and poor oil quality [125,126]. Among the cultivated species, B. napus is more sensitive to HS than B. juncea and B. campestris [126]. Proteins such as HSPs (e.g., HSP101, HSP70 and HSP17.6) [125], gucosinolates, including glucobrassican, glucocarphanin and glucoraphasatin, gluconapin, sinigrin, and progoitrin [127] and glyoxalase I [128] contribute to thermotolerance. Phytohormone signaling pathways also play a critical role in HS-tolerance.
Proteomic study of the effect of HS on Brassica started only a decade ago. The first proteomic study on HS in B. napus found ascorbate peroxidase as a key protein for thermotolerance in seedlings using 2-DE [129]. Increased glyoxalase-I enhanced seed thermotolerance by detoxifying methylglyoxal, maintaining redox balance, and increasing SOD activity [128].
Beyond canola, proteomic studies have also been conducted on other oilseed Brassica species. Over the last five years, the advancement of proteomic techniques has accelerated in HS responses in oilseed Brassica (Table 1). Heat stress causes dead organs such as seed coat and pericarp affecting yield [130], while label-free proteomics identified the role of protease accumulation in dead pericarps in B. juncea [131]. A combined proteomic and metabolomic study on B. juncea under HS found the high abundance of several proteins, including HSP70-5, HSP104, and multiple sHSPs, with proteases being the most abundant in the pericarp [131]. Heat primarily induced the accumulation of cysteine proteases, 25 HS-induced proteins, and sugars on its dead pericarp.
Increased antioxidants and flavonoids contribute to thermotolerance in B. juncea seedling [114]. Phenylalanine ammonia lyase and respiratory burst oxidase homolog protein-A improve thermotolerance by synthesizing metabolites (e.g., flavonoids and lignins) and producing ROS, respectively. A combined proteomics and peptidomics—a comprehensive analysis of all peptides of an organism [132])—study in oilseed Brassica identified thermotolerant DAPs. Key thermotolerance-associated proteins, such as HSPs (21.7, 14.7 and 17.5 kDa HSP groups), sHSPs, and TFs, including HS transcription factor A-4a, Myb family Atlg14600, and WRKY, are related to signal transductions, regulating transcriptions, repairing DNA, and processing RNA. In B. campestris seedling, thermotolerance under both heat and cold stress involves several molecular pathways, including redox homeostasis, photosynthesis, chaperons, HSPs, carbohydrate metabolism, and signal transduction [116]. Under both stresses, 1022 DAPs (172 upregulated and 324 downregulated) and 1784 DAPs under HS were found using a combined TMT and LC-MS. The critical role of redox homeostasis in thermotolerance was validated through the reduced glutathione-to-oxidized glutathione ratio and decreased oxidative damage in transgenic Arabidopsis overexpressing GLU1, a gene encoding a redox-associated protein.
3.3. Cotton
Heat stress critically affects all the developmental stages of cotton, with the reproductive stage being the most sensitive [133]. It impairs seed germination, shoot and root growth, and induces male sterility, ultimately reducing yield and fiber quality. Increased leaf temperature due to HS disrupts photosynthesis by inhibiting RuBisCO activation [134]. In G. barbadense, HS restricts photosynthesis by limiting the regeneration capacity of ribulose-1,5-bisphosphate and impeding electron transport [135]. Traits such as membrane thermostability, high proline and soluble sugar can improve thermotolerance in cotton [136]. Additionally, the regulation of microRNAs, signaling pathways, including calcium, ROS, carbohydrate, transcription factors, phytohormones and gene regulation pathway, and HSPs play critical role in its thermotolerance [133]. Despite numerous studies on the physiological impacts of HS on cotton, proteomic studies remain limited. However, recent proteomic studies have provided valuable insights into thermotolerance in cotton (Table 1).
Heat stress increases ROS and mitochondrial acetylation activity and decreases ATP levels in the cotton seedling’s leaves leading to the HS-sensitivity through the expression of the mitochondrial protein, GhHSP24.7 [111]. However, suppressed GhHSP24.7 enhanced thermotolerance by decreasing stomatal conductance and H2O2, activating GhHDA14, maintaining chloroplast structure, and improving ROS scavenging.
The reproductive stage of cotton is particularly vulnerable to HS [137]. Upregulated protective proteins such as HSP (15.7 kDa) and peptidylprolyl isomerase recovers pollen viability at the tetrad stage under 38 °C [50]. However, under 40 °C, at the tetrad stage, mature pollen grain size and pollen tube are reduced by 36% and 120 µm, respectively, resulting in a yield reduction of two-thirds [111]. Protein export and folding, and their processing pathways protect pollen from HS through upregulation of HSP70, isoforms of heat shock cognate 70 kDa protein 2-like, luminal-binding protein 5-like, and luminal-binding protein 5-like, HSP70 protein 14–15-like and HSP70-90 organizing protein 3-like and downregulation of late embryogenesis abundant protein (LEA) 2.
A recent proteomic study identified over 8,000 HS-induced DAPs at the flowering stage [109]. Increased abundance of β-glucosidase, different photosynthetic proteins, NDH subunit of subcomplex B1, NADH ubiquinone oxidoreductase (Complex I), stress-responsive proteins, 1-aminocyclopropane-1-carboxylate oxidase, and HSPs (e.g., Hsp70, Hsp90 and Hsp80) is crucial for thermotolerance. These proteins contribute to HS-tolerance by maintaining membrane and cell wall integrity, producing energy, maintaining osmotic balance, enhancing proline accumulation and ROS scavenging, and regulating electron transport in photosynthesis channels, carbohydrate metabolism, and protein folding, and preventing protein aggregation. The role of HSP70 has also been reported for HS and drought tolerance in rice through increasing proline accumulation and SOD activities, respectively [138].
Heat stress also reduces insecticidal proteins in Bt cotton’s bolls [112]. Under combined heat (38 °C) and drought (40% field capacity) stress, the insecticidal proteins led to a decrease in the fresh weight of boll shell by 38.3 and 30.7 ng/g in 2026 and 2017, respectively. That reduction may be the result of the downregulation of signal recognition particles (SRPs) and SRP receptors, restriction of peptide chain translocation into the endoplasmic reticulum, and increase in ubiquitin-mediated proteolysis.
Overall, proteins associated with photosynthesis, carbohydrate metabolism, stress response, ROS regulation, protein folding and aggregation, and electron transport play pivotal roles in cotton growth and HS tolerance.
4. Proteomic Studies for Biotic Stress
Biotic stress—caused by living organisms such as bacteria, fungi, viruses, and insects—significantly threatens global food security [139]. Plant resistance to pathogens is a complex trait governed by a multifaceted interplay of morphological, genetic, biochemical, and molecular processes, with proteomic insights playing pivotal roles [140,141,142]. Therefore, a comprehensive understanding of these mechanisms is essential in order to address biotic stress in complex plant genomes. Proteins and PTMs contribute directly to plant immunity through activating stress-associated defense responses, while subcellular protein localization provides key insights into how plants respond to biotic stress. Therefore, proteomics—a powerful and evolving analytical tool—offers substantial potential for developing disease resistance, particularly in polyploid crops [143,144].
4.1. Wheat
Wheat production is mostly challenged by fungal pathogens such as rusts, mildew, and blights [145]. Numerous proteomic studies have explored the mechanisms underlying wheat–pathogen interactions to understand both compatible and incompatible responses [146,147,148,149,150]. For example, a comprehensive proteomic revealed key virulence and host defense proteins—including PR1, PR4, and GST—associated with wheat rust resistance [150]. In the wheat apoplast, an increased level of protein-modifying enzymes, particularly serine proteases, mitigated infection-induced damage by Magnaporthe oryzae [151]. Upregulation of antioxidant-related proteins and downregulation of plant–pathogen interactions and glutathione metabolism were found due to Tilletia controversa and T. foetida infection in wheat [152]. However, thaumatin-like proteins and HSPs contributed to increasing the resistance against these pathogens.
Separately, 366 DAPs for wheat infected by Fusarium pseudograminearum (causal agent of Fusarium crown rot) were identified using the TMT tool [153]. Cellular activities—glucoside, electron transport, cellulose synthase and oxidoreductase—metabolic processes, plant–pathogen interactions, and antioxidant activities contribute to resistance to the disease [153,154]. In the resistant variety Xinong 538, unique proteins encoded by Chitinase IV, Thaumatin-like 1, PR1.1, and PR1.2 genes were strongly associated with resistance to Fusarium head blight (FHB) [154]. Furthermore, an FHB resistant network—Ca2+, phytohormone- and phenylalanine-signaling pathways for stomatal closure, disease resistance and thickening cell wall, respectively—was proposed. Although wheat cultivars vary in FHB susceptibility, they share a core set of defense-related proteins [155]. During FHB infection, pectin-derived oligogalacturonides (produced via homogalacturonan degradation) act as damage-associated molecular patterns. They are recognized by wall-associated kinase 1 in Arabidopsis, a plant receptor kinase functioning as a pattern recognition receptor. Similarly, the Stb6 gene in wheat encodes a wall-associated kinase-like protein that recognizes AvrStb6, the fungal avirulence effector, thereby conferring resistance to Septoria tritici blotch [146]. In the resilient wheat cultivar “Xingmin318”, 741 DAPs had distinct defense strategies against Puccinia striiformis and Blumeria graminis infections [156], while 394 DAPs showed resistance in response to Blumeria graminis f. sp. tritici infection [157]. Among these, a few DAPs were primarily associated with pathogenesis-related processes, oxidative stress responses, and primary metabolism. Key metabolic pathways for plant’s defense response include phenylpropanoid biosynthesis, phenylalanine metabolism, and photosynthesis-antenna proteins. The identification of protein motifs rich in leucine repeats and histidine sites, along with eight predicted PPI networks, provides insights into the potential molecular mechanisms underlying resistance to powdery mildew.
Although proteomics has significantly advanced our understanding of plant–pathogen interactions at the molecular level, the narrow genetic diversity of modern wheat varieties remains a major challenge. Continuous research and breeding programs are essential to enhance wheat’s resistance to evolving pathogens.
In summary, proteins involved in antioxidant defense, glutathione metabolism, carbohydrate metabolism, photosynthesis, and calcium-, phytohormone-, and phenylalanine-mediated signaling pathways play vital roles in wheat’s resistance to biotic stress.
4.2. Oilseed Brassica Crops
Biotic stress activates detoxification, redox homeostasis, and defense-related pathways in oilseed Brassica. Black rot, caused by Xanthomonas campestris pv. campestris (Xcc), severely affects cruciferous vegetables, including Brassica napus [158]. Five major functional protein groups—antioxidative systems, proteolysis, photosynthesis, redox, and innate immunity—were identified in Xcc-responsive DAPs in the susceptible cultivar (Mosa) and the resistant cultivar (Capitol) using label-free proteomics. Mosa showed increased protein degradation and oxidative stress markers, while Capitol exhibited high redox potential and an enhanced innate immune response. Downregulation of photosystem II-related proteins improved disease resistance.
Using LC with tandem MS, 73 putative proteins were identified on the quantitative trait loci in chromosomes A3 and A8 in the clubroot (caused by Plasmodiophora brassicae) resistant B. napus cultivar [159]. The abundance of the proteins associated with pathways related to calcium signaling, reactive oxygen species, dehydrins, lignin, and phytohormones changes due to the pathogenic infestation. Additionally, 73 orthologous proteins, including calcium signaling associated proteins (BnaA02T0335400WE, BnaA03T0374600WE, BnaA03T0262200WE, and BnaA03T0464700WE) play a crucial role in defense against Plasmodiophora brassicae. In B. oleracea, proteins associated with ABA and glucose signaling, and fructose-bisphosphate aldolase are critical for clubroot defense [160]. Most of the abundant proteins were localized to outside the nucleus, particularly in the chloroplast, thylakoid, stroma, and membranes. Additionally, the restriction of energy metabolism enhances resistance.
Stem rot, caused by Sclerotinia sclerotiorum, is another major disease of Brassica [161]. In the first proteomic study on S. sclerotiorum infection in B. napus, upregulated antioxidant enzymes peroxidase and SOD along with proteins related to photosynthesis, metabolism, hormone signaling, and protein folding were reported for the disease resistance [162]. Several proteomic studies have expanded the understanding of stem rot resistance mechanisms in B. napus [161,163,164,165]. A TMT-based analysis identified 221 unique proteins and 173 DAPs in response to stem rot infection, including proteins involved in ROS homeostasis, lipid signaling, DNA methylation, histone modification, defense responses, and cyanate lyase activity [165].
In B. juncea, nano-LC-MS revealed peptidyl-prolyl cis–trans isomerase as the key protein for stem rot defense [166]. Furthermore, interacting with other proteins related to protein importation into the nucleus and RNA exportation, including GTP binding nuclear proteins, 60S ribosomal protein and protein kinase play important roles in disease defense. Alternative splicing on introns or alternative 3′ splice sites in S. sclerotiorum-infected B. napus led to 130 genes with 98 differentially expressed genes for defense [167]. However, further proteomic investigations focusing on PTMs are needed to deepen our understanding of host–pathogen interactions.
Overall, proteins associated with photosynthesis, ROS homeostasis, lignin synthesis, lipid- and Ca2+- signaling, DNA methylation, and histone modification contribute to the biotic stress resistance in oilseed Brassica.
4.3. Cotton
Phytopathogens—fungi, bacteria, and viruses—cause significant yield loss in cotton [168]. Among the fungi, Verticilium dahlia, Fusarium oxysporum, Thielaviopsis basicola [169], and Rhizoctonia solani [170] are major causal agents for cotton diseases. Bacterial blight by Xanthomonas campestris also causes significant yield loss in cotton [171]. Cotton proteomics has advanced significantly over the past decade, improving the understanding of pathogenicity and plant defense responses at the molecular level [53].
Numerous proteomic studies have focused on unraveling the molecular mechanism of Verticillium wilt (VW), a devastating soil-borne disease caused by V. dahlia that severely affects cotton yield and quality [172]. The first root proteomic study on VW in cotton (G. barbadense) identified 51 upregulated and 17 downregulated proteins using 2-DE [173]. This study highlighted the roles of the ethylene signaling pathway, pentose phosphate pathway, and Bet v 1 family proteins in defense. The proteins of Bet v 1 family proteins, PR10 (GbPR10.5D1), increased resistance by modulating lipid metabolism and defense signaling pathways [174]. A DIA-based comparative proteomics identified 885 DAPs associated with disease resistance in G. barbadense [175]. Silencing ascorbate peroxidase proteins reduced VW resistance, underscoring the importance of APX-mediated redox metabolism in plant defense. Gene co-expression network analysis identified oxidoreductase and peroxidase activities as significantly enriched pathways for VW resistance.
Silencing a nuclear protein, GthGAPC2, in Gossypium thurberi increased susceptibility of VW by reducing lignin content and disrupting redox balance [176]. On the other hand, overexpression of GthGAPC2 in tobacco and Arabidopsis confirmed its role in disease resistance, highlighting its potential as a promising candidate for breeding programs. Mi et al. (2024) [177] demonstrated that the GhMPK9-GhRAF39_1-GhWRKY40a signaling pathway enhanced cotton disease resistance by regulating the GhERF1b- and GhABF2-mediated pathways. GhMAC3e, a homologous gene of a key component of the MOS4-associated complex, was implicated in growth and defense responses against VW [178]. A phosphoproteomic study identified 359 and 287 differential phosphoproteins at 1 and 3 days post-VW inoculation in the resistant line, respectively [179]. Analysis of PTMs revealed 37 enriched serine (Ser) and 4 threonine (Thr) motifs, of which 19 Ser and 2 Thr were novel. Moreover, the upregulation of burst oxidase homolog D (GhRbohD) increased phosphorylation after VW infection, increasing resistance by decreasing ROS, H2O2, nitric oxide, and calcium levels while promoting lignin and cellulose accumulation [180].
Fusarium wilt (FW), caused by F. oxysporum, also results in considerable yield losses in cotton [181], though limited proteomic studies have explored this disease. Proteins associated with polyketides and cellular processes (e.g., lipid biosynthesis, protein and nucleotide metaboSerlism, protein folding, carbohydrate metabolism and oxidative stress response), HSP70, and protease are significantly enriched in the F. oxysporum f. sp. vasinfectum [182]. These proteins are crucial in mediating host–pathogen interactions via extracellular vesicles. In contrast, the MAPK cascade involving GhMPK20, GhMKK4, and GhWRKY40 negatively regulated FW resistance, while GhMPK20 plays a key role in the defense signal transduction pathway to improve FW resistance in cotton [183]. Furthermore, a phrosphoproteomics identified GhMORG1 (a MAPK scaffold protein of the GhMKK6–GhMPK4 cascade) as enhancing FW resistance in cotton [184].
In response to Rhizoctonia solani infection, iTRAQ analysis identified 174 DAPs, with proteins involved in ROS homeostasis—such as glutathione S-transferases and glutathione peroxidase—and those linked to lignin and phenylpropanoid biosynthesis, DNA methylation, and histone modification significantly upregulated [185].
Defense mechanisms for bacterial blight, caused by Xanthomonas campestris pv. Malvacearum, include the downregulation of photosynthesis-related proteins, such as ribulose-biphosphate carboxylase activase, ATP synthase β subunit, RuBisCo large subunit-binding protein, and glyceraldehyde-3-phosphate dehydrogenase B [186]. Furthermore, ROS-regulating proteins, including peroxidase, peroxiredoxins, and Prx type 2-Cys, play important roles in enhancing resistance.
Overall, proteins associated with signaling pathways—ethylene, calcium, and defense—as well as lipid and carbohydrate metabolism, DNA-methylation, histone modification, and peroxidase activity contribute to the biotic stress resistance in cotton.
5. Challenges of Proteomics in Polyploid Crops
Proteomics has been used in allopolyploids, including wheat, oilseed Brassica, and cotton, to harness the molecular insights of abiotic and biotic stress tolerance. However, these studies face several challenges, primarily due to the complex nature of polyploid genomes, difficulties in protein identification, reproducibility, and the lack of comprehensive protein databases.
The genomic complexity of an allopolyploid is the result of the combination of divergent genomes through interspecific hybridization. Gene loss during interspecific hybridization or further polyploidization (e.g., in wheat), continued recombination between the homoeologous chromosomes (e.g., Brassica), and chromosomal translocations between subgenomes increase the complexity of the genomic landscape [187,188]. While proteomics analysis can detect chromosomal rearrangements by comparing DAPs or proteoforms between progenitor lines, the specific parental contributions often remain unresolved [45].
For example, bread wheat has a large and complex genome (~17 Gb across three sets of chromosomes) [69], with 55–63% comprising repetitive sequences [189]. Paralogous genes in allopolyploids often share > 90% amino acid sequence identity. This high redundancy makes it difficult to identify novel proteins and protein–protein interactions associated with distinct phenotypes [45,190]. Studies have shown that 65–70% of transcriptomic data does not correspond to proteomic outcomes [41], potentially due to its amino acid composition and alternative splicing or PTMs. Protein isoforms—protein sequences altered by alternate splicing—and PTMs are two major proteoforms for determining biological functions of proteins in plants [36].
Besides genetically functional proteins, the identification of proteofroms is crucial due to their important role in allopolyploid evolution and stress tolerance. Homologous genes—orthologous and paralogous—and homeologous genes are a significant part of polyploid evolution. Homeologous genes or homeologs are the homologous gene pairs originated from different species but merged to an allopolyploid genome by alloplolyploidization [191], while protein isoforms are formed due to their expression [192]. For example, 18.7% of expressed genes of allotetraploid wheat (AADD) have a similar type of alternate splicing in two subgenomes [192], while 90 novel protein isoforms of a HSP, TaHSP90, developed from three homeologs were found in hexaploid wheat [193]. The protein isoform of TaYRG1.1 (TaYRG1.6) increases susceptibility to yellow rust disease in wheat [194], while TaHSFA6e, originated from the alternative splicing of a HS transcription factor gene increases HS-tolerance [195]. However, the identification of protein isoforms is challenging in allopolyploids due to a lack of complete protein databases, a higher chance of shared peptides compared to diploids due to their homeologous genes, and variability of the isoforms according to developmental stages of crops, tissue types, and external stimulus [196]. Long-read proteogenomic pipelines or prediction models using artificial intelligence (AI) can help overcome the challenges.
Similarly, PTMs also play an important role in stress tolerance in polyploids. Recently, the model of controlling cellular functions by PTM was proposed as follows; enzymes such as kinases, “writer”, catalyze the PTM addition to amino acid residues, while other enzymes such as phosphatases de-ubiquitinases and -acetylases, “erasers”, play the role of deleting PTMs from target amino acid residues, while a protein domains, “readers”, play the role of interacting between the PTMs and the other proteins [197]. However, this PTM crosstalk is underexplored in plant proteomes, which can be addressed by incorporating AI. The use of AI in identifying critical PTMs has been significantly advanced in human proteomics. There are many AI-based models, including machine learning and deep learning models, that have been developed to predict sequence for PTM sites, as well as their structure and their functions [198]. For example, a protein language-based model, PTMGPT2, precisely identified 19 disease and drug-associated PTMs—amino acid distributions and positions [199]. Compared to MS and deep learning-based AI tools (PTM prediction-structure version (MIND-S) framework), the crosstalk between different PTMs, including phosphorylation on HSP90 and their role in drug binding, was precisely predicted by AI [200]. Thus, training data for PTM crosstalk on stress- and yield-associated proteins in polyploids can advance their improvement; however, to avoid false negatives in training data, a combination of AI model prediction and experimental data is recommended for precise outcomes.
Under different external stimuli, a single protein can develop the same or different kinds of PTMs, interacting with different target motifs [36]. For example, different temperature stresses activated phosphatase and kinase and produced different phosphofroms of a target protein in wheat [122]. However, progress in allopolyploid proteomics for stress-related proteoform identification lags far behind compared with other plants and human proteomics due to the inefficiency of the current proteomic approaches. Unlike for other diploids or humans, the advanced computational algorithm of the proteomics (e.g., label free proteomics) often fails precise quantification of stress associated proteins from the multiple paralogus genes of polyploids that encode > 90% identical amino acids and to identify protein variation in a specific locus due to allelic diversity [201,202]. It is also associated with incorrect or incomplete gene models, lack of annotations [203], and lack of reliable bioinformatic tools [202].
Protein enrichment protocol is also important to identify proteoforms, as 1 to 5% proteoforms of the total protein in wheat can be identified using a protein enrichment protocol [204]. However, with necessary modifications, adapting nano-particle enrichment MS proteomics used in human proteomics [205] can improve the proteoform identification in allopolyploids. Furthermore, despite having poor reproducibility and limited ability to detect low-abundance and low-molecular-weight proteins precisely, 2-DE coupled with other proteomic approaches, such as LC-MS/MS, can identify proteoforms (Table 2) [206]. In addition, 2-DE can identify the isoelectric point and relative mass of proteoforms combining with other approaches, such as Orbitrap MS, iTRAQ, TMT, and stable isotope labeling of amino acids in cell-culture (SILAC) [206,207], sometimes used in plant proteomics. An advanced proteomic technique, DIA, can quantify proteins and PTMs precisely, but it requires sophisticated software to overcome the data complexity challenges [208]. DIA-MS identifies peptides using spectral libraries originating from DDA-MS or DIA-MS dataset search or deep-learning models and protein sequence databases (Table 2) [209]. Therefore, the identification of unknown (not listed in the genomic or protein databases) multiple proteoforms with closer structures and unique peptides is challenging in DIA [210]. Recently, DIA has been advanced due to its incorporation into Orbitrap (a high-resolution mass analyzer) [208]—mostly used for DDA—trapped ion mobility spectrometry [211] and high-field asymmetric waveform ion mobility spectrometry [212], but rarely applied in plant proteomics. Another revolutionary DIA is DIA-PASEF, which has significantly seen an increase in its proteomic depth (83% than the previous version), experimental speed and precision, and reduced experimental sample amounts [213]. Improved algorithms of DIA-NN and Spectronaut software (Spectronaut 20: https://biognosys.com/software/spectronaut, accessed on 3 October 2025) in library-free methods lead to more accurate protein and peptide quantification and data consistency than other methods, such as library-based methods [22]. DIA-NN and Spectronaut identified 120.2% and 69.8%, and 30.2% and 11.6% higher peptides and proteins than the library-based methods, respectively. Furthermore, with optimization, this method can be adapted for improved protein quantification from the complex polyploid tissues.

Table 2.
Advantages and limitations of the major proteomic methods for allopolyploid proteomics.
In addition, sample preparation methods and different proteomic approaches influence true proteoform identification. Several recent review articles have discussed the technical challenges of using top-down and bottom-up approaches for proteoform identifications [214,215,216,217,218]. Though top-down proteomics with MS identifies intact proteoforms [214], it faces the challenge of a proteoform mass limit of 30 kDa [219], and requirements of compulsory reproducible samples and a high level of sample fractions [220], resulting in difficulties in finding exact localization [221]. However, improved liquid/gas-phase separation of MS instruments and bioinformatic tools [221]—e.g., TDPortal, Proteoform Suite and MetaMorpheus—alongside false-discovery rate determination, proteoform annotations and databases, and proteome validation [220] are required to overcome the challenges. “Super charging”—forcefully bringing a single charge state of all the molecules of a proteoform within the mass range of the MS instrument—and modified 2-DE-PAGE improve the sensitivity of the top-down approach for higher proteoform coverage [215]. In contrast, though bottom-up proteomics is a more sensitive, faster, and easier approach than top-down proteomics to identify high- and low-abundance proteoforms [206,217], it has limitations in identifying complete proteform information and distinguishing between multiple proteoforms originating from a single protein due to protein digestion and a lack of proteoform database [220,222]. SWATH-MS, and a combination of bottom-up and 2-DE approaches, can be useful for high-resolution proteome identification in bottom-up proteomics [217].
Data reproducibility is still a key challenge for proteomics. Though addressing the challenges in human proteomics has been initiated, it remains under-represented in polyploid proteomics. In human proteomics, reproducibility and data accuracy in DIA-MS have been improved by short-time data acquisition from different instruments in triplicate [223]. Furthermore, a pipeline (ProNorM), developed using computational modules, mitigates instrumental variations, resulting in high reproducibility and statistical analysis potential. On the other hand, DDA’s reproducibility can be improved by a dual-search approach—a spectral library is built using the searched sequence data, and further the same data is searched in the library [224]. This method also minimizes the requirement of large database size, but two different sizes libraries result in significant differences in protein and peptide identification. Improved software tools are required for the practical use of the proposed approach (Table 2). Though the progress of top-down and bottom-up proteomics for proteform identification techniques is mostly explored in animal proteomics, with an improved protein extraction protocol, complete protein and proteoform databases, and bioinformatic tools, they can be explored in polyploid proteomics.
The protein extraction protocol from allopolyploid tissues is still being optimized. The complex cell wall composition and presence of secondary metabolites can hinder complete protein extraction [40,225]. For example, wheat tissues have polysaccharides, lipids, polyphenols, and other secondary metabolites [226], while cotton fibers contain polyphenols and lipids in the cell wall [53]. An improved TCA-B (trichloroacetic acid/acetone-borax/PVPP/phenol) protocol of protein extraction from polysachharide and polyphenol rich tissues for further 2-DE and LC-MS/MS has been proposed [227]. Combination of two different protein extraction methods with an additional phenol extraction yielded high-quality protein from cotton leaves. A combination of multiple protein extraction methods also facilitates the achievement of greater protein coverage. Using a combination of two protein extraction buffers, isoelectric focusing and phenol, and pairing pressure cycling technology for further label-free quantification yielded 66 more proteins than the commonly used phenol extraction method in cotton fiber [228]. Protein extraction buffer and digestion enzymes can be modified according to the crops and tissues used for proteomics. Testing seven different protein extraction protocols in polyploid grains, including wheat and barley, Tris-HCL or urea-based extraction buffer coupled with trypsin-based membrane digestion with filter-aided sample preparation (FASP) found effective for a higher number of protein and peptide identifications than the conventional method [229]. However, trypsin digestion is limited to a shortage of lysine and arginine cleavage sites, often restricting precise proteoform identification. On the contrary, multiple enzymatic digestions—trypsin and glutamyl endoproteinase—improve grain and metabolic proteins identification in wheat [230]. High-quality single-seed storage protein extraction from oilseed Brassica—B. rapa, B. nigra, B. juncea, and B. fruticulosa—for 1D-SDS-PAGE based proteomics was improved by using a ball grinder with stainless steel beads in 2.0 mL Safe-Lock tubes with rounded bottoms and hinged lock lids with methanol for 1.2–5.5 mg of single seed grinding [231]. Tris-glycine- and tris-tricine-based extraction buffers were found to be efficient for high- (25–120 kDa) and low- (2–25 kDa) molecular-weight protein separation. Thus, optimized protein extraction protocols are crucial for successful proteomic studies in allopolyploids [40,53,231], while the combination of different protein extraction strategies could be more efficient than a single method.
Another associated challenge is less precise and incomplete protein annotations. Like other diploids, annotation databases of allopolyploids are often incomplete [41,45]. Despite significant advancement of next-generation sequencing, the repetitive genomes, gene duplications, transposable elements and homeologous genes of allopolyploid often challenges genomic annotation of the crops [232]. Although several genomic databases are available for allopolyploid crops, such as Wheat@URGI [233], Bolbase [234], BRAD [235], and CottonGen [236], they often fall short of providing a comprehensive match between transcriptomic and proteomic data. The Plant Public RNA-seq database (PPRD, http://ipf.sustech.edu.cn/pub/plantrna/, accessed on 3 October 2025), a comprehensive web-based platform, offers a common RNAseq libraries for crops, including wheat and cotton, reducing the inequality of data quantification resulting from different bioinformatic tools [237]. To understand the molecular mechanisms of stress tolerance and evolution of polyploids, a common reference standard is recommended. In human omics, standard transcriptomic and proteomic reference datasets have been proposed addressing the variabilities of the two omics [238]. However, developing such reference datasets is challenging for polyploids due to their large genome size with homeologous and duplicated genes, i.e., genetic redundancy, transposable elements, higher level of heterozygosity and chromosomal translocations, and lack of bioinformatic tools for differentiating nearly identical homologs and homeologs and species-specific standard genome assembly [239,240].
Inadequate annotation restricts the identification of protein localization, PTMs, and protein variants in allopolyploids like wheat [225]. Multiomics databases—Wheatomics, BnIR and COTTONOMICS for wheat [241], B. napus [242] and cotton [243], respectively—offer better annotation, while plant stress proteome database (PlantPReS; www.proteome.ir) has >10,600 unique stress proteins. However, individual high-quality protein databases remain essential. For example, the wheat proteome database remains incomplete [40,244], limiting our ability to investigate complex, polygenic traits like abiotic stress tolerance [54], organelle-specific proteomics [chloroplast [245], mitochondria [246] and transmembrane [247], and single-cell proteomics [248]. Incorporating the information of uncharacterized proteins and protein–protein interaction, improving conceptual biasness and computational tools, and integration of different databases can improve the protein annotation dataset of the allopolyploids [249].
Furthermore, while the crop databases are incomplete, deciphering information about unknown proteins, which might play a key role in crop improvement, from the existing protein databases is challenging. However, identifying protein function through experiments is expensive and time-consuming, while computational methods can predict it, which is often less effective, as small changes in amino acid sequences can affect protein structure and functions [250]. Deep learning models such as AlfaFold [251] can help overcome the limitations of computational methods. Though deep-learning models have been introduced in plant proteomics (e.g., sequence-based protein language), but they are restricted to identifying DNA-binding proteins [252], and they are also expensive. A recently proposed conventional machine learning-based model, the LightGBM-based method (FUJISAN), in human proteomics offers opportunities for structural similarities of protein domains and pocket-forming residues identification to predict the functionality of unknown proteins [250].
As functional diversification and neofunctionalization of gene products (protein) are common in allopolyploids due to polyploidization, developing machine learning based models will be beneficial to characterize unknown proteins in allopolyploids. It will also open the avenue of protein engineering to improve protein functionality for improving stress resilience and yield in allopolyploids. However, protein engineering—modification of the amino acid sequence of a protein by mutation or inserting or substituting new sequences through site-directed mutagenesis or direct evolution methods to alter the structure and function of proteins aiming modified phenotypes [253]—in allopolyploid is challenging. While locus-specific mutagenesis is rare in plant protein engineering [254], regulation of protein isoforms, complex protein–protein interaction, and non-equilibrium homeologous proteins in the allopolyploids [45] may hinder successful protein engineering.
Difficulties in pathogenic protein identification, and lack of optimized in vitro mimicking media as well as in vivo models [255] often limit proteomics to understand the molecular mechanism of biotic stress tolerance of crops. Pathogenic protein extraction complexity from the host cell [256], MS-based instrument’s limitations, including ionization suppression [257] and lack of complete genome sequence of the pathogens [258] are the major challenges of pathogenic protein identification. Consequently, protein abundance comparison becomes difficult due to the disproportionate host and pathogenic proteins [258], leading to challenges in proteoforms and PTMs identification. The challenges could be intensified if new pathogenic strains evolved. Use of advanced proteomic approaches, such as DIA, reducing heterogeneity among the infected cells through improving pathogen inoculation methods, using improved mimicking media, and improving pathogenic annotation databases, can improve the understanding of host–pathogen interactions. For example, a recently optimized in vitro-mimicking media improved the protein dynamics in Botrytis cinerea [259], a major pathogen of many crops, including cotton [260]. Protein–protein interaction (PPI) also plays a crucial role in host–pathogen interactions through controlling cellular mechanisms. Recently developed TurboID-based proximity labeling method overcomes the limitations of low efficiency of PPI identification, using the conventional method [261], which has been proposed for a polyploid, potato [262], indicating the prospect of using this method for other polyploids with developing protocols. However, the requirement of wild variety as control, and optimization of biotin addition time and enzyme (Turbo) [261] needs to be considered during protocol development.
Proteomics often fails to contribute to breeding due to a lack of validation of the protein or proteoform biomarkers. Popular validation approach using antibody-based methods such as Western blotting remains extremely challenging [201] due to a lack of strong affinity to the target protein or amino acid residues and the ability to mark only the target, selectivity [263]. While researchers are searching for an efficient protein validation method, using CRISPR/Cas knockdown/knockout of the target-protein encoding functional genes is a promising approach [264]. For proteoform validation, the use of immunoassays [265] and Proteoform-predictor [266] can be explored in polyploids.
6. Prospects of Proteomics in Polyploid Breeding
Rapid advances in genome sequencing technologies have increased the availability of gene sequences for most of the crops. However, the identification of protein coding regions in the genome and their functions in crop performance is essential. With large sequence data in databases, geneticists face the challenge of deciphering the protein products of gene expression. Proteomics, the large-scale study of proteins, holds great promise for revolutionizing plant breeding by enhancing our understanding of the genetic factors underlying complex traits in polyploid crops [45]. Despite major progress over the last two decades in understanding genetic and genomic consequences of polyploidy, research into proteomic-level changes in polyploids remains in its early stages. Protein isoforms and PTMs are key regulators of protein function, activity, localization, and interactions. Current research efforts focus increasingly on linking genotype to proteome to phenotype, and proteomics is expected to emerge as a critical field in polyploid breeding over the coming decade.
During flowering and grain filling, ROS causes oxidative stress and reduces yield [267]. These stresses affect photosynthesis and increase photorespiration by altering cellular homeostasis. However, the complex interactions among gene copy numbers, stress-induced expression changes, and the resulting impacts on the transcriptome, translatome, proteome, metabolome, phenotype, and plant fitness are not fully understood. Investigating the effect of gene content and expression changes in the proteome of autopolyploids and allopolyploids is crucial. Identification of biologically important proteins is also important. To achieve a better understanding of the biologically significant low-abundance proteins, researchers often reduce sample complexity or remove high-abundance proteins.
Polyploidy involves whole-genome duplications, which enables newly formed angiosperm polyploids to tolerate extensive genomic restructuring [188]. These large-scale genome alternation within just one or a few generations result in significant changes to the transcriptome, metabolome, and proteome, ultimately leading to altered phenotypes.
Beyond the evolutionary research challenge, subcellular proteins or multiplex proteins, which are often located in two or more locations, are important for stress tolerance, identification remains challenging [268]. In wheat, isolating specific organelles from whole tissue complicates subcellular proteomic analyses. As a result, many subcellular proteins—particularly stress-related and housekeeping proteins—remain unclassified. A recent AI-based predictor, pLoc-mPlant, has been advanced to pLoc bal-mPlant to identify subcellular proteins in plants using Gene Ontology (GO) annotations and balanced training datasets [269].
The subcellular location resources in The Crop Proteins of Annotated Location database (https://croppal.org/) can be used to improve the model and develop crop-specific datasets for wheat and canola, but need to include other polyploids, including cotton. To understand the host–pathogen interactions, coupled with crop predictors, pathogen-specific subcellular protein predictors such as Gram-LocEN [270] and FunSecKB2 [271] can be considered. Furthermore, the recently proposed dual-perspective protein profiling in clinical proteomics [272], coupled with bioinformatic tools, can be applied in crops including polyploids to identify novel resistance mechanisms, host–pathogen relationships, and disease protection strategies [273]. Therefore, AI-based predictors can help identify organelle-specific proteins, improving our understanding of stress responses and cellular functions in polyploids.
Proteomics helps understand polyploidization, a complementary tool in crop improvement, particularly in identifying differential proteins and their roles in functional expression and evolution. Studying proteome alterations caused by polyploidization gives breeders insights into developing crops with improved traits, boosting productivity and resilience. For example, proteomic studies on hexaploid Brassica identified 452 DAPs with a significant expression bias toward the tetraploid progenitor [274]. In wheat, comparative proteomic analyses across diploid, tetraploid, and hexaploid genotypes revealed that protein expression in hexaploid wheat is influenced not just by individual donor genomes but also by complex interactions among these genomes [275]. Similarly, in cotton, allopolyploidization has led to altered gene expression patterns and diversified proteomic profiles. Hu et al. (2015) [276] reported that 4.4–12.8% of differential protein expression between allopolyploids and their diploid progenitors with A and D genome while over 80% of them were additive in nature. Additionally, proteogenomics—the combination of proteomics, genomics, and transcriptomics—is a highly promising method to refine the gene annotations of these complex polyploids, discover protein coding regions in the genes, and identify novel peptides [277]. For example, using proteogenomics, tissue-specific proteomes including novel proteins and their subcellular locations were identified in the 24 organs of T. aestivum [244]. Thus, proteomics, combining with other omics, multi-omics, can provide better insights into the molecular mechanisms of the stress response of the polyploids for improving their stress-resilience.
Allopolyplodization also offers an opportunity for allele-specific proteomics and protein modification at their active sites. Multiple genome combination creates gene variant patchworks, providing gene expression flexibility in genotype–environment interactions, leading to improved stress tolerance compared to their progenitors [278]. Stress-resilient phenotypes often differ by a tissue-specific single amino acid polymorphism, which can be identified by genotype-specific peptides. Recently, Carpentier (2020) proposed protocols for allele-specific protein identification for allopolyploids, which may overcome the reference genome biasness. However, besides the proposed proteomics, a crop- and genotype-specific genomic library will add more precise protein information. On the other hand, subgenomes of the allopolyploids do not generally recombine and the duplicate genes often help to play multiple roles without affecting each other [279]. For example, the gene family of a metal transporter protein class, a natural resistance-associated macrophage protein (NRAMP), regulates cadmium toxicity in wheat without affecting other metal ion transportation [279], while it affects manganese, iron, and cadmium transportation in rice [280]. Therefore, using CRISPR/Cas9 coupled with computational models, engineering of the active sites of the proteins through in vivo targeted mutagenesis could develop stable and target specific phenotypes in allopolyploids [254]. Though genome engineering in allopolyploids has been advanced significantly [281,282], protein engineering has not been started yet. Recently, using an in vivo mutagenesis, a potential lysine binding sites of dihydrodipicolinate synthase enhanced salinity and drought stress tolerance and yield in rice [283].
Comparative proteomic studies have significantly contributed to identifying and validating candidate genes for crop improvement. These analyses provide molecular insights into grain yield increase and stress tolerance mechanisms, helping breeders to develop high-yielding varieties with improved stress resilience. For example, in wheat, Daba et al. (2020) [284] identified 3182 DAPs involved in tillering, spike initiation, and kernel development after anthesis using quantitative proteomics. These proteins are promising targets for boosting yield potential. Moreover, DAPs have been identified in relation to various stress tolerances, including heat [114], salinity [285], cold [286], drought [287], and disease resistance [159,288]. In cotton, proteomic research revealed key proteins associated with high-quality fiber development [53,289], and DAPs linked to drought [290,291], cold [292], and salinity tolerance [293].
Overall, proteomics helps elucidate how environmental stresses modify protein structure in polyploid crops. Future advancements in functional and subcellular proteomics will enhance our understanding of stress response pathways and facilitate the development of resilient crop varieties. Notably, breakthroughs in wheat genomics have greatly supported breeding efforts, and the integration of new stress-tolerant genes into breeding pipelines will accelerate the development of homozygous lines with high genetic gains. As proteomic separation techniques improve, proteomics will become increasingly central to modern crop breeding programs.
7. Conclusions
Allopolyploid crops such as wheat, oilseed Brassica, and cotton are vital for global food security and economic growth. However, HS and pathogenic infections drastically reduce grain yield, highlighting the need to improve climate resilience and yield stability. The advancement of proteomic approaches has been utilized to unravel the molecular mechanisms underpinning stress responses in these crops. Among the three allopolyploids, proteomic research in wheat has progressed the most. Across these allopolyploid crops, several proteins—particularly those involved in photosynthesis, ROS scavenging, carbohydrate metabolism, peroxidase activity, and HSPs—and protein isoforms have been commonly associated with HS tolerance without grain yield penalty. On the other hand, the proteins involved in responses to biotic stress vary according to the disease specific pathogens. Nonetheless, proteins linked to photosynthesis, defense mechanisms, calcium signaling, lipid metabolism, and lignin biosynthesis commonly contribute to pathogen resistance in the allopolyploids.
Understanding stress-induced proteins at different growth stages is critical for breeding climate-resistant cultivars. In wheat, for example, upregulated proteins such as RuBisCo activase A, and PEP carboxylase, along with antioxidant enzymes like SOD, CAT, and GSH, contribute to HS tolerance in seedlings. Additionally, proteins related to starch biosynthesis, low-molecular-weight glutenin subunits, and gliadin help maintain grain yield and quality under HS. Despite the promising potential of proteomics to enhance our understanding of HS responses and host–pathogen interactions in allopolyploid crops, several limitations remain. A lack of improved protein extraction methodologies, comprehensive protein annotation databases, bioinformatic tools, and pipelines for investigation into protein isoforms, PTMs, and PPI, along with challenges regarding reproducibility, functional validation of candidate protein biomarkers, and subcellular proteomics, are major challenges of proteomics. Combinations of multiple protein extraction methods can offer better protein coverage than the single extraction method. The inefficiency of the current proteomic approaches in overcoming the genetic redundancy of allopolyploids aiming to identify stress-induced proteins, proteoforms, and peptides is another major challenge. Advanced DIA or double-search DDA used in other biological systems can be adapted for improved proteoform identification and reproducibility. An improved TurboID-based proximity labeling method can decipher the PPI to understand the cellular functions of the crops during stress conditions. CRISPR/Cas, immunoassays, and Proteoform-predictor can be potential proteoform validation methods.
Despite challenges, proteomics has great prospects in polyploid breeding. Proteomics can not only be successfully used to discover the evolution of crops and stress-associated genes but also identify the subcellular localization of the candidate proteins. The integration of AI into the existing MS-based proteomic approaches and other predictive tools are promising for identifying stress-induced proteoforms and their subcellular locations, improving protein databases, understanding the crosstalk between PTMs on stress-associated proteins, and protein engineering. Proteomics coupled with other -omics can also be useful for clear understanding of the stress-resilience mechanisms of the allopolyploids. Moreover, addressing the current challenges and gaps of proteomics will be the key to translating molecular insights into practical tools for stress-resilient breeding programs in allopolyploid crops.
Author Contributions
Conceptualization, writing, reviewing, supervision of majority sections of the original draft through interpretating literature, and software, T.H.; writing a section, reviewing, and editing, R.B.; writing another section, M.N.S. and M.O.K.; reviewing, commenting, and editing the manuscript critically, K.H.M.S.; reviewing, providing ideas for figure preparation, and editing G.Y.; reviewing, commenting, and editing, S.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The author is cordially thankful to Samalka Wijeweera, Postdoctoral Research Associate at the University of Western Australia, for helping search for relevant studies.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HS | Heat stress |
| HSPs | Heat shock proteins |
| ROS | Reactive oxygen species |
| MAPK | Mitogen-activated protein kinase |
| SOD | Superoxide dismutase |
| PPI | Protein–protein interaction |
| AI | Artificial intelligence |
References
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Rédei, G.P. Encyclopedia of Genetics, Genomics, Proteomics, and Informatics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef]
- Kui, L.; Majeed, A.; Ahmed, S.; Khan, M.S.S.; Islam, F.; Chen, J.; Dong, Y. Solanum tuberosum (potato). Trends Genet. 2022, 38, 1193–1195. [Google Scholar] [CrossRef]
- Sforça, D.A.; Vautrin, S.; Cardoso-Silva, C.B.; Mancini, M.C.; Romero-da Cruz, M.V.; Pereira, G.d.S.; Conte, M.; Bellec, A.; Dahmer, N.; Fourment, J. Gene duplication in the sugarcane genome: A case study of allele interactions and evolutionary patterns in two genic regions. Front. Plant Sci. 2019, 10, 553. [Google Scholar] [CrossRef]
- El Baidouri, M.; Murat, F.; Veyssiere, M.; Molinier, M.; Flores, R.; Burlot, L.; Alaux, M.; Quesneville, H.; Pont, C.; Salse, J. Reconciling the evolutionary origin of bread wheat (Triticum aestivum). New Phytol. 2017, 213, 1477–1486. [Google Scholar] [CrossRef]
- Branca, F.; Cartea, E. Brassica. In Wild Crop Relatives: Genomic and Breeding Resources: Oilseeds; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–36. [Google Scholar]
- Akagi, T.; Jung, K.; Masuda, K.; Shimizu, K.K. Polyploidy before and after domestication of crop species. Curr. Opin. Plant Biol. 2022, 69, 102255. [Google Scholar] [CrossRef]
- FAO. World Food Situation: FAO Cereal Supply and Demand Brief; FAO (Food and Agriculture Organization of the United States): Roam, Italy, 2025. [Google Scholar]
- Friedt, W.; Tu, J.; Fu, T. Academic and economic importance of Brassica napus rapeseed. In The Brassica Napus Genome; Liu, S., Snowdon, R., Chalhoub, Eds.; Springer: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Rathore, S.S.; Babu, S.; Shekhawat, K.; Singh, V.K.; Upadhyay, P.K.; Singh, R.K.; Raj, R.; Singh, H.; Zaki, F.M. Oilseed Brassica species diversification and crop geometry influence the productivity, economics, and environmental footprints under semi-arid regions. Sustainability 2022, 14, 2230. [Google Scholar] [CrossRef]
- Rai, P.; Yadav, P.; Kumar, A.; Sharma, A.; Kumar, V.; Rai, P. Brassica juncea: A crop for food and health. In The Brassica Juncea Genome; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–13. [Google Scholar]
- USDA. Oilseeds: World Markets and Trade; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2025; pp. 13–21. [Google Scholar]
- Akın, S. The strategic importance of cotton production for the world and Türkiye. In Best Crop Management and Processing Practices for Sustainable Cotton Production; Gürsoy, S., Akın, S., Eds.; IntechOpen: London, UK, 2024. [Google Scholar]
- Khan, M.A.; Wahid, A.; Ahmad, M.; Tahir, M.T.; Ahmed, M.; Ahmad, S.; Hasanuzzaman, M. World cotton production and consumption: An overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Ahmad, S., Hasanuzzaman, M., Eds.; Springer: Singapore, 2020; pp. 1–7. [Google Scholar]
- ICAC. Current Global Cotton Market Outlook for 2024/2025; International Cotton Advisory Commitee: Washington, DC, USA, 2025. [Google Scholar]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cereal Production, Utilization, and Trade Reaching Record Levels in 2021/22; Food and Agriculture Organization: Rome, Italy, 3 February 2022. [Google Scholar]
- CIMMYT. Wheat Research. Available online: https://www.cimmyt.org/work/wheat-research/ (accessed on 15 February 2025).
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; pp. 236–247. [Google Scholar] [CrossRef]
- OECD-FAO. OECD-FAO Agricultural Outlook 2021–2030; Organisation for Economic Co-operation and Development (OECD) and the Food and Agriculture Organization (FAO): Paris, France, 2021. [Google Scholar]
- Zhang, F.; Ge, W.; Huang, L.; Li, D.; Liu, L.; Dong, Z.; Xu, L.; Ding, X.; Zhang, C.; Sun, Y. A comparative analysis of data analysis tools for data-independent acquisition mass spectrometry. Mol. Cell. Proteom. 2023, 22, 100623. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Change 2014, 5, 143–147. [Google Scholar] [CrossRef]
- Kutcher, H.; Warland, J.; Brandt, S. Temperature and precipitation effects on canola yields in Saskatchewan, Canada. Agric. For. Meteorol. 2010, 150, 161–165. [Google Scholar] [CrossRef]
- Sharma, R.K.; Dhillon, J.; Kumar, P.; Reddy, K.R.; Reed, V.; Dodds, D.M.; Reddy, K.N. Modelling the climate change and cotton yield relationship in Mississippi: Autoregressive distributed lag approach. Ecol. Indic. 2024, 166, 112573. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield, oil, and protein: A meta-analysis. Field Crops Res. 2023, 293, 108848. [Google Scholar] [CrossRef]
- Bista, M.K.; Adhikari, B.; Sankarapillai, L.V.; Pieralisi, B.; Reddy, K.R.; Jenkins, J.; Bheemanahalli, R. Drought and heat stress induce differential physiological and agronomic trait responses in cotton. Ind. Crops Prod. 2024, 222, 119540. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Xie, L.; Wu, H.; Han, S.; Hu, L.; Zhang, F.; Wang, H. Quantitative proteomic analysis reveals hub proteins for high temperature-induced male sterility in bread wheat (Triticum aestivum L.). Front. Plant Sci. 2024, 15, 1426832. [Google Scholar] [CrossRef]
- Choudhary, M.; Yan, G.; Siddique, K.H.; Cowling, W.A. Heat stress during meiosis has lasting impacts on plant growth and reproduction in wheat (Triticum aestivum L.). Agronomy 2022, 12, 987. [Google Scholar] [CrossRef]
- Onyemaobi, I.; Liu, H.; Siddique, K.H.; Yan, G. Both male and female malfunction contributes to yield reduction under water stress during meiosis in bread wheat. Front. Plant Sci. 2017, 7, 2071. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Ullah, A.; Siddique, K.H.; Farooq, M. Grain development in wheat under combined heat and drought stress: Plant responses and management. Environ. Exp. Bot. 2021, 188, 104517. [Google Scholar] [CrossRef]
- Bregaglio, S.; Willocquet, L.; Kersebaum, K.C.; Ferrise, R.; Stella, T.; Ferreira, T.B.; Pavan, W.; Asseng, S.; Savary, S. Comparing process-based wheat growth models in their simulation of yield losses caused by plant diseases. Field Crops Res. 2021, 265, 108108. [Google Scholar] [CrossRef]
- Strehlow, B.; de Mol, F.; Struck, C. Risk potential of clubroot disease on winter oilseed rape. Plant Dis. 2015, 99, 667–675. [Google Scholar] [CrossRef]
- Parkash, V.; Sharma, D.B.; Snider, J.; Bag, S.; Roberts, P.; Tabassum, A.; West, D.; Khanal, S.; Suassuna, N.; Chee, P. Effect of cotton leafroll dwarf virus on physiological processes and yield of individual cotton plants. Front. Plant Sci. 2021, 12, 734386. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant abiotic stress proteomics: The major factors determining alterations in cellular proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Klíma, M.; Renaut, J. Plant proteoforms under environmental stress: Functional proteins arising from a single gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Smith, L.M.; Kelleher, N.L. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Vila, M.; Laguna, A. Systems Medicine in Parkinson’s Disease: Joining Efforts to Change History. In Systems Medicine Integrative, Qualitative and Computational Approaches; Wolkenhauer, O., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 2, pp. 1–14. [Google Scholar]
- Singh, B.; Mishra, S.; Bohra, A.; Joshi, R.; Siddique, K.H. Crop phenomics for abiotic stress tolerance in crop plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 277–296. [Google Scholar]
- Halder, T.; Choudhary, M.; Liu, H.; Chen, Y.; Yan, G.; Siddique, K.H. Wheat proteomics for abiotic stress tolerance and root system architecture: Current status and future prospects. Proteomes 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Stroeher, E.; Liu, H.; Chen, Y.; Yan, G.; Siddique, K.H.M. Protein biomarkers for root length and root dry mass on chromosomes 4A and 7A in wheat. J. Proteom. 2024, 291, 105044. [Google Scholar] [CrossRef]
- Fu, X.; Fu, N.; Guo, S.; Yan, Z.; Xu, Y.; Hu, H.; Menzel, C.; Chen, W.; Li, Y.; Zeng, R. Estimating accuracy of RNA-Seq and microarrays with proteomics. BMC Genom. 2009, 10, 161. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.H.; Rasool, S.; Akram, N.A.; Ashraf, M.; Gucel, S. Role of proteomics in crop stress tolerance. Front. Plant Sci. 2016, 7, 1336. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Gayen, D. Decoding post-translational modifications for understanding stress tolerance in plant. Crop Des. 2024, 100077. [Google Scholar] [CrossRef]
- Soltis, D.E.; Misra, B.B.; Shan, S.; Chen, S.; Soltis, P.S. Polyploidy and the proteome. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2016, 1864, 896–907. [Google Scholar] [CrossRef]
- Thomson, J.J. Bakerian Lecture:—Rays of positive electricity. In Proceedings of the Royal Society, London, UK, 1 August 1913; pp. 1–20. [Google Scholar]
- Müller, F.; Kolbowski, L.; Bernhardt, O.M.; Reiter, L.; Rappsilber, J. Data-independent acquisition improves quantitative cross-linking mass spectrometry. Mol. Cell. Proteom. 2019, 18, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, W.; Liu, Y.; Shen, Y.; Li, W. Unlocking Wheat’s Heat Stress Survival Secrets: A Comprehensive Study of Spike Development’s Metabolic Responses. J. Plant Growth Regul. 2024, 43, 1875–1890. [Google Scholar] [CrossRef]
- Shen, C.-C.; Chen, M.-X.; Xiao, T.; Zhang, C.; Shang, J.; Zhang, K.-L.; Zhu, F.-Y. Global proteome response to Pb (II) toxicity in poplar using SWATH-MS-based quantitative proteomics investigation. Ecotoxicol. Environ. Saf. 2021, 220, 112410. [Google Scholar] [CrossRef]
- Masoomi-Aladizgeh, F.; McKay, M.J.; Asar, Y.; Haynes, P.A.; Atwell, B.J. Patterns of gene expression in pollen of cotton (Gossypium hirsutum) indicate downregulation as a feature of thermotolerance. Plant J. 2022, 109, 965–979. [Google Scholar] [CrossRef] [PubMed]
- Gris, C.F.; Baldoni, A. Proteomics and its use in obtaining superior soybean genotypes. In A Comprehensive Survey of International Soybean Research-Genetics, Physiology, Agronomy and Nitrogen Relationships; IntechOpen: New York, NY, USA, 2013. [Google Scholar]
- Nadeem, M.; Anjum, F.M.; Khan, M.R.; Sajjad, M.; Hussain, S.; Arshad, M.S. Electrophoretic characteristics of gluten proteins as influenced by crop year and variety. Int. J. Food Prop. 2016, 19, 897–910. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, G.; Sun, Z.; Tang, Y.; Wu, Y. Cotton proteomics for deciphering the mechanism of environment stress response and fiber development. J. Proteom. 2014, 105, 74–84. [Google Scholar] [CrossRef]
- Barkla, B.J.; Vera-Estrella, R.; Pantoja, O. Progress and challenges for abiotic stress proteomics of crop plants. Proteomics 2013, 13, 1801–1815. [Google Scholar] [CrossRef]
- Sharif, I.; Aleem, S.; Junaid, J.A.; Ali, Z.; Aleem, M.; Shahzad, R.; Farooq, J.; Khan, M.I.; Arshad, W.; Ellahi, F. Multiomics approaches to explore drought tolerance in cotton. J. Cotton Res. 2024, 7, 32. [Google Scholar] [CrossRef]
- D’Agostino, N.; Fasano, C. Editorial: Genetics and Genomics of Polyploid Plants. Genes 2024, 15, 1377. [Google Scholar] [CrossRef] [PubMed]
- Bird, K.A.; VanBuren, R.; Puzey, J.R.; Edger, P.P. The causes and consequences of subgenome dominance in hybrids and recent polyploids. New Phytol. 2018, 220, 87–93. [Google Scholar] [CrossRef]
- Leitch, A.; Leitch, I. Genomic plasticity and the diversity of polyploid plants. Science 2008, 320, 481–483. [Google Scholar] [CrossRef]
- Sloan, D.B.; Warren, J.M.; Williams, A.M.; Wu, Z.; Abdel-Ghany, S.E.; Chicco, A.J.; Havird, J.C. Cytonuclear integration and co-evolution. Nat. Rev. Genet. 2018, 19, 635–648. [Google Scholar] [CrossRef]
- Grover, C.E.; Forsythe, E.S.; Sharbrough, J.; Miller, E.R.; Conover, J.L.; DeTar, R.A.; Chavarro, C.; Arick, M.A.; Peterson, D.G.; Leal-Bertioli, S.C. Variation in cytonuclear expression accommodation among allopolyploid plants. Genetics 2022, 222, iyac118. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Koh, J.; Yoo, M.-J.; Chen, S.; Wendel, J.F. Gene-expression novelty in allopolyploid cotton: A proteomic perspective. Genetics 2015, 200, 91–104. [Google Scholar] [CrossRef]
- Song, Q.; Chen, Z.J. Epigenetic and developmental regulation in plant polyploids. Curr. Opin. Plant Biol. 2015, 24, 101–109. [Google Scholar] [CrossRef]
- Aufiero, G.; Fruggiero, C.; D’Angelo, D.; D’Agostino, N. Homoeologs in Allopolyploids: Navigating Redundancy as Both an Evolutionary Opportunity and a Technical Challenge—A Transcriptomics Perspective. Genes 2024, 15, 977. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.; Gallagher, J.; Szadkowski, E.; Yoo, M.; Flagel, L.; Wendel, J. Homoeolog expression bias and expression level dominance in allopolyploids. New Phytol. 2012, 196, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, Y.; Zhang, T.; Chen, M.; Song, H. Proteome evaluation of homolog abundance patterns in Arachis hypogaea cv. Tifrunner. Plant Methods 2022, 18, 6. [Google Scholar] [CrossRef]
- Zhang, B.; Kuster, B. Proteomics is not an island: Multi-omics integration is the key to understanding biological systems. Mol. Cell. Proteom. 2019, 18, S1–S4. [Google Scholar] [CrossRef]
- Peng, J.H.; Sun, D.; Nevo, E. Domestication evolution, genetics and genomics in wheat. Mol. Breed. 2011, 28, 281–301. [Google Scholar] [CrossRef]
- Borrill, P.; Harrington, S.A.; Uauy, C. Applying the latest advances in genomics and phenomics for trait discovery in polyploid wheat. Plant J. 2019, 97, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Avni, R.; Nave, M.; Barad, O.; Baruch, K.; Twardziok, S.O.; Gundlach, H.; Hale, I.; Mascher, M.; Spannagl, M.; Wiebe, K. Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 2017, 357, 93–97. [Google Scholar] [CrossRef]
- Ling, H.-Q.; Zhao, S.; Liu, D.; Wang, J.; Sun, H.; Zhang, C.; Fan, H.; Li, D.; Dong, L.; Tao, Y. Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 2013, 496, 87–90. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef]
- Vélez-Gavilán, J. Brassica rapa (field mustard). In CABI Compendium; CABI International: Wallingford, UK, 2022; Volume 10. [Google Scholar]
- Cai, C.; Wang, X.; Liu, B.; Wu, J.; Liang, J.; Cui, Y.; Cheng, F.; Wang, X. Brassica rapa genome 2.0: A reference upgrade through sequence re-assembly and gene re-annotation. Mol. Plant 2017, 10, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, J.; Chen, H.; Zhang, Z.; Wu, J.; Wang, X. A near-complete genome assembly of Brassica rapa provides new insights into the evolution of centromeres. Plant Biotechnol. J. 2023, 21, 1022–1032. [Google Scholar] [CrossRef]
- Guo, N.; Wang, S.; Gao, L.; Liu, Y.; Wang, X.; Lai, E.; Duan, M.; Wang, G.; Li, J.; Yang, M. Genome sequencing sheds light on the contribution of structural variants to Brassica oleracea diversification. BMC Biol. 2021, 19, 93. [Google Scholar] [CrossRef]
- Ji, G.; Long, Y.; Cai, G.; Wang, A.; Yan, G.; Li, H.; Gao, G.; Xu, K.; Huang, Q.; Chen, B. A new chromosome-scale genome of wild Brassica oleracea provides insights into the domestication of Brassica crops. J. Exp. Bot. 2024, 75, 2882–2899. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Koh, C.S.; Jin, L.; Buchwaldt, M.; Higgins, E.E.; Zheng, C.; Sankoff, D.; Robinson, S.J.; Kagale, S.; Navabi, Z.-K. A high-contiguity Brassica nigra genome localizes active centromeres and defines the ancestral Brassica genome. Nat. Plants 2020, 6, 929–941. [Google Scholar] [CrossRef]
- Paritosh, K.; Pradhan, A.K.; Pental, D. A highly contiguous genome assembly of Brassica nigra (BB) and revised nomenclature for the pseudochromosomes. BMC Genom. 2020, 21, 887. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232. [Google Scholar] [CrossRef]
- Heng, S.; Cui, M.; Li, X.; Zhang, S.; Mao, G.; Xing, F.; Wan, Z.; Wen, J.; Shen, J.; Fu, T. High quality genome of potherb mustard XC (Brassica juncea var. multiceps) provides new insight into leaf shape variation1. J. Integr. Agric. 2025; 24, 1461–1476. [Google Scholar]
- Niu, Y.; Liu, Q.; He, Z.; Raman, R.; Wang, H.; Long, X.; Qin, H.; Raman, H.; Parkin, I.A.; Bancroft, I. A Brassica carinata pan-genome platform for Brassica crop improvement. Plant Commun. 2024, 5, 100725. [Google Scholar] [CrossRef]
- Emani, C. Gossypium. In Wild Crop Relatives: Genomic and Breeding Resources: Industrial Crops; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–122. [Google Scholar]
- Egbuta, M.A.; McIntosh, S.; Waters, D.L.; Vancov, T.; Liu, L. Biological importance of cotton by-products relative to chemical constituents of the cotton plant. Molecules 2017, 22, 93. [Google Scholar] [CrossRef]
- Wegier, A.; Alavez, V.; Piñero, D. Cotton: Traditional and modern uses. In Ethnobotany of Mexico; Springer: New York, NY, USA, 2016; pp. 439–456. [Google Scholar]
- Adams, K.L.; Wendel, J.F. Exploring the genomic mysteries of polyploidy in cotton. Biol. J. Linn. Soc. 2004, 82, 573–581. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, C.; He, L.; Guo, Q.; Zhang, X.; Xu, P.; Su, H.; Gong, Y.; Ni, W.; Shen, X. Morphological, cytological and molecular analyses of a synthetic hexaploid derived from an interspecific hybrid between Gossypium hirsutum and Gossypium anomalum. Crop J. 2014, 2, 272–277. [Google Scholar] [CrossRef]
- Percival, A.; Wendel, J.; Stewart, J. Taxonomy and germplasm resources. Cotton Orig. Hist. Technol. Prod. 1999, 33, 63. [Google Scholar]
- Wendel, J.; Clark Cronn, R.; Clark, R.; Cronn, R. Polyploidy and the Evolutionary History of Cotton. Adv. Agron. 2003, 78, 139–186. [Google Scholar]
- Chen, Z.J.; Sreedasyam, A.; Ando, A.; Song, Q.; De Santiago, L.M.; Hulse-Kemp, A.M.; Ding, M.; Ye, W.; Kirkbride, R.C.; Jenkins, J. Genomic diversifications of five Gossypium allopolyploid species and their impact on cotton improvement. Nat. Genet. 2020, 52, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Xu, Y.; Tian, S.; Unver, T.; Liu, Z.; Zhou, Z.; Cai, X.; Wang, K.; Wei, Y.; Liu, Y. Evolutionary divergence of duplicated genomes in newly described allotetraploid cottons. Proc. Natl. Acad. Sci. USA 2022, 119, e2208496119. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, G.; Wang, K.; Sun, F.; Yuan, Y.; Song, G.; Li, Q.; Ma, Z.; Lu, C.; Zou, C. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 2014, 46, 567–572. [Google Scholar] [CrossRef]
- Ramaraj, T.; Grover, C.E.; Mendoza, A.C.; Arick, M.A.; Jareczek, J.J.; Leach, A.G.; Peterson, D.G.; Wendel, J.F.; Udall, J.A. The Gossypium herbaceum L. Wagad genome as a resource for understanding cotton domestication. G3 2023, 13, jkac308. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, B.; Stewart, J.M. Estimation of the nuclear DNA content of Gossypium species. Ann. Bot. 2005, 95, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.E.; Gallagher, J.P.; Jareczek, J.J.; Page, J.T.; Udall, J.A.; Gore, M.A.; Wendel, J.F. Re-evaluating the phylogeny of allopolyploid Gossypium L. Mol. Phylogenetics Evol. 2015, 92, 45–52. [Google Scholar] [CrossRef]
- Wendel, J.F.; Grover, C.E. Taxonomy and evolution of the cotton genus, Gossypium. Cotton 2015, 57, 25–44. [Google Scholar]
- Meng, Q.; Gu, J.; Xu, Z.; Zhang, J.; Tang, J.; Wang, A.; Wang, P.; Liu, Z.; Rong, Y.; Xie, P. Comparative analysis of genome sequences of the two cultivated tetraploid cottons, Gossypium hirsutum (L.) and G. barbadense (L.). Ind. Crops Prod. 2023, 196, 116471. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, M.; Ahmed, M.; Iftikhar Hussain, M. Rising atmospheric temperature impact on wheat and thermotolerance strategies. Plants 2020, 10, 43. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef]
- Feng, J.; Jia, Y.; Xu, B.; Bi, X.; Ge, Z.; Ma, G.; Xie, Y.; Wang, C.; Ma, D. Quantitative proteomic analysis for characterization of protein components related to dough quality and celiac disease in wheat flour, dough, and heat-treated dough. Food Chem. 2024, 461, 140924. [Google Scholar] [CrossRef]
- Fei, L.; Chu, J.; Zhang, X.; Dong, S.; Dai, X.; He, M. Physiological and proteomic analyses indicate delayed sowing improves photosynthetic capacity in wheat flag leaves under heat stress. Front. Plant Sci. 2022, 13, 848464. [Google Scholar] [CrossRef] [PubMed]
- Chunduri, V.; Kaur, A.; Kaur, S.; Kumar, A.; Sharma, S.; Sharma, N.; Singh, P.; Kapoor, P.; Kaur, S.; Kumari, A. Gene expression and proteomics studies suggest an involvement of multiple pathways under day and day–night combined heat stresses during grain filling in wheat. Front. Plant Sci. 2021, 12, 660446. [Google Scholar] [CrossRef]
- Kumar, R.R.; Dubey, K.; Arora, K.; Dalal, M.; Rai, G.K.; Mishra, D.; Chaturvedi, K.K.; Rai, A.; Kumar, S.N.; Singh, B. Characterizing the putative mitogen-activated protein kinase (MAPK) and their protective role in oxidative stress tolerance and carbon assimilation in wheat under terminal heat stress. Biotechnol. Rep. 2021, 29, e00597. [Google Scholar] [CrossRef]
- Wu, B.; Qiao, J.; Wang, X.; Liu, M.; Xu, S.; Sun, D. Factors affecting the rapid changes of protein under short-term heat stress. BMC Genom. 2021, 22, 263. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Dong, J.; Tian, X.; Wang, J.; Palta, J.A.; Xu, S.; Fang, Y.; Wang, Z. Over-expression of the heat-responsive wheat gene TaHSP23. 9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Front. Plant Sci. 2020, 11, 243. [Google Scholar]
- Kumar, R.R.; Singh, K.; Ahuja, S.; Tasleem, M.; Singh, I.; Kumar, S.; Grover, M.; Mishra, D.; Rai, G.K.; Goswami, S. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct. Integr. Genom. 2019, 19, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, H.; Guo, D.; Zhang, R.; Su, M.; Hou, Z.; Zhou, H.; Liang, R.; Xie, C.; You, M. Identifying changes in the wheat kernel proteome under heat stress using iTRAQ. Crop J. 2018, 6, 600–610. [Google Scholar] [CrossRef]
- Perveen, A.; Sheheryar, S.; Ahmad, F.; Mustafa, G.; Moura, A.A.; Campos, F.A.; Domont, G.B.; Nishan, U.; Ullah, R.; Ibrahim, M.A. Integrative physiological, biochemical, and proteomic analysis of the leaves of two cotton genotypes under heat stress. PLoS ONE 2025, 20, e0316630. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Liu, F.; Zhang, T.; Guan, X. GhHSP24. 7 mediates mitochondrial protein acetylation to regulate stomatal conductance in response to abiotic stress in cotton. Crop J. 2023, 11, 1128–1139. [Google Scholar] [CrossRef]
- Masoomi-Aladizgeh, F.; Najeeb, U.; Hamzelou, S.; Pascovici, D.; Amirkhani, A.; Tan, D.K.; Mirzaei, M.; Haynes, P.A.; Atwell, B.J. Pollen development in cotton (Gossypium hirsutum) is highly sensitive to heat exposure during the tetrad stage. Plant Cell Environ. 2021, 44, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Q.; Zhao, Z.; Dong, Z.; Chen, Y.; Chen, D. Analysis of differentially expressed proteins affecting insecticidal protein content in Bt cotton under high-temperature and water deficit stress using label-free quantitation. J. Agron. Crop Sci. 2021, 207, 1–11. [Google Scholar] [CrossRef]
- Masoomi-Aladizgeh, F.; Kamath, K.S.; Haynes, P.A.; Atwell, B.J. Genome survey sequencing of wild cotton (Gossypium robinsonii) reveals insights into proteomic responses of pollen to extreme heat. Plant Cell Environ. 2022, 45, 1242–1256. [Google Scholar] [CrossRef]
- Rani, R.; Mawlong, I.; Balbeer, B.; Kumar, M.S.; Rai, P.K.; Singh, V.V. Proteomic, biochemical and peptidomics based analysis reveals heat responsive changes in the seedlings of Brassica juncea. J. Plant Biochem. Biotechnol. 2024, 33, 570–589. [Google Scholar] [CrossRef]
- Singiri, J.R.; Swetha, B.; Sikron-Persi, N.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xie, S.; Zhao, M.; Nie, L.; Zheng, Y.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Comparative proteomics indicates that redox homeostasis is involved in high-and low-temperature stress tolerance in a novel Wucai (Brassica campestris L.) genotype. Int. J. Mol. Sci. 2019, 20, 3760. [Google Scholar] [CrossRef]
- Mishra, D.; Shekhar, S.; Chakraborty, S.; Chakraborty, N. Wheat 2-Cys peroxiredoxin plays a dual role in chlorophyll biosynthesis and adaptation to high temperature. Plant J. 2021, 105, 1374–1389. [Google Scholar] [CrossRef]
- Gupta, O.P.; Mishra, V.; Singh, N.; Tiwari, R.; Sharma, P.; Gupta, R.; Sharma, I. Deciphering the dynamics of changing proteins of tolerant and intolerant wheat seedlings subjected to heat stress. Mol. Biol. Rep. 2015, 42, 43–51. [Google Scholar] [CrossRef]
- Cao, J.; Qin, Z.; Cui, G.; Chen, Z.; Cheng, X.; Peng, H.; Yao, Y.; Hu, Z.; Guo, W.; Ni, Z. Natural variation of STKc_GSK3 kinase TaSG-D1 contributes to heat stress tolerance in Indian dwarf wheat. Nat. Commun. 2024, 15, 2097. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Zhang, B.; Zhou, J.; Wang, Y.; Yang, Q.; Ke, Y.; He, H. Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci. 2011, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Qian, Y.; Zhang, W.; Qian, L.; Wang, Y.; Cailin, G.; Ding, H. Mitogen-activated protein kinase action in plant response to high-temperature stress: A mini review. Protoplasma 2021, 258, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Zhu, T.; Verstraeten, I.; Van De Cotte, B.; Consortium, I.W.G.S.; Gevaert, K.; De Smet, I. Temperature-induced changes in the wheat phosphoproteome reveal temperature-regulated interconversion of phosphoforms. J. Exp. Bot. 2018, 69, 4609–4624. [Google Scholar] [CrossRef] [PubMed]
- Majoul, T.; Bancel, E.; Triboï, E.; Ben Hamida, J.; Branlard, G. Proteomic analysis of the effect of heat stress on hexaploid wheat grain: Characterization of heat-responsive proteins from total endosperm. Proteomics 2003, 3, 175–183. [Google Scholar] [CrossRef]
- Skylas, D.; Cordwell, S.; Hains, P.; Larsen, M.; Basseal, D.; Walsh, B.; Blumenthal, C.; Rathmell, W.; Copeland, L.; Wrigley, C. Heat shock of wheat during grain filling: Proteins associated with heat-tolerance. J. Cereal Sci. 2002, 35, 175–188. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef]
- Angadi, S.; Cutforth, H.; Miller, P.; McConkey, B.; Entz, M.; Brandt, S.; Volkmar, K. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Skalicky, M.; Hussain, S.; Zulfiqar, U.; Anjum, M.Z.; Habib ur Rahman, M.; Brestic, M.; Ratnasekera, D.; Lamilla-Tamayo, L. Adaptation strategies to improve the resistance of oilseed crops to heat stress under a changing climate: An overview. Front. Plant Sci. 2021, 12, 767150. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Lv, X.; Gao, G.; Li, F.; Li, J.; Qiao, J.; Xu, K.; Chen, B.; Wang, L.; Xiao, X. Identification and characterization of a glyoxalase I gene in a rapeseed cultivar with seed thermotolerance. Front. Plant Sci. 2016, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, A.; Salavati, A.; Pour Mohammadi, P. A comparative proteomic analysis of responses to high temperature stress in hypocotyl of canola (Brassica napus L.). Protein Pept. Lett. 2015, 22, 285–299. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Li, Y.; Ma, Y.; Zhao, Y.; Wang, C.; Chi, H.; Chen, M.; Ding, Y.; Guo, X. Proteomic analysis reveals that sugar and fatty acid metabolisms play a central role in sterility of the male-sterile line 1355A of cotton. J. Biol. Chem. 2019, 294, 7057–7067. [Google Scholar] [CrossRef]
- Swetha, B.; Singiri, J.R.; Novoplansky, N.; Grandhi, R.; Srinivasan, J.; Khadka, J.; Galis, I.; Grafi, G. Single and combined salinity and heat stresses impact yield and dead pericarp priming activity. Plants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Schulte, I.; Tammen, H.; Selle, H.; Schulz-Knappe, P. Peptides in body fluids and tissues as markers of disease. Expert Rev. Mol. Diagn. 2005, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y. Response and tolerance mechanism of cotton Gossypium hirsutum L. to elevated temperature stress: A review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef]
- Wise, R.; Olson, A.; Schrader, S.; Sharkey, T. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Ashraf, M.; Saeed, M.; Qureshi, M. Tolerance to high temperature in cotton (Gossypium hirsutum L.) at initial growth stages. Environ. Exp. Bot. 1994, 34, 275–283. [Google Scholar] [CrossRef]
- Majeed, S.; Rana, I.A.; Mubarik, M.S.; Atif, R.M.; Yang, S.-H.; Chung, G.; Jia, Y.; Du, X.; Hinze, L.; Azhar, M.T. Heat stress in cotton: A review on predicted and unpredicted growth-yield anomalies and mitigating breeding strategies. Agronomy 2021, 11, 1825. [Google Scholar] [CrossRef]
- Kou, S.-Y.; Wu, Z.-G.; Li, H.-Y.; Chen, X.; Liu, W.-H.; Yuan, P.-R.; Zhu, Z.-H.; Yang, X.; Li, H.-H.; Huang, P. Heterologous expression of heat-shock protein PpHSP70 improves high temperature and drought tolerance in rice. Plant Stress 2023, 10, 100273. [Google Scholar] [CrossRef]
- Sahu, B.; Choudhary, V.K.; Sahu, M.; Kumar, K.K.; Sujayanand, G.; Gopi, R.; Prakasam, V.; Sridhar, J.; Mallikarjuna, J.; Singh, H. Biotic Stress Management. In Trajectory of 75 Years of Indian Agriculture After Independence; Springer: Berlin/Heidelberg, Germany, 2023; pp. 619–653. [Google Scholar]
- Dixit, S.; Sivalingam, P.N.; Baskaran, R.M.; Senthil-Kumar, M.; Ghosh, P.K. Plant responses to concurrent abiotic and biotic stress: Unravelling physiological and morphological mechanisms. Plant Physiol. Rep. 2024, 29, 6–17. [Google Scholar] [CrossRef]
- Kayess, O.; Ashrafuzzaman, M.; Khan, M.A.R.; Siddiqui, M.N. Functional phenomics and genomics: Unravelling heat stress responses in wheat. Plant Stress 2024, 14, 100601. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Mmbando, G.S. Omics: A new, promising technologies for boosting crop yield and stress resilience in African agriculture. Plant Stress 2024, 11, 100366. [Google Scholar] [CrossRef]
- Haq, S.A.U.; Bashir, T.; Roberts, T.H.; Husaini, A.M. Ameliorating the effects of multiple stresses on agronomic traits in crops: Modern biotechnological and omics approaches. Mol. Biol. Rep. 2024, 51, 41. [Google Scholar] [CrossRef] [PubMed]
- Bakala, H.S.; Mandahal, K.S.; Sarao, L.K.; Srivastava, P. Breeding wheat for biotic stress resistance: Achievements, challenges and prospects. Curr. Trends Wheat Res. 2021, 12, 11–34. [Google Scholar]
- Kema, G.H.; Mirzadi Gohari, A.; Aouini, L.; Gibriel, H.A.; Ware, S.B.; van Den Bosch, F.; Manning-Smith, R.; Alonso-Chavez, V.; Helps, J.; Ben M’Barek, S. Stress and sexual reproduction affect the dynamics of the wheat pathogen effector AvrStb6 and strobilurin resistance. Nat. Genet. 2018, 50, 375–380. [Google Scholar] [CrossRef]
- Yang, F.; Melo-Braga, M.N.; Larsen, M.R.; Jørgensen, H.J.; Palmisano, G. Battle through signaling between wheat and the fungal pathogen Septoria tritici revealed by proteomics and phosphoproteomics. Mol. Cell. Proteom. 2013, 12, 2497–2508. [Google Scholar] [CrossRef]
- Yang, F.; Yin, Q. Comprehensive proteomic analysis of the wheat pathogenic fungus Zymoseptoria tritici. Proteomics 2016, 16, 98–101. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Fiorilli, V.; Seco, D.G.; Novero, M.; Marsoni, M.; Wisniewski-Dye, F.; Bracale, M.; Moulin, L.; Bonfante, P. Proteomic analysis reveals how pairing of a Mycorrhizal fungus with plant growth-promoting bacteria modulates growth and defense in wheat. Plant Cell Environ. 2021, 44, 1946–1960. [Google Scholar] [CrossRef]
- Maytalman, D.; Mert, Z.; Baykal, A.; Inan, C.; Gunel, A.; Hasançebi, S. Proteomic analysis of early responsive resistance proteins of wheat (Triticum aestivum) to yellow rust (Puccinia striiformis f. sp. tritici) using ProteomeLab PF2D. Plant Omics 2013, 6, 24–35. [Google Scholar]
- Wang, J.; Diaz, J.; Hua, K.; Bellizzi, M.; Qi, L.; Zhu, L.; Qu, M.; Wang, G.-L. Proteomic identification of apoplastic proteins from rice, wheat, and barley after Magnaporthe oryzae infection. Phytopathol. Res. 2024, 6, 55. [Google Scholar] [CrossRef]
- He, T.; Xu, T.; Muhae-Ud-Din, G.; Guo, Q.; Liu, T.; Chen, W.; Gao, L. iTRAQ-based proteomic analysis of wheat (Triticum aestivum) spikes in response to Tilletia controversa Kühn and Tilletia foetida Kühn infection, causal organisms of dwarf bunt and common bunt of wheat. Biology 2022, 11, 865. [Google Scholar] [CrossRef]
- Qiao, F.; Yang, X.; Xu, F.; Huang, Y.; Zhang, J.; Song, M.; Zhou, S.; Zhang, M.; He, D. TMT-based quantitative proteomic analysis reveals defense mechanism of wheat against the crown rot pathogen Fusarium pseudograminearum. BMC Plant Biol. 2021, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, X.; Dong, J.; Zhao, W.; Alam, T.; Thomashow, L.S.; Weller, D.M.; Gao, X.; Rustgi, S.; Wen, S. Proteomics reveals the changes that contribute to Fusarium head blight resistance in wheat. Phytopathology 2021, 111, 386–397. [Google Scholar] [CrossRef]
- Fabre, F.; Urbach, S.; Roche, S.; Langin, T.; Bonhomme, L. Proteomics-based data integration of wheat cultivars facing Fusarium graminearum strains revealed a core-responsive pattern controlling Fusarium head blight. Front. Plant Sci. 2021, 12, 644810. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Jin, P.; Guo, M.; Guo, J.; Cheng, P.; Li, Q.; Wang, B. Glutathione S-transferase interactions enhance wheat resistance to powdery mildew but not wheat stripe rust. Plant Physiol. 2022, 190, 1418–1439. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, H.; Mandal, S.N.; Wang, C.; Chen, C.; Ji, W. Quantitative proteomics reveals the central changes of wheat in response to powdery mildew. J. Proteom. 2016, 130, 108–119. [Google Scholar] [CrossRef]
- Islam, M.T.; Lee, B.-R.; La, V.H.; Bae, D.-W.; Jung, W.-J.; Kim, T.-H. Label-free quantitative proteomics analysis in susceptible and resistant Brassica napus cultivars infected with Xanthomonas campestris pv. campestris. Microorganisms 2021, 9, 253. [Google Scholar] [CrossRef]
- Adhikary, D.; Mehta, D.; Uhrig, R.G.; Rahman, H.; Kav, N.N. A Proteome-level investigation into Plasmodiophora brassicae resistance in Brassica napus canola. Front. Plant Sci. 2022, 13, 860393. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kim, S.T.; Choi, G.J.; Kwon, S.-Y.; Cho, H.S.; Kim, H.-S.; Moon, J.S.; Park, J.M. Comparative proteomic analysis of host responses to Plasmodiophora brassicae infection in susceptible and resistant Brassica oleracea. Plant Biotechnol. Rep. 2020, 14, 263–274. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, P.; Budhlakoti, N.; Mishra, D.C.; Bhardwaj, N.R.; Rao, M.; Sharma, P.; Gupta, N.C. Exploration of the Sclerotinia sclerotiorum-Brassica pathosystem: Advances and perspectives in omics studies. Mol. Biol. Rep. 2024, 51, 1097. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Srivastava, S.; Rahman, M.H.; Strelkov, S.E.; Kav, N.N. Proteome changes in leaves of Brassica napus L. as a result of Sclerotinia sclerotiorum challenge. J. Agric. Food Chem. 2008, 56, 1963–1976. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Li, H.; Sivasithamparam, K.; Barbetti, M.J. Differentially expressed proteins and associated histological and disease progression changes in cotyledon tissue of a resistant and susceptible genotype of Brassica napus infected with Sclerotinia sclerotiorum. PLoS ONE 2013, 8, e65205. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Rahman, M.H.; Strelkov, S.E.; Kav, N.N. Developmentally induced changes in the sclerotial proteome of Sclerotinia sclerotiorum. Fungal Biol. 2010, 114, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-Y.; Xu, Y.-P.; Cai, X.-Z. TMT-based quantitative proteomics analyses reveal novel defense mechanisms of Brassica napus against the devastating necrotrophic pathogen Sclerotinia sclerotiorum. J. Proteom. 2016, 143, 265–277. [Google Scholar] [CrossRef]
- Singh, M.; Avtar, R.; Lakra, N.; Hooda, E.; Singh, V.K.; Bishnoi, M.; Kumari, N.; Punia, R.; Kumar, N.; Choudhary, R.R. Genetic and proteomic basis of sclerotinia stem rot resistance in Indian mustard [Brassica juncea (L.) czern & coss.]. Genes 2021, 12, 1784. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, C.; Gao, L.; Zeng, L.; Xu, Y.; Liu, F.; Huang, J.; Liu, L.; Liu, S.; Zhang, X. Alternative splicing reprogramming in fungal pathogen Sclerotinia sclerotiorum at different infection stages on Brassica napus. Front. Plant Sci. 2022, 13, 1008665. [Google Scholar] [CrossRef]
- Kamburova, V.; Salakhutdinov, I.; Abdurakhmonov, I.Y. Cotton breeding in the view of abiotic and biotic stresses: Challenges and perspectives. In Cotton; Abdurakhmonov, I.Y., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Beckman, C. Cell irritability and localization of vascular infections in plants. Phytopathology 1966, 56, 821–824. [Google Scholar]
- Aydın, M.H. Rhizoctonia solani and its biological control. Türkiye Tarımsal Araştırmalar Derg. 2022, 9, 118–135. [Google Scholar] [CrossRef]
- Jalloul, A.; Sayegh, M.; Champion, A.; Nicole, M. Bacterial blight of cotton. Phytopathol. Mediterr. 2015, 54, 3–20. [Google Scholar]
- Palanga, K.K.; Liu, R.; Ge, Q.; Gong, J.; Li, J.; Lu, Q.; Li, P.; Yuan, Y.; Gong, W. Current advances in pathogen-plant interaction between Verticillium dahliae and cotton provide new insight in the disease management. J. Cotton Res. 2021, 4, 25. [Google Scholar] [CrossRef]
- Wang, F.X.; Ma, Y.P.; Yang, C.L.; Zhao, P.M.; Yao, Y.; Jian, G.L.; Luo, Y.M.; Xia, G.X. Proteomic analysis of the sea-island cotton roots infected by wilt pathogen Verticillium dahliae. Proteomics 2011, 11, 4296–4309. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, P.; Yuan, L.; Xia, G.; Zhang, H.; Li, J.; Wang, F. Revealing the contribution of GbPR10. 5D1 to resistance against Verticillium dahliae and its regulation for structural defense and immune signaling. Plant Genome 2022, 15, e20271. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, L.; Liang, Y.; Wang, F.; Cao, A.; Xie, S.; Chen, X.; Shen, H.; Wang, B.; Hu, M. Comparative proteomic analysis reveals the ascorbate peroxidase-mediated plant resistance to Verticillium dahliae in Gossypium barbadense. Front. Plant Sci. 2022, 13, 877146. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.J.; Batool, R.; Yang, M.; Zheng, J.; Nazir, M.F.; Wang, H.; Cai, X.; Hou, Y.; Xu, Y.; Wang, Y. Unravelling the Functional Role of GthGAPC2 in Cotton’s Defense Against Verticillium dahliae through Proteome. Physiol. Plant. 2024, 176, e14127. [Google Scholar] [CrossRef]
- Mi, X.; Li, W.; Chen, C.; Xu, H.; Wang, G.; Jin, X.; Zhang, D.; Guo, W. GhMPK9-GhRAF39_1-GhWRKY40a regulates the GhERF1b- and GhABF2-mediated pathways to increase cotton disease resistance. Adv. Sci. 2024, 11, 2404400. [Google Scholar] [CrossRef]
- Han, Z.; Qiu, Y.; Pan, T.; Wang, L.; Wang, J.; Liu, K. GhMAC3e is involved in plant growth and defense response to Verticillium dahliae. Plant Cell Rep. 2024, 43, 259. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Zhao, L.; Wei, F.; Feng, Z.; Feng, H. Phosphoproteomics profiling of cotton (Gossypium hirsutum L.) roots in response to Verticillium dahliae inoculation. ACS Omega 2019, 4, 18434–18443. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Zhou, J.; Wei, F.; Feng, Z.; Zhao, L.; Shi, Y.; Feng, H.; Zhu, H. The respiratory burst oxidase homolog protein D (GhRbohD) positively regulates the cotton resistance to Verticillium dahliae. Int. J. Mol. Sci. 2021, 22, 13041. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.A.; Waheed, U.; Raheel, M.; Khan, Z.; Alrefaei, A.F.; Alkhamis, H.H. Morphological and genetic characterization of Fusarium oxysporum and its management using weed extracts in cotton. J. King Saud Univ.-Sci. 2021, 33, 101299. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Samuel, M.; Garcia-Ceron, D.; McKenna, J.A.; Lowe, R.G.; Pathan, M.; Zhao, K.; Ang, C.-S.; Mathivanan, S.; Anderson, M.A. Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants. Front. Plant Sci. 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, X.; Li, Y.; Wang, L.; Guo, X.; Guo, X. The cotton MAPK kinase GhMPK20 negatively regulates resistance to Fusarium oxysporum by mediating the MKK4–MPK20–WRKY40 cascade. Mol. Plant Pathol. 2018, 19, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, H.; He, X.; Zhang, S.; Wang, J.; Wang, L.; Guo, D.; Guo, X. Scaffold protein GhMORG1 enhances the resistance of cotton to Fusarium oxysporum by facilitating the MKK6-MPK4 cascade. Plant Biotechnol. J. 2020, 18, 1421–1433. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.-T.; Wang, H.-Y.; Wu, J.-H.; Luo, Y.-M.; Wang, Q.; Wang, F.-X.; Xia, G.-X. iTRAQ-based proteomic analysis of defence responses triggered by the necrotrophic pathogen Rhizoctonia solani in cotton. J. Proteom. 2017, 152, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.R.; Rios, T.B.; Maximiano, M.R.; Coutinho, W.M.; De Lima, L.M.; Silva, L.P.; Oliveira-Neto, O.B.; Mehta, A. Proteomic screening for the identification of proteins involved in resistance to Xanthomonas campestris pv. malvacearum in cotton. Physiol. Mol. Plant Pathol. 2021, 113, 101562. [Google Scholar] [CrossRef]
- Hegarty, M.J.; Hiscock, S.J. The complex nature of allopolyploid plant genomes. Heredity 2009, 103, 100–101. [Google Scholar] [CrossRef]
- Lim, K.Y.; Kovarik, A.; Matyasek, R.; Chase, M.W.; Clarkson, J.J.; Grandbastien, M.; Leitch, A.R. Sequence of events leading to near-complete genome turnover in allopolyploid Nicotiana within five million years. New Phytol. 2007, 175, 756–763. [Google Scholar] [CrossRef]
- Garbus, I.; Romero, J.R.; Valarik, M.; Vanžurová, H.; Karafiátová, M.; Cáccamo, M.; Doležel, J.; Tranquilli, G.; Helguera, M.; Echenique, V. Characterization of repetitive DNA landscape in wheat homeologous group 4 chromosomes. BMC Genom. 2015, 16, 375. [Google Scholar] [CrossRef]
- Vasupalli, N.; Bhat, J.A.; Jain, P.; Sri, T.; Islam, M.A.; Shivaraj, S.; Singh, S.K.; Deshmukh, R.; Sonah, H.; Lin, X. Omics big data for crop improvement: Opportunities and challenges. Crop J. 2024, 12, 1517–1532. [Google Scholar] [CrossRef]
- Glover, N.M.; Redestig, H.; Dessimoz, C. Homoeologs: What are they and how do we infer them? Trends Plant Sci. 2016, 21, 609–621. [Google Scholar] [CrossRef]
- Zhang, Z.; Xun, H.; Lv, R.; Gou, X.; Ma, X.; Li, J.; Zhao, J.; Li, N.; Gong, L.; Liu, B. Effects of homoeologous exchange on gene expression and alternative splicing in a newly formed allotetraploid wheat. Plant J. 2022, 111, 1267–1282. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, P.; Zhang, A.; Ma, L.; Xu, S.; Wang, X. Alternative splicing diversified the heat response and evolutionary strategy of conserved heat shock protein 90s in hexaploid wheat (Triticum aestivum L.). Front. Genet. 2020, 11, 577897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Q.; Wang, C.; Lv, S.; Wang, Y.; Wang, J.; Wang, Y.; Yuan, J.; Zhang, H. An alternative splicing isoform of wheat TaYRG1 resistance protein activates immunity by interacting with dynamin-related proteins. J. Exp. Bot. 2022, 73, 5474–5489. [Google Scholar] [CrossRef]
- Wen, J.; Qin, Z.; Sun, L.; Zhang, Y.; Wang, D.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; Sun, Q. Alternative splicing of TaHSFA6e modulates heat shock protein–mediated translational regulation in response to heat stress in wheat. New Phytol. 2023, 239, 2235–2247. [Google Scholar] [CrossRef]
- Miller, R.M.; Jordan, B.T.; Mehlferber, M.M.; Jeffery, E.D.; Chatzipantsiou, C.; Kaur, S.; Millikin, R.J.; Dai, Y.; Tiberi, S.; Castaldi, P.J. Enhanced protein isoform characterization through long-read proteogenomics. Genome Biol. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding post-translational modification crosstalk with proteomics. Mol. Cell. Proteom. 2021, 20, 100129. [Google Scholar] [CrossRef]
- Kim, D.N.; Yin, T.; Zhang, T.; Im, A.K.; Cort, J.R.; Rozum, J.C.; Pollock, D.; Qian, W.-J.; Feng, S. Artificial intelligence transforming post-translational modification research. Bioengineering 2024, 12, 26. [Google Scholar] [CrossRef]
- Shrestha, P.; Kandel, J.; Tayara, H.; Chong, K.T. Post-translational modification prediction via prompt-based fine-tuning of a GPT-2 model. Nat. Commun. 2024, 15, 6699. [Google Scholar] [CrossRef] [PubMed]
- Heritz, J.A.; Meluni, K.A.; Backe, S.J.; Cayaban, S.J.; Wengert, L.A.; Kunz, M.; Woodford, M.R.; Bourboulia, D.; Mollapour, M. Integrating deep learning for post-translational modifications crosstalk on Hsp90 and drug binding. J. Biol. Chem. 2025, 301. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.E.; Chu, Y.; Ozias-Akins, P.; Thelen, J.J. Validation of gel-free, label-free quantitative proteomics approaches: Applications for seed allergen profiling. J. Proteom. 2009, 72, 555–566. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Z. Effective omics tools are still lacking for improvement of stress tolerance in polyploid crops. Front. Plant Sci. 2023, 14, 1295528. [Google Scholar] [CrossRef]
- Kourelis, J.; Kaschani, F.; Grosse-Holz, F.M.; Homma, F.; Kaiser, M.; van der Hoorn, R.A. A homology-guided, genome-based proteome for improved proteomics in the alloploid Nicotiana benthamiana. BMC Genom. 2019, 20, 722. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Klíma, M.; Nesvadba, Z.; Vítámvás, P.; Ovesná, J. Proteomics of wheat and barley cereals in response to environmental stresses: Current state and future challenges. J. Proteom. 2023, 282, 104923. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Venkataraman, G.R.; Guturu, H.; Halama, A.; Stephan, N.; Thareja, G.; Sarwath, H.; Motamedchaboki, K.; Donovan, M.K.; Siddiqui, A. Nanoparticle enrichment mass-spectrometry proteomics identifies protein-altering variants for precise pQTL mapping. Nat. Commun. 2024, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, B.; Zhan, X.; Schlüter, H.; Jungblut, P.R.; Coorssen, J.R. Innovating the concept and practice of two-dimensional gel electrophoresis in the analysis of proteomes at the proteoform level. Proteomes 2019, 7, 36. [Google Scholar] [CrossRef]
- Zhan, X.; Li, N.; Zhan, X.; Qian, S. Revival of 2DE-LC/MS in proteomics and its potential for large-scale study of human proteoforms. Med One 2018, 3, e180008. [Google Scholar]
- Fröhlich, K.; Fahrner, M.; Brombacher, E.; Seredynska, A.; Maldacker, M.; Kreutz, C.; Schmidt, A.; Schilling, O. Data-independent acquisition: A Milestone and prospect in clinical mass spectrometry–based proteomics. Mol. Cell. Proteom. 2024, 23, 100800. [Google Scholar] [CrossRef]
- Basharat, A.R.; Xiong, X.; Xu, T.; Zang, Y.; Sun, L.; Liu, X. TopDIA: A software tool for top-down data-independent acquisition proteomics. J. Proteome Res. 2024, 24, 55–64. [Google Scholar] [CrossRef]
- Regnier, F.E.; Kim, J. Proteins and proteoforms: New separation challenges. Anal. Chem. 2017, 90, 361. [Google Scholar] [CrossRef]
- Charkow, J.; Röst, H.L. Trapped ion mobility spectrometry reduces spectral complexity in mass spectrometry-based proteomics. Anal. Chem. 2021, 93, 16751–16758. [Google Scholar] [CrossRef]
- Ai, L.; Binek, A.; Kreimer, S.; Ayres, M.; Stotland, A.; Van Eyk, J.E. High-field asymmetric waveform Ion mobility spectrometry: Practical alternative for cardiac proteome sample processing. J. Proteome Res. 2023, 22, 2124–2130. [Google Scholar] [CrossRef]
- Demichev, V.; Szyrwiel, L.; Yu, F.; Teo, G.; Rosenberger, G.; Niewienda, A. dia-PASEF data analysis using FragPipe and DIA-NN for deep proteomics of low sample amounts. Nat. Commun. 2022, 13, 3944. [Google Scholar] [CrossRef] [PubMed]
- Kaulich, P.T.; Jeong, K.; Kohlbacher, O.; Tholey, A. Influence of different sample preparation approaches on proteoform identification by top-down proteomics. Nat. Methods 2024, 21, 2397–2407. [Google Scholar] [CrossRef]
- Padula, M.P.; Berry, I.J.; O′ Rourke, M.B.; Raymond, B.B.; Santos, J.; Djordjevic, S.P. A comprehensive guide for performing sample preparation and top-down protein analysis. Proteomes 2017, 5, 11. [Google Scholar] [CrossRef]
- Duong, V.-A.; Lee, H. Bottom-up proteomics: Advancements in sample preparation. Int. J. Mol. Sci. 2023, 24, 5350. [Google Scholar] [CrossRef]
- Carbonara, K.; Andonovski, M.; Coorssen, J.R. Proteomes are of proteoforms: Embracing the complexity. Proteomes 2021, 9, 38. [Google Scholar] [CrossRef]
- Coorssen, J.R.; Padula, M.P. Proteomics—The state of the field: The definition and analysis of proteomes should be based in reality, not convenience. Proteomes 2024, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, L.; Toby, T.K. Characterization of large intact protein ions by mass spectrometry: What directions should we follow? Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2022, 1870, 140758. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.V.; Millikin, R.J.; Miller, R.M.; Anderson, L.C.; Fellers, R.T.; Ge, Y.; Kelleher, N.L.; LeDuc, R.D.; Liu, X.; Payne, S.H. Identification and quantification of proteoforms by mass spectrometry. Proteomics 2019, 19, 1800361. [Google Scholar] [CrossRef]
- Kaulich, P.T.; Tholey, A. Top-Down Proteomics: Why and When? Proteomics 2025, e202400338. [Google Scholar] [CrossRef]
- Cassidy, L.; Kaulich, P.T.; Tholey, A. Proteoforms expand the world of microproteins and short open reading frame-encoded peptides. iScience 2023, 26, 106069. [Google Scholar] [CrossRef]
- Poulos, R.C.; Hains, P.G.; Shah, R.; Lucas, N.; Xavier, D.; Manda, S.S.; Anees, A.; Koh, J.M.; Mahboob, S.; Wittman, M. Strategies to enable large-scale proteomics for reproducible research. Nat. Commun. 2020, 11, 3793. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Costa, C.; Martínez-Bartolomé, S.; McClatchy, D.; Yates, J.R., III. Improving proteomics data reproducibility with a dual-search strategy. Anal. Chem. 2019, 92, 1697–1701. [Google Scholar] [CrossRef]
- Bacala, R.; Hatcher, D.W.; Perreault, H.; Fu, B.X. Challenges and opportunities for proteomics and the improvement of bread wheat quality. J. Plant Physiol. 2022, 275, 153743. [Google Scholar] [CrossRef]
- Niu, L.; Yuan, H.; Gong, F.; Wu, X.; Wang, W. Protein extraction methods shape much of the extracted proteomes. Front. Plant Sci. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, L.; Tao, C.; Xie, Q.; Xu, X.; Chang, L.; Tan, Y.; Ding, G.; Li, H.; Wang, X. An improved protein extraction method applied to cotton leaves is compatible with 2-DE and LC-MS. BMC Genom. 2019, 20, 285. [Google Scholar] [CrossRef]
- Mujahid, H.; Pendarvis, K.; Reddy, J.S.; Nallamilli, B.R.R.; Reddy, K.; Nanduri, B.; Peng, Z. Comparative proteomic analysis of cotton fiber development and protein extraction method comparison in late stage fibers. Proteomes 2016, 4, 7. [Google Scholar] [CrossRef]
- Bose, U.; Broadbent, J.A.; Byrne, K.; Hasan, S.; Howitt, C.A.; Colgrave, M.L. Optimisation of protein extraction for in-depth profiling of the cereal grain proteome. J. Proteom. 2019, 197, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Z.; Mao, J.; Zhang, Y.; Zhou, L.; Wei, J.; Liu, Y.; Wang, L.; Liu, F.; Cui, F. Accelerating wheat (Triticum aestivum) proteomics using a tandem digestion approach. J. Cereal Sci. 2025, 123, 104133. [Google Scholar] [CrossRef]
- Rahman, M.; Liu, L.; Barkla, B.J. A single seed protein extraction protocol for characterizing Brassica seed storage proteins. Agronomy 2021, 11, 107. [Google Scholar] [CrossRef]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current strategies of polyploid plant genome sequence assembly. Front. Plant Sci. 2018, 9, 1660. [Google Scholar] [CrossRef]
- Alaux, M.; Rogers, J.; Letellier, T.; Flores, R.; Alfama, F.; Pommier, C.; Mohellibi, N.; Durand, S.; Kimmel, E.; Michotey, C. Linking the International Wheat Genome Sequencing Consortium bread wheat reference genome sequence to wheat genetic and phenomic data. Genome Biol. 2018, 19, 111. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, M.; Wang, X.; Tong, C.; Huang, S.; Tehrim, S.; Liu, Y.; Hua, W.; Liu, S. Bolbase: A comprehensive genomics database for Brassica oleracea. BMC Genom. 2013, 14, 664. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; He, X.; Cai, X.; Lin, R.; Liang, J.; Wu, J.; King, G.; Wang, X. BRAD V3.0: An upgraded Brassicaceae database. Nucleic Acids Res. 2022, 50, D1432–D1441. [Google Scholar] [CrossRef]
- Yu, J.; Jung, S.; Cheng, C.-H.; Lee, T.; Zheng, P.; Buble, K.; Crabb, J.; Humann, J.; Hough, H.; Jones, D. CottonGen: The community database for cotton genomics, genetics, and breeding research. Plants 2021, 10, 2805. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Long, Y.; Shu, Y.; Zhai, J. Plant public RNA-seq database: A comprehensive online database for expression analysis of~ 45 000 plant public RNA-seq libraries. Plant Biotechnol. J. 2022, 20, 806. [Google Scholar] [CrossRef]
- Lu, S.; Lu, H.; Zheng, T.; Yuan, H.; Du, H.; Gao, Y.; Liu, Y.; Pan, X.; Zhang, W.; Fu, S. A multi-omics dataset of human transcriptome and proteome stable reference. Sci. Data 2023, 10, 455. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, L.; Zhu, Q.-H.; Fan, L.; Guo, L. Twenty years of plant genome sequencing: Achievements and challenges. Trends Plant Sci. 2022, 27, 391–401. [Google Scholar] [CrossRef]
- Lv, R.; Gou, X.; Li, N.; Zhang, Z.; Wang, C.; Wang, R.; Wang, B.; Yang, C.; Gong, L.; Zhang, H. Chromosome translocation affects multiple phenotypes, causes genome-wide dysregulation of gene expression, and remodels metabolome in hexaploid wheat. Plant J. 2023, 115, 1564–1582. [Google Scholar] [CrossRef]
- Ma, S.; Wang, M.; Wu, J.; Guo, W.; Chen, Y.; Li, G.; Wang, Y.; Shi, W.; Xia, G.; Fu, D. WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 2021, 14, 1965–1968. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Wei, L.; Huang, Y.; Liu, D.; Jia, Y.; Luo, C.; Lin, Y.; Liang, C.; Hu, Y. BnIR: A multi-omics database with various tools for Brassica napus research and breeding. Mol. Plant 2023, 16, 775–789. [Google Scholar] [CrossRef]
- Dai, F.; Chen, J.; Zhang, Z.; Liu, F.; Li, J.; Zhao, T.; Hu, Y.; Zhang, T.; Fang, L. COTTONOMICS: A comprehensive cotton multi-omics database. Database 2022, 2022, baac080. [Google Scholar] [CrossRef]
- Duncan, O.; Trösch, J.; Fenske, R.; Taylor, N.L.; Millar, A.H. Resource: Mapping the Triticum aestivum proteome. Plant J. 2017, 89, 601–616. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Cho, K.; Choi, J.-S.; Bae, K.-H.; Komatsu, S.; Uozumi, N.; Woo, S.H. The wheat chloroplastic proteome. J. Proteom. 2013, 93, 326–342. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Opportunities for wheat proteomics to discover the biomarkers for respiration-dependent biomass production, stress tolerance and cytoplasmic male sterility. J. Proteom. 2016, 143, 36–44. [Google Scholar] [CrossRef][Green Version]
- Takemori, N.; Takemori, A.; Matsuoka, K.; Morishita, R.; Matsushita, N.; Aoshima, M.; Takeda, H.; Sawasaki, T.; Endo, Y.; Higashiyama, S. High-throughput synthesis of stable isotope-labeled transmembrane proteins for targeted transmembrane proteomics using a wheat germ cell-free protein synthesis system. Mol. Biosyst. 2015, 11, 361–365. [Google Scholar] [CrossRef]
- Schoof, E.M.; Furtwängler, B.; Üresin, N.; Rapin, N.; Savickas, S.; Gentil, C.; Lechman, E.; Keller, U.A.D.; Dick, J.E.; Porse, B.T. Quantitative single-cell proteomics as a tool to characterize cellular hierarchies. Nat. Commun. 2021, 12, 3341. [Google Scholar] [CrossRef]
- Kustatscher, G.; Collins, T.; Gingras, A.-C.; Guo, T.; Hermjakob, H.; Ideker, T.; Lilley, K.S.; Lundberg, E.; Marcotte, E.M.; Ralser, M. Understudied proteins: Opportunities and challenges for functional proteomics. Nat. Methods 2022, 19, 774–779. [Google Scholar] [CrossRef]
- Fujita, S.; Terada, T. Enhanced prediction of protein functional identity through the integration of sequence and structural features. Comput. Struct. Biotechnol. J. 2024, 23, 4124–4130. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Pokharel, S.; Barasa, K.; Pratyush, P.; Kc, D.B. PLM-DBPs: Enhancing plant DNA-binding protein prediction by integrating sequence-based and structure-aware protein language models. Brief. Bioinform. 2025, 26, bbaf245. [Google Scholar] [CrossRef]
- Julia, E. Protein Engineering: Principles and Applications in Biotechnology. J. Bioeng. Biomed. Sci. 2025, 15, 1. [Google Scholar]
- Engqvist, M.K.; Rabe, K.S. Applications of protein engineering and directed evolution in plant research. Plant Physiol. 2019, 179, 907–917. [Google Scholar] [CrossRef]
- Fels, U.; Gevaert, K.; Van Damme, P. Proteogenomics in aid of host–pathogen interaction studies: A bacterial perspective. Proteomes 2017, 5, 26. [Google Scholar] [CrossRef]
- Balotf, S.; Wilson, R.; Tegg, R.S.; Nichols, D.S.; Wilson, C.R. Shotgun proteomics as a powerful tool for the study of the proteomes of plants, their pathogens, and plant–pathogen interactions. Proteomes 2022, 10, 5. [Google Scholar] [CrossRef]
- Elmore, J.M.; Griffin, B.D.; Walley, J.W. Advances in functional proteomics to study plant-pathogen interactions. Curr. Opin. Plant Biol. 2021, 63, 102061. [Google Scholar] [CrossRef]
- Ravikumar, V.; Jers, C.; Mijakovic, I. Elucidating host–pathogen interactions based on post-translational modifications using proteomics approaches. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Singh, S.; Hegde, M.; Kaur, I.; Adlakha, N. Temporal proteome profiling of Botrytis cinerea reveals proteins involved in plant invasion and survival. Sci. Rep. 2025, 15, 11857. [Google Scholar] [CrossRef]
- Gao, W.; Long, L.; Xu, L.; Lindsey, K.; Zhang, X.; Zhu, L. Suppression of the homeobox gene HDTF1 enhances resistance to Verticillium dahliae and Botrytis cinerea in cotton. J. Integr. Plant Biol. 2016, 58, 503–513. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, J.; Zhu, D.; Gao, C. TurboID-based proximity labeling accelerates discovery of neighboring proteins in plants. Trends Plant Sci. 2024, 29, 383–384. [Google Scholar] [CrossRef]
- Shi, L.; Ferrando, T.M.; Villanueva, S.L.; Joosten, M.H.; Vleeshouwers, V.G.; Bachem, C.W. Protocol to identify protein-protein interaction networks in Solanum tuberosum using transient TurboID-based proximity labeling. STAR Protoc. 2023, 4, 102577. [Google Scholar] [CrossRef]
- Kwon, D. The antibodies don’t work! The race to rid labs of molecules that ruin experiments. Nature 2024, 635, 26–28. [Google Scholar] [CrossRef]
- Fr, T.; Molek, O. When antibodies mislead: The quest for validation. Nature 2020, 585, 313. [Google Scholar] [CrossRef]
- Nierves, L.A.; Lin, T.-T.; Moradian, A.; Shen, Q.; Sechi, S.; MacCoss, M.J.; Qu, J.; van Eyk, J.E.; Hoofnagle, A.N.; Qian, W.-J. Biomarkers, Proteoforms, and Mass Spectrometry–based Assays for Diabetes Clinical Research. J. Clin. Endocrinol. Metab. 2025, 110, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Fellers, R.T.; Greer, J.B.; LeDuc, R.D.; Thomas, P.M.; Kelleher, N.L. Proteoform-predictor: Increasing the Phylogenetic Reach of Top-Down Proteomics. J. Proteome Res. 2025, 24, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.; Alharbi, S.; Alotaibi, M.; Al Mosallam, M.; Motawei, M.; Alrajhi, A. Wheat omics: Classical breeding to new breeding technologies. Saudi J. Biol. Sci. 2021, 28, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Cheng, X.; Xiao, X.; Chou, K.-C. pLoc_bal-mPlant: Predict subcellular localization of plant proteins by general PseAAC and balancing training dataset. Curr. Pharm. Des. 2018, 24, 4013–4022. [Google Scholar] [CrossRef]
- Wan, S.; Mak, M.-W.; Kung, S.-Y. Gram-LocEN: Interpretable prediction of subcellular multi-localization of Gram-positive and Gram-negative bacterial proteins. Chemom. Intell. Lab. Syst. 2017, 162, 1–9. [Google Scholar] [CrossRef]
- Meinken, J.; Asch, D.K.; Neizer-Ashun, K.A.; Chang, G.H.; Cooper JR, C.R.; Min, X.J. FunSecKB2: A fungal protein subcellular location knowledgebase. Comput. Mol. Biol. 2014, 4, 1–17. [Google Scholar] [CrossRef]
- Sukumaran, A.; Woroszchuk, E.; Ross, T.; Geddes-McAlister, J. Proteomics of host–bacterial interactions: New insights from dual perspectives. Can. J. Microbiol. 2021, 67, 213–225. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Uhrig, R.G. The plant proteome delivers from discovery to innovation. Trends Plant Sci. 2025, 30, 837–845. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Zou, J.; Meng, J.; Wang, J. Comparative proteomic study on Brassica hexaploid and its parents provides new insights into the effects of polyploidization. J. Proteom. 2015, 112, 274–284. [Google Scholar] [CrossRef]
- Islam, N.; Tsujimoto, H.; Hirano, H. Proteome analysis of diploid, tetraploid and hexaploid wheat: Towards understanding genome interaction in protein expression. Proteomics 2003, 3, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Rampitsch, C.; Bykova, N.V. Advances in plant proteomics toward improvement of crop productivity and stress resistance. Front. Plant Sci. 2015, 6, 209. [Google Scholar] [CrossRef]
- Song, Y.-C.; Das, D.; Zhang, Y.; Chen, M.-X.; Fernie, A.R.; Zhu, F.-Y.; Han, J. Proteogenomics-based functional genome research: Approaches, applications, and perspectives in plants. Trends Biotechnol. 2023, 41, 1532–1548. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, S.C. The use of proteomics in search of allele-specific proteins in (Allo) polyploid crops. In Plant Proteomics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 297–308. [Google Scholar]
- Yu, Y.; Rong, K.; Sui, X.; Zhang, L.; Zhang, M.; Hu, H.; Jia, J.; Wu, J.; Li, C. Analysis of NRAMP genes in the Triticeae reveals that TaNRAMP5 positively regulates cadmium (Cd) tolerance in wheat (Triticum aestivum). Plant Physiol. Biochem. 2025, 219, 109321. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Ni, P.; Zhao, Y.; Zhou, X.; Liu, Z.; Huang, Z.; Ni, Z.; Sun, Q.; Zong, Y. Efficient and versatile multiplex prime editing in hexaploid wheat. Genome Biol. 2023, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.S.; Mishra, M.; Pareek, A.; Singla-Pareek, S.L. Grain lysine enrichment and improved stress tolerance in rice through protein engineering. J. Exp. Bot. 2025, 76, 1408–1426. [Google Scholar] [CrossRef]
- Daba, S.D.; Liu, X.; Aryal, U.; Mohammadi, M. A proteomic analysis of grain yield-related traits in wheat. AoB Plants 2020, 12, plaa042. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Ma, X.; Ma, H.; Huang, Q.; Zhang, Y.; Guan, M.; Guan, C. Transcriptomic, proteomic, metabolomic, and functional genomic approaches of Brassica napus L. during salt stress. PLoS ONE 2022, 17, e0262587. [Google Scholar] [CrossRef]
- Mi, W.; Liu, Z.; Jin, J.; Dong, X.; Xu, C.; Zou, Y.; Xu, M.; Zheng, G.; Cao, X.; Fang, X. Comparative proteomics analysis reveals the molecular mechanism of enhanced cold tolerance through ROS scavenging in winter rapeseed (Brassica napus L.). PLoS ONE 2021, 16, e0243292. [Google Scholar] [CrossRef]
- Koh, J.; Chen, G.; Yoo, M.-J.; Zhu, N.; Dufresne, D.; Erickson, J.E.; Shao, H.; Chen, S. Comparative proteomic analysis of Brassica napus in response to drought stress. J. Proteome Res. 2015, 14, 3068–3081. [Google Scholar] [CrossRef]
- Lan, M.; Li, G.; Hu, J.; Yang, H.; Zhang, L.; Xu, X.; Liu, J.; He, J.; Sun, R. iTRAQ-based quantitative analysis reveals proteomic changes in Chinese cabbage (Brassica rapa L.) in response to Plasmodiophora brassicae infection. Sci. Rep. 2019, 9, 12058. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, Z.; Ma, F.; Liu, K. Identification of key proteins related to high-quality fiber in Upland cotton via proteomics analysis. Plant Cell Rep. 2022, 41, 893–904. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Liu, Y.; Li, C. Tandem mass tag-based (TMT) quantitative proteomics analysis reveals the response of fine roots to drought stress in cotton (Gossypium hirsutum L.). BMC Plant Biol. 2020, 20, 328. [Google Scholar] [CrossRef]
- Zheng, M.; Meng, Y.; Yang, C.; Zhou, Z.; Wang, Y.; Chen, B. Protein expression changes during cotton fiber elongation in response to drought stress and recovery. Proteomics 2014, 14, 1776–1795. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Khan, A.; Ullah, N.; Li, Z.; Zaheer, S.; Zhou, R.; Zhang, Z. Quantitative proteomics-based analysis reveals molecular mechanisms of chilling tolerance in grafted cotton seedlings. Agronomy 2022, 12, 1152. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Gong, W.; Xu, F.; Pan, Z.; Jia, Y.; Geng, X.; Du, X. Integration of proteomic and transcriptomic profiles reveals multiple levels of genetic regulation of salt tolerance in cotton. BMC Plant Biol. 2018, 18, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).