Deciphering Radiotherapy Resistance: A Proteomic Perspective
Abstract
1. Introduction
2. Mechanisms Involved in Radiotherapy Resistance
2.1. DNA Damage and Repair
2.2. Apoptosis
2.3. Hypoxia
2.4. Metabolic Reprogramming
2.5. Tumor Microenvironment
2.6. Cancer Stem Cells
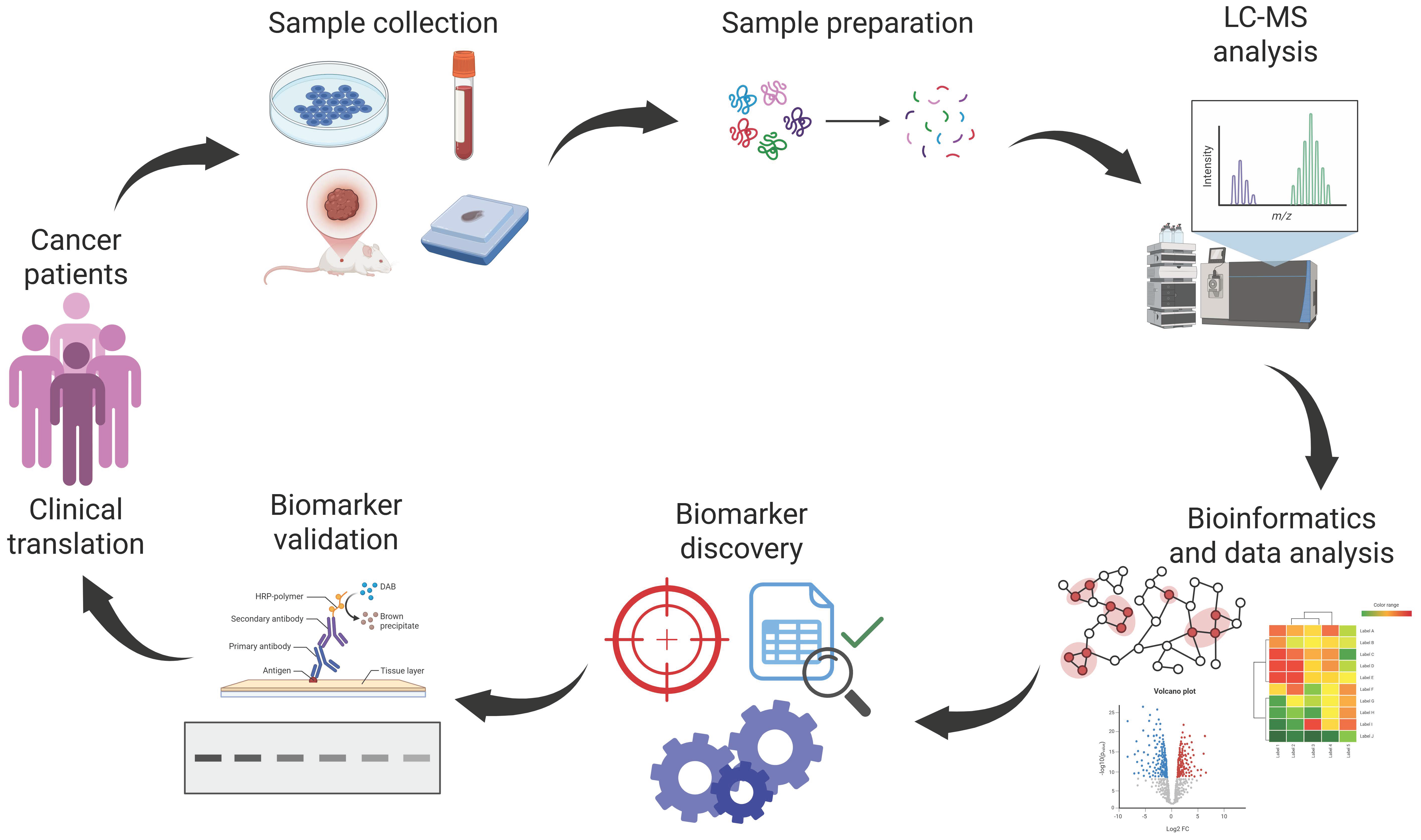
3. Proteomic Technologies and Applications in Cancer
3.1. Proteomic Workflow
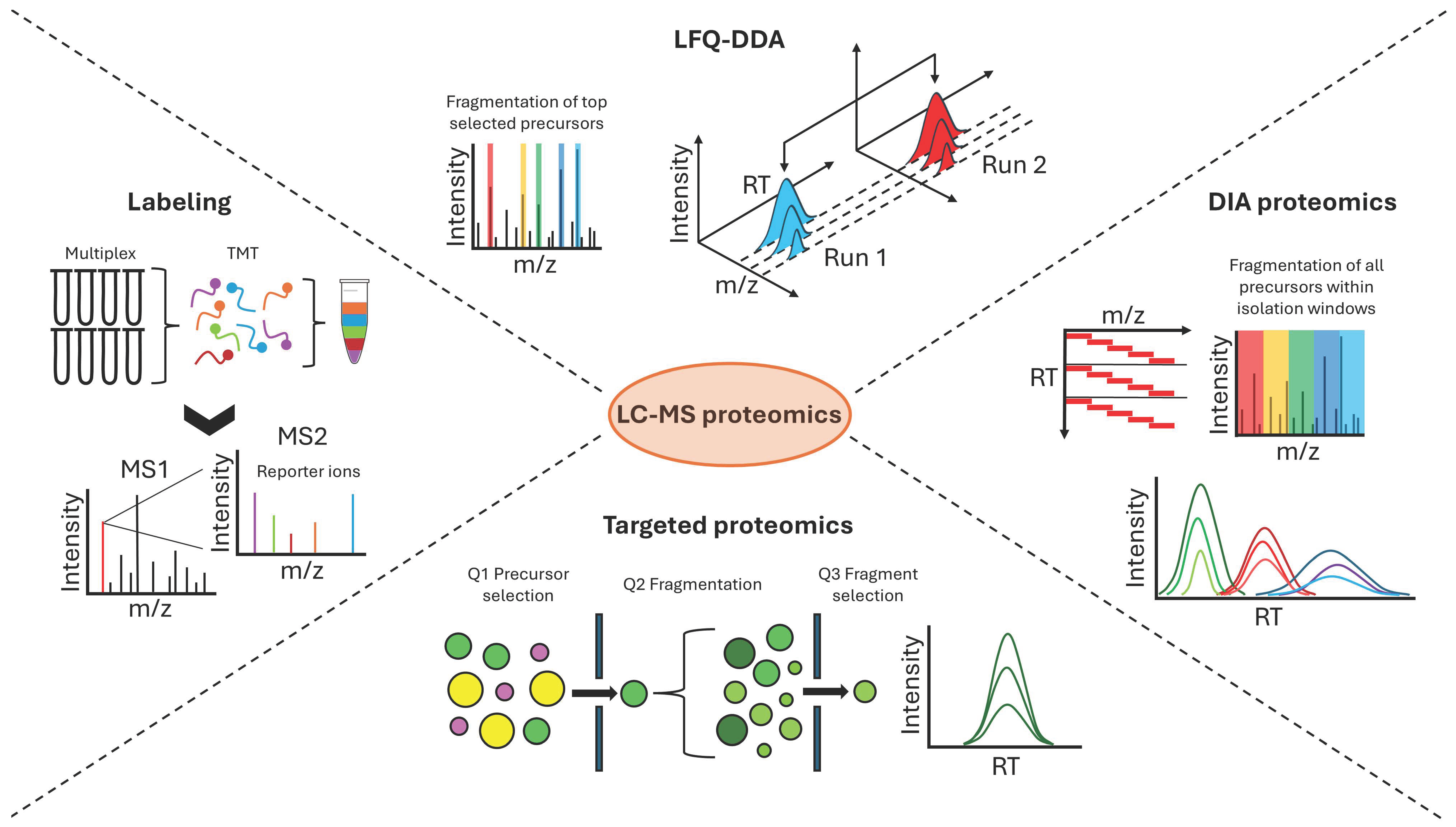
3.2. Label-Based Quantitative Proteomics
3.3. Label-Free Quantification with Data-Dependent and Data-Independent Acquisition
3.4. Targeted Proteomics
4. Identification of Radiotherapy Resistance Biomarkers by Proteomics
4.1. Head and Neck Cancers
4.2. Breast Cancer
4.3. Lung Cancer
4.4. Prostate Cancer
4.5. Other Cancers
| Potential Biomarker | Quantitative Change | Biological Function | Tumor | Sample | Proteomic Method | Validation Method | Reference |
|---|---|---|---|---|---|---|---|
| CD166 | Upregulated | Cell adhesion | NPC | Sensitive and resistant CNE2 cell secretome | iTRAQ labeling | WB | [134] |
| CFL2 | Upregulated | Cytoskeletal remodeling | |||||
| FBN2 | Downregulated | Cell adhesion, migration | |||||
| QSOX1 | Downregulated | ECM remodeling, redox regulation | |||||
| MAPK15 | Upregulated | DNA repair, oxidative stress response | NPC | Sensitive and resistant CNE2 cells | TMT labeling | WB | [135] |
| SPARC | Upregulated | ECM remodeling, EMT | NPC | Serum of responder and non-responder patients | TMT labeling | Not validated | [136] |
| SERPIND1 | Upregulated | Coagulation, immune response | |||||
| C4B | Upregulated | Complement activation, immune evasion | |||||
| PPIB | Upregulated | Protein folding, ER stress | |||||
| FAM173A | Downregulated | Mitochondrial regulation | |||||
| LGALS7 | Downregulated | Cell adhesion, apoptosis, inflammation | OSCC | FFPE tissues of responder and non-responder patients | LFQ-DDA | WB in cells and IHC in tissues | [141] |
| KRT17 | Upregulated | Cytoskeleton organization, immune evasion | HPV+ OPSCC | FFPE tissues from patients with and without recurrence | LFQ-DIA | Not validated | [143] |
| MMP2 | Upregulated | ECM remodeling, EMT, invasion | |||||
| S100A4 | Upregulated | EMT, migration | |||||
| LMNB1 | Upregulated | Chromatin remodeling, apoptosis, DNA repair | |||||
| CDS2 | Downregulated | Phospholipid biosynthesis, metabolism | |||||
| GAPVD1 | Downregulated | Vesicle trafficking, proliferation | HPV+ OPSCC | FFPE tissues from patients with and without recurrence | LFQ-DIA | Not validated | [143] |
| WDR81 | Downregulated | Autophagy, stress response | |||||
| RRAS | Upregulated | Survival signaling, proliferation | OSCC | Frozen tissue of patients with and without recurrence | TMT labeling | IHC | [148] |
| CTSD | Upregulated | Proteolysis, apoptosis, inflammation | Triple negative breast cancer | MDA-MB-231 cells treated with single or fractionated RT | SILAC labeling | WB | [150] |
| GSN | Upregulated | Cytoskeleton organization, immune response | |||||
| MRC2 | Upregulated | ECM remodeling, cell motility | |||||
| CHK1 | Upregulated | DDR, cell cycle | ER+ breast cancer | Sensitive and resistant MCF-7 cells | Labeling and MRM | WB | [151] |
| CDK1 | Upregulated | DDR, cell cycle | |||||
| CDK2 | Upregulated | DDR, cell cycle | |||||
| TAF9 | Upregulated | Transcription regulation | Triple negative and ER+ breast cancer | Sensitive and resistant MCF-7 and MDA-MB-231 cells | SILAC labeling and PRM | WB | [152] |
| DEK (P) | Downregulated | Chromatin remodeling, DNA repair | Triple negative breast cancer | MDA-MB-231 and MDA-MB-468 cells irradiated and treated with radiosensitizer | Phospho-proteomics | Not validated | [153] |
| NCL (P) | Downregulated | Ribosome biogenesis, DDR | |||||
| XRCC1 (P) | Downregulated | DNA repair | |||||
| TOP2A (P) | Downregulated | DNA topology, DDR | |||||
| PRKDC | Upregulated | DNA repair | ER+ Breast cancer | MCF-7 and T47D mammospheres treated with irradiation and doxycycline | LFQ-DDA | WB | [154] |
| ERK2 | Upregulated | Survival signaling, proliferation | HER2+ breast cancer | Hypoxic and normoxic EVs from SKBR3 cells | SILAC labeling | Not validated | [155] |
| GSK3A | Upregulated | Survival signaling, apoptosis | |||||
| GSK3B | Upregulated | Survival signaling, apoptosis | |||||
| MBD4 | Upregulated | DNA repair | NSCLC | Sensitive and resistant H2170 cells | TMT labeling | Not validated | [158] |
| TIMP3 | Upregulated | ECM remodeling, invasion | NSCLC | Sensitive and resistant H2170 cells | TMT labeling | Not validated | [158] |
| PODXL | Upregulated | Cell adhesion, EMT | |||||
| EGFR | Upregulated | Survival signaling, proliferation, angiogenesis | NSCLC | Sensitive and resistant A549 cells | LFQ-DDA | WB | [159] |
| PRKCA | Downregulated | Survival signaling, proliferation | |||||
| FN1 | Upregulated | ECM remodeling, migration, TME modulation | NSCLC | H460 cells with and without irradiation | TMT labeling | Not validated | [161] |
| THBS1 | Upregulated | ECM remodeling, migration, TME modulation | |||||
| PPAT | Downregulated | Nucleotide biosynthesis, proliferation | NSCLC | Resistant H358 and H157 cells treated with radiosensitizer | SILAC labeling | WB | [162] |
| SERPINA1 | Upregulated | Immune modulation, inflammation | NSCLC | Serum of responder and non-responder patients | LFQ-DDA | ELISA | [164] |
| CRP | Upregulated | Inflammation, invasion | NSCLC | Plasma of patients before and during RT | iTRAQ labeling | ELISA | [166] |
| LRG1 | Upregulated | Angiogenesis | |||||
| ALDOA | Upregulated | Glycolysis, metabolism | Prostate cancer | Sensitive and resistant PC-3, DU145, LNCaP cells | LFQ-DDA | ALDOA validated by WB | [168] |
| AHSG | Upregulated | Inflammation | |||||
| VIM | Upregulated | EMT, invasion | |||||
| YWHAE | Upregulated | Stress response, cell cycle regulation | |||||
| PRDX6 | Upregulated | ROS detoxification | |||||
| CD44 | Upregulated | Cell adhesion, migration | Prostate cancer | Sensitive and resistant DU145 cells | LFQ-DDA | WB | [170] |
| ASNS | Upregulated | UPR, ER stress | Prostate cancer | 22Rv1 cells with and without irradiation | LFQ-DIA | Not validated | [171] |
| PPAT | Downregulated | Nucleotide biosynthesis, proliferation | NSCLC | Resistant H358 and H157 cells treated with radiosensitizer | SILAC labeling | WB | [162] |
| SERPINA1 | Upregulated | Immune modulation, inflammation | NSCLC | Serum of responder and non-responder patients | LFQ-DDA | ELISA | [164] |
| CRP | Upregulated | Inflammation, invasion | NSCLC | Plasma of patients before and during RT | iTRAQ labeling | ELISA | [166] |
| LRG1 | Upregulated | Angiogenesis | |||||
| ALDOA | Upregulated | Glycolysis, metabolism | Prostate cancer | Sensitive and resistant PC-3, DU145, LNCaP cells | LFQ-DDA | ALDOA validated by WB | [168] |
| AHSG | Upregulated | Inflammation | |||||
| VIM | Upregulated | EMT, invasion | |||||
| YWHAE | Upregulated | Stress response, cell cycle regulation | |||||
| PRDX6 | Upregulated | ROS detoxification | |||||
| CD44 | Upregulated | Cell adhesion, migration | Prostate cancer | Sensitive and resistant DU145 cells | LFQ-DDA | WB | [170] |
| ASNS | Upregulated | UPR, ER stress | Prostate cancer | 22Rv1 cells with and without irradiation | LFQ-DIA | Not validated | [171] |
| LDHA | Upregulated | Glycolysis, metabolism | Prostate cancer | Sensitive and resistant mouse xenograft | LFQ-DDA | WB and IHC | [172] |
| FTL | Upregulated | Iron homeostasis, ROS detoxification | Prostate cancer | Frozen and FFPE tissues of patients before and after RT | LFQ-DIA | WB | [173] |
| FGG | Upregulated | Wound healing, angiogenesis, inflammation | |||||
| ACPP | Downregulated | Signal transduction, proliferation | |||||
| TOP2A | Upregulated | DNA topology, DDR | Prostate cancer | FFPE tissues of multiresistant cancer | LFQ-DIA | Not validated | [177] |
| S100A8 | Upregulated | Inflammation, TME modulation | |||||
| S110A9 | Upregulated | Inflammation, TME modulation | |||||
| KPNA2 | Upregulated | Nuclear transport, EMT | |||||
| SPON2 | Upregulated | Cell adhesion, immune response | |||||
| AGR2 | Downregulated | Protein folding, stress response | Prostate cancer | FFPE tissues of multiresistant cancer | LFQ-DIA | Not validated | [177] |
| ABCC4 | Downregulated | Transport, detoxification | |||||
| ALOX15B | Downregulated | Lipid metabolism, inflammation | |||||
| S100A6 | Upregulated | DNA repair, EMT | ESCC | Sensitive and resistant TE-1 and KYSE-150 cells | TMT labeling | WB | [178] |
| TGM2 | Upregulated | Autophagy, stress response | |||||
| PYGB | Upregulated | Metabolism | |||||
| CST5 | Upregulated | Migration, EMT | CRC | SW480 cells irradiated and treated with radiosensitizer | LFQ-DDA | WB in cells and IHC in xenografts | [181] |
| PAI1 | Upregulated | Migration, EMT | |||||
| RAB5C | Upregulated | Endocytosis, DDR | Rectal cancer | Sensitive and resistant SW837 cells plus FFPE tissues of patients before and after RT | LFQ-DDA | WB in cells and IHC in tissues | [182] |
| XRCC5 | Upregulated | DNA repair | |||||
| XRCC6 | Upregulated | DNA repair | |||||
| PSME1 | Upregulated | Proteostasis, survival signaling | GB | Sensitive and resistant U87MG and SF268 cells | iTRAQ labeling | WB | [184] |
| PSMA7 | Upregulated | Proteostasis, survival signaling | |||||
| PSMB4 | Upregulated | Proteostasis, survival signaling | |||||
| MDH1 | Upregulated | Metabolism, redox regulation | GB | FFPE tissues and serum of short-term and long-term survivors after RT | LFQ-DIA | FABP7 validated by IHC | [185] |
| RNH1 | Upregulated | RNA stability, ROS detoxification | |||||
| FABP7 | Downregulated | Lipid metabolism, ROS detoxification |
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.A.; Keane, F.K. Contemporary radiotherapy: Present and future. Lancet 2021, 398, 171–184. [Google Scholar] [CrossRef]
- Ji, D.; Song, C. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J. Immunother. Cancer 2020, 8, e000826. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Trainor, C.; Butterworth, K.T. DNA damage responses following exposure to modulated radiation fields. PLoS ONE 2012, 7, e43326. [Google Scholar] [CrossRef]
- Talarico, C.; Dattilo, V. SI113, a SGK1 inhibitor, potentiates the effects of radiotherapy, modulates the response to oxidative stress and induces cytotoxic autophagy in human glioblastoma multiforme cells. Oncotarget 2016, 7, 15868–15884. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E. Predictive biomarkers of resistance to hypofractionated radiotherapy in high grade glioma. Radiat. Oncol. 2017, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Rakina, M. Radiotherapy resistance: Identifying universal biomarkers for various human cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 1015–1031. [Google Scholar] [CrossRef]
- Yard, B.; Chie, E.K. Radiotherapy in the Era of Precision Medicine. Semin. Radiat. Oncol. 2015, 25, 227–236. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Research on radiotherapy related genes and prognostic target identification of rectal cancer based on multi-omics. J. Transl. Med. 2023, 21, 856. [Google Scholar] [CrossRef]
- Chen, R.; Snyder, M. Systems biology: Personalized medicine for the future? Curr. Opin. Pharmacol. 2012, 12, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Merrick, B.A.; London, R.E. Platforms for biomarker analysis using high-throughput approaches in genomics, transcriptomics, proteomics, metabolomics, and bioinformatics. IARC Sci. Publ. 2011, 163, 121–142. [Google Scholar]
- Chaudhary, K.; Poirion, O.B. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin. Cancer Res. 2018, 24, 1248–1259. [Google Scholar] [CrossRef]
- Qian, W.J.; Jacobs, J.M. Advances and challenges in liquid chromatography-mass spectrometry-based proteomics profiling for clinical applications. Mol. Cell Proteomics 2006, 5, 1727–1744. [Google Scholar] [CrossRef]
- Rozanova, S.; Barkovits, K. Quantitative Mass Spectrometry-Based Proteomics: An Overview. Methods Mol. Biol. 2021, 2228, 85–116. [Google Scholar] [PubMed]
- Swietlik, J.J.; Bärthel, S. Cell-selective proteomics segregates pancreatic cancer subtypes by extracellular proteins in tumors and circulation. Nat. Commun. 2023, 14, 2642. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, H. Identifying new biomarkers and potential therapeutic targets for breast cancer through the integration of human plasma proteomics: A Mendelian randomization study and colocalization analysis. Front. Endocrinol. 2024, 15, 1449668. [Google Scholar] [CrossRef]
- Domon, B.; Aebersold, R. Mass spectrometry and protein analysis. Science 2006, 312, 212–217. [Google Scholar] [CrossRef]
- Scaife, L.; Hodgkinson, V.C. Differential proteomics in the search for biomarkers of radiotherapy resistance. Expert Rev. Proteomics. 2011, 8, 535–552. [Google Scholar] [CrossRef]
- Jeon, H.; Byun, J. Proteomic analysis predicts anti-angiogenic resistance in recurred glioblastoma. J. Transl. Med. 2023, 21, 69. [Google Scholar] [CrossRef]
- Giglia-Mari, G.; Zotter, A. DNA damage response. Cold Spring Harb. Perspect. Biol. 2011, 3, a000745. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Montori, A.; Germani, A. Somatic NGS Analysis of DNA Damage Response (DDR) Genes ATM, MRE11A, RAD50, NBN, and ATR in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemo-Radiotherapy. Biomedicines 2022, 10, 3247. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.; Köcher, S. Functional crosstalk between DNA damage response proteins 53BP1 and BRCA1 regulates double strand break repair choice. Radiother. Oncol. 2016, 119, 276–281. [Google Scholar] [CrossRef]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in cancer and aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Desideri, I. BRCA mutation in breast cancer patients: Prognostic impact and implications on clinical management. Breast J. 2018, 24, 1019–1023. [Google Scholar] [CrossRef]
- Qin, C.J.; Song, X.M. XRCC2 as a predictive biomarker for radioresistance in locally advanced rectal cancer patients undergoing preoperative radiotherapy. Oncotarget 2015, 6, 32193–32204. [Google Scholar] [CrossRef]
- Moeller, B.J.; Yordy, J.S. DNA repair biomarker profiling of head and neck cancer: Ku80 expression predicts locoregional failure and death following radiotherapy. Clin. Cancer Res. 2011, 17, 2035–2043. [Google Scholar] [CrossRef]
- Tennstedt, P.; Fresow, R. RAD51 overexpression is a negative prognostic marker for colorectal adenocarcinoma. Int. J. Cancer 2013, 132, 2118–2126. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, Y. RPA3 is a potential marker of prognosis and radioresistance for nasopharyngeal carcinoma. J. Cell Mol. Med. 2017, 21, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Kleiblova, P.; Stolarova, L. Identification of deleterious germline CHEK2 mutations and their association with breast and ovarian cancer. Int. J. Cancer 2019, 145, 1782–1797. [Google Scholar] [CrossRef] [PubMed]
- Gallo, O.; Chiarelli, I. Cumulative prognostic value of p53 mutations and bcl-2 protein expression in head-and-neck cancer treated by radiotherapy. Int. J. Cancer 1999, 84, 573–579. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z. Potential Functional Variants in DNA Repair Genes Are Associated with Efficacy and Toxicity of Radiotherapy in Patients with Non-Small-Cell Lung Cancer. J. Oncol. 2020, 2020, 3132786. [Google Scholar] [CrossRef]
- Tu, Z.; Xu, B. BRCC3 acts as a prognostic marker in nasopharyngeal carcinoma patients treated with radiotherapy and mediates radiation resistance in vitro. Radiat. Oncol. 2015, 10, 123. [Google Scholar] [CrossRef]
- Turgeon, G.A.; Weickhardt, A. Radiotherapy and immunotherapy: A synergistic effect in cancer care. Med. J. Aust. 2019, 210, 47–53. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kristeleit, R.; Lisyanskaya, A. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 465–478. [Google Scholar] [CrossRef]
- Moutafi, M.; Economopoulou, P. PARP inhibitors in head and neck cancer: Molecular mechanisms, preclinical and clinical data. Oral. Oncol. 2021, 117, 105292. [Google Scholar] [CrossRef]
- Olivares-Hernández, A.; Roldán-Ruiz, J. Efficacy and safety of PARP inhibitor in non-small cell lung cancer: A systematic review with meta-analysis. Chin. Clin. Oncol. 2023, 12, 62. [Google Scholar] [CrossRef]
- Anderson, V.E.; Walton, M.I. CCT241533 is a potent and selective inhibitor of CHK2 that potentiates the cytotoxicity of PARP inhibitors. Cancer Res. 2011, 71, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Roulston, A.; Zimmermann, M. RP-3500: A Novel, Potent, and Selective ATR Inhibitor that is Effective in Preclinical Models as a Monotherapy and in Combination with PARP Inhibitors. Mol. Cancer Ther. 2022, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H. Hsp90 Inhibitor Ganetespib Sensitizes Non-Small Cell Lung Cancer to Radiation but Has Variable Effects with Chemoradiation. Clin. Cancer Res. 2016, 22, 5876–5886. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, F. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Sun, S.Y.; Yue, P. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem. Biophys. Res. Commun. 2001, 280, 788–797. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- O’Steen, S.; Green, D.J. Venetoclax Synergizes with Radiotherapy for Treatment of B-cell Lymphomas. Cancer Res. 2017, 77, 3885–3893. [Google Scholar] [CrossRef]
- Michaeli, O.; Luz, I. APR-246 as a radiosensitization strategy for mutant p53 cancers treated with alpha-particles-based radiotherapy. Cell Death Dis. 2024, 15, 426. [Google Scholar] [CrossRef]
- Yang, L.; Kumar, B. LCL161, a SMAC-mimetic, Preferentially Radiosensitizes Human Papillomavirus-negative Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2019, 18, 1025–1035. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, T.R. The sequential treatment with ionizing radiation followed by TRAIL/Apo-2L reduces tumor growth and induces apoptosis of breast tumor xenografts in nude mice. Int. J. Oncol. 2004, 24, 1133–1140. [Google Scholar] [CrossRef]
- Ma, D.J.; Galanis, E. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015, 17, 1261–1269. [Google Scholar] [CrossRef]
- Beckers, C.; Pruschy, M. Tumor hypoxia and radiotherapy: A major driver of resistance even for novel radiotherapy modalities. Semin. Cancer Biol. 2024, 98, 19–30. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Acosta, P.; Vallard, A. Biomarkers of resistance to radiation therapy: A prospective study in cervical carcinoma. Radiat. Oncol. 2017, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, R.; Goto, Y. UCHL1-HIF-1 axis-mediated antioxidant property of cancer cells as a therapeutic target for radiosensitization. Sci. Rep. 2017, 7, 6879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, X. HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Xueguan, L.; Xiaoshen, W. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor expression are associated with a poor prognosis in patients with nasopharyngeal carcinoma receiving radiotherapy with carbogen and nicotinamide. Clin. Oncol. 2008, 20, 606–612. [Google Scholar] [CrossRef]
- Thomson, D.; Yang, H. NIMRAD—A phase III trial to investigate the use of nimorazole hypoxia modification with intensity-modulated radiotherapy in head and neck cancer. Clin. Oncol. 2014, 26, 344–347. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—A systematic review and meta-analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef]
- Silva, V.L.; Ruiz, A. Hypoxia-targeted cupric-tirapazamine liposomes potentiate radiotherapy in prostate cancer spheroids. Int. J. Pharm. 2021, 607, 121018. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.L.; Powis, G. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol. Cancer Ther. 2009, 8, 947–958. [Google Scholar] [CrossRef]
- Tsien, C.I.; Pugh, S.L. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J. Clin. Oncol. 2023, 41, 1285–1295. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y. Lactate-Modulated Immunosuppression of Myeloid-Derived Suppressor Cells Contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol. Res. 2020, 8, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wei, R. CPT1A/2-Mediated FAO Enhancement-A Metabolic Target in Radioresistant Breast Cancer. Front. Oncol. 2019, 9, 1201. [Google Scholar] [CrossRef]
- Jiang, N.; Xie, B. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat. Commun. 2022, 13, 1511. [Google Scholar] [CrossRef]
- Fu, S.; Li, Z. Glutamine Synthetase Promotes Radiation Resistance via Facilitating Nucleotide Metabolism and Subsequent DNA Damage Repair. Cell Rep. 2019, 28, 1136–1143.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yao, Y. Purine metabolism regulates DNA repair and therapy resistance in glioblastoma. Nat. Commun. 2020, 11, 3811. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Shi, R.; Tang, Y.Q. Metabolism in tumor microenvironment: Implications for cancer immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Tao, T. Cancer-Associated Fibroblast-Induced Remodeling of Tumor Microenvironment in Recurrent Bladder Cancer. Adv. Sci. 2023, 10, e2303230. [Google Scholar] [CrossRef] [PubMed]
- Rødningen, O.K.; Overgaard, J. Microarray analysis of the transcriptional response to single or multiple doses of ionizing radiation in human subcutaneous fibroblasts. Radiother. Oncol. 2005, 77, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Yun, H.S. PLOD3 suppression exerts an anti-tumor effect on human lung cancer cells by modulating the PKC-delta signaling pathway. Cell Death Dis. 2019, 10, 156. [Google Scholar] [CrossRef]
- Saigusa, S.; Toiyama, Y. Cancer-associated fibroblasts correlate with poor prognosis in rectal cancer after chemoradiotherapy. Int. J. Oncol. 2011, 38, 655–663. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, S. Radiotherapy-sensitized cancer immunotherapy via cGAS-STING immune pathway by activatable nanocascade reaction. J. Nanobiotechnol. 2024, 22, 234. [Google Scholar] [CrossRef]
- Larionova, I.; Cherdyntseva, N. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 2019, 8, 1596004. [Google Scholar] [CrossRef]
- Ai, D.; Dou, Y. CD68+ Macrophage Infiltration Associates With Poor Outcome of HPV Negative Oral Squamous Carcinoma Patients Receiving Radiation: Poly(I:C) Enhances Radiosensitivity of CAL-27 Cells but Promotes Macrophage Recruitment Through HMGB1. Front. Oncol. 2021, 11, 740622. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer 2014, 111, 1509–1518. [Google Scholar] [CrossRef]
- Lippens, L.; Van Bockstal, M. Immunologic impact of chemoradiation in cervical cancer and how immune cell infiltration could lead toward personalized treatment. Int. J. Cancer 2020, 147, 554–564. [Google Scholar] [CrossRef]
- Wang, N.H.; Lei, Z. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: A narrative review. Ann. Transl. Med. 2022, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Komar, C. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017, 23, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017, 8, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Moustafa, M. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell 2021, 39, 1388–1403.e10. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 2016, 95 (Suppl. S1), S20–S25. [Google Scholar] [CrossRef]
- Gillespie, M.S.; Ward, C.M. DNA Repair and Therapeutic Strategies in Cancer Stem Cells. Cancers 2023, 15, 1897. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X. Correction: Radiochemotherapy-induced DNA repair promotes the biogenesis of gastric cancer stem cells. Stem Cell Res. Ther. 2024, 15, 358. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Ko, H.J. Ionizing Radiation Induces Resistant Glioblastoma Stem-Like Cells by Promoting Autophagy via the Wnt/β-Catenin Pathway. Life 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Linge, A. SLC3A2/CD98hc, autophagy and tumor radioresistance: A link confirmed. Autophagy 2019, 15, 1850–1851. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Bauer, K.M. Comparison of bottom-up proteomic approaches for LC-MS analysis of complex proteomes. Anal. Methods 2013, 5, 4615–4621. [Google Scholar] [CrossRef]
- Jumel, T.; Shevchenko, A. Multispecies Benchmark Analysis for LC-MS/MS Validation and Performance Evaluation in Bottom-Up Proteomics. J. Proteome Res. 2024, 23, 684–691. [Google Scholar] [CrossRef]
- Hamza, G.M.; Raghunathan, R. Proteomics of prostate cancer serum and plasma using low and high throughput approaches. Clin. Proteom. 2024, 21, 21. [Google Scholar] [CrossRef]
- Barrachina, M.N.; García, Á. Clinical Proteomics for the Analysis of Circulating Extracellular Vesicles. Methods Mol. Biol. 2021, 2259, 13–23. [Google Scholar]
- Beekhof, R.; Bertotti, A. Phosphoproteomics of patient-derived xenografts identifies targets and markers associated with sensitivity and resistance to EGFR blockade in colorectal cancer. Sci. Transl. Med. 2023, 15, eabm3687. [Google Scholar] [CrossRef]
- Zheng, R.; Matzinger, M. A High-Sensitivity Low-Nanoflow LC-MS Configuration for High-Throughput Sample-Limited Proteomics. Anal. Chem. 2023, 95, 18673–18678. [Google Scholar] [CrossRef]
- Lenčo, J.; Jadeja, S. Reversed-Phase Liquid Chromatography of Peptides for Bottom-Up Proteomics: A Tutorial. J. Proteome Res. 2022, 21, 2846–2892. [Google Scholar] [CrossRef]
- Glish, G.L.; Burinsky, D.J. Hybrid mass spectrometers for tandem mass spectrometry. J. Am. Soc. Mass. Spectrom. 2008, 19, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Shen, H. Identification, Quantification, and Site Localization of Protein Posttranslational Modifications via Mass Spectrometry-Based Proteomics. Adv. Exp. Med. Biol. 2016, 919, 345–382. [Google Scholar] [PubMed]
- Debrie, E.; Malfait, M. Quality Control for the Target Decoy Approach for Peptide Identification. J. Proteome Res. 2023, 22, 350–358. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Huang, T. Protein Inference. Adv. Exp. Med. Biol. 2016, 919, 237–242. [Google Scholar]
- Lemeer, S.; Hahne, H. Software tools for MS-based quantitative proteomics: A brief overview. Methods Mol. Biol. 2012, 893, 489–499. [Google Scholar]
- Lou, R.; Cao, Y. Benchmarking commonly used software suites and analysis workflows for DIA proteomics and phosphoproteomics. Nat. Commun. 2023, 14, 94. [Google Scholar] [CrossRef]
- Rho, S.; You, S. From proteomics toward systems biology: Integration of different types of proteomics data into network models. BMB Rep. 2008, 41, 184–193. [Google Scholar] [CrossRef]
- Ogata, S.; Masuda, T. Targeted proteomics for cancer biomarker verification and validation. Cancer Biomark. 2022, 33, 427–436. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, L. Proteomics of post-translational modifications in colorectal cancer: Discovery of new biomarkers. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188735. [Google Scholar] [CrossRef]
- Na, S.; Paek, E. Software eyes for protein post-translational modifications. Mass. Spectrom. Rev. 2015, 34, 133–147. [Google Scholar] [CrossRef]
- Petelski, A.A.; Emmott, E. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc. 2021, 16, 5398–5425. [Google Scholar] [CrossRef] [PubMed]
- Szyrwiel, L.; Gille, C. Fast proteomics with dia-PASEF and analytical flow-rate chromatography. Proteomics 2024, 24, e2300100. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, K.H. Mass spectrometry imaging for spatially resolved multi-omics molecular mapping. npj Imaging 2024, 2, 20. [Google Scholar] [CrossRef]
- Po, A.; Eyers, C.E. Top-Down Proteomics and the Challenges of True Proteoform Characterization. J. Proteome Res. 2023, 22, 3663–3675. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y. Quantitative Proteomics Using Isobaric Labeling: A Practical Guide. Genom. Proteom. Bioinform. 2021, 19, 689–706. [Google Scholar] [CrossRef]
- Swiatly, A.; Horala, A. Understanding Ovarian Cancer: iTRAQ-Based Proteomics for Biomarker Discovery. Int. J. Mol. Sci. 2018, 19, 2240. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, X. Quantitative proteomic analysis of cervical cancer based on TMT-labeled quantitative proteomics. J. Proteom. 2022, 252, 104453. [Google Scholar] [CrossRef]
- Kani, K. Quantitative Proteomics Using SILAC. Methods Mol. Biol. 2017, 1550, 171–184. [Google Scholar]
- Megger, D.A.; Bracht, T. Label-free quantification in clinical proteomics. Biochim. Biophys. Acta 2013, 1834, 1581–1590. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Dasari, S. Refining comparative proteomics by spectral counting to account for shared peptides and multiple search engines. Anal. Bioanal. Chem. 2012, 404, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Easterly, C.W. Precursor Intensity-Based Label-Free Quantification Software Tools for Proteomic and Multi-Omic Analysis within the Galaxy Platform. Proteomes 2020, 8, 15. [Google Scholar] [CrossRef]
- Meyer, J.G. Qualitative and Quantitative Shotgun Proteomics Data Analysis from Data-Dependent Acquisition Mass Spectrometry. Methods Mol. Biol. 2021, 2259, 297–308. [Google Scholar]
- Ludwig, C.; Gillet, L. Data-independent acquisition-based SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef]
- Palomba, A.; Abbondio, M. Comparative Evaluation of MaxQuant and Proteome Discoverer MS1-Based Protein Quantification Tools. J. Proteome Res. 2021, 20, 3497–3507. [Google Scholar] [CrossRef]
- Rice, S.J.; Belani, C.P. Optimizing data-independent acquisition (DIA) spectral library workflows for plasma proteomics studies. Proteomics 2022, 22, e2200125. [Google Scholar] [CrossRef] [PubMed]
- Pino, L.K.; Just, S.C. Acquiring and Analyzing Data Independent Acquisition Proteomics Experiments without Spectrum Libraries. Mol. Cell Proteom. 2020, 19, 1088–1103. [Google Scholar] [CrossRef]
- Manes, N.P.; Nita-Lazar, A. Application of targeted mass spectrometry in bottom-up proteomics for systems biology research. J. Proteom. 2018, 189, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Blonder, J. Targeted proteomics for validation of biomarkers in clinical samples. Brief. Funct. Genom. Proteom. 2009, 8, 126–135. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Sanni, A. Targeted Glycoproteomics Analysis Using MRM/PRM Approaches. Methods Mol. Biol. 2024, 2762, 231–250. [Google Scholar]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, Y. Clinical proteomics in cancer research-promises and limitations of current two-dimensional gel electrophoresis. Curr. Med. Chem. 2008, 15, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P. Proteomics discovery of radioresistant cancer biomarkers for radiotherapy. Cancer Lett. 2015, 369, 289–297. [Google Scholar] [CrossRef]
- Chen, Z.T.; Li, L. Analysis of the differential secretome of nasopharyngeal carcinoma cell lines CNE-2R and CNE-2. Oncol. Rep. 2015, 34, 2477–2488. [Google Scholar] [CrossRef]
- Li, Z.; Li, N. Quantitative Proteomic Analysis Identifies MAPK15 as a Potential Regulator of Radioresistance in Nasopharyngeal Carcinoma Cells. Front. Oncol. 2018, 8, 548. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, K. Serum proteomics identify potential biomarkers for nasopharyngeal carcinoma sensitivity to radiotherapy. Biosci. Rep. 2019, 39, BSR20190027. [Google Scholar] [CrossRef]
- Kochanek, D.M.; Ghouse, S.M. Complementing Cancer Metastasis. Front. Immunol. 2018, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yan, Y. SPARC expression in tumor microenvironment induces partial epithelial-to-mesenchymal transition of esophageal adenocarcinoma cells via cooperating with TGF-β signaling. Cell Biol. Int. 2023, 47, 250–259. [Google Scholar] [CrossRef]
- Williams, P.D.; Owens, C.R. Cyclophilin B expression is associated with in vitro radioresistance and clinical outcome after radiotherapy. Neoplasia 2011, 13, 1122–1131. [Google Scholar] [CrossRef][Green Version]
- Małecki, J.M.; Willemen, H.L.D.M. Human FAM173A is a mitochondrial lysine-specific methyltransferase that targets adenine nucleotide translocase and affects mitochondrial respiration. J. Biol. Chem. 2019, 294, 11654–11664. [Google Scholar] [CrossRef]
- Matsukawa, S.; Morita, K. Galectin-7 as a potential predictive marker of chemo- and/or radio-therapy resistance in oral squamous cell carcinoma. Cancer Med. 2014, 3, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Camby, I. Galectins as modulators of tumor progression in head and neck squamous cell carcinomas. Head. Neck 2007, 29, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.C.; Liu, J.J. A proteomic signature for human papillomavirus-associated oropharyngeal squamous cell carcinoma predicts patients at high risk of recurrence. Cancer Res. Commun. 2025, 5, 580–593. [Google Scholar] [CrossRef]
- Rasanen, K.; Sriswasdi, S. Comparative secretome analysis of epithelial and mesenchymal subpopulations of head and neck squamous cell carcinoma identifies S100A4 as a potential therapeutic target. Mol. Cell Proteom. 2013, 12, 3778–3792. [Google Scholar] [CrossRef]
- Lesko, J.; Triebl, A. Phospholipid dynamics in ex vivo lung cancer and normal lung explants. Exp. Mol. Med. 2021, 53, 81–90. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L. GAPVD1 Promotes the Proliferation of Triple-negative Breast Cancer Cells by Regulating the ERK/MAPK Signaling Pathway. Curr. Cancer Drug Targets 2025, 25, 509–519. [Google Scholar] [CrossRef]
- Tadayoni Nia, A.; Bazi, Z. WDR81 Gene Silencing Can Reduce Exosome Levels in Human U87-MG Glioblastoma Cells. J. Mol. Neurosci. 2021, 71, 1696–1702. [Google Scholar] [CrossRef]
- Wu, C.S.; Li, H.P. Integrated multi-omics analyses of oral squamous cell carcinoma reveal precision patient stratification and personalized treatment strategies. Cancer Lett. 2025, 614, 217482. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Yalamarty, S.S.K. Recent strategies to overcome breast cancer resistance. Crit. Rev. Oncol. Hematol. 2024, 197, 104351. [Google Scholar] [CrossRef]
- Kim, M.H.; Jung, S.Y. Quantitative proteomic analysis of single or fractionated radiation-induced proteins in human breast cancer MDA-MB-231 cells. Cell Biosci. 2015, 5, 2. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, Y. Profiling global kinome signatures of the radioresistant MCF-7/C6 breast cancer cells using MRM-based targeted proteomics. J. Proteome Res. 2015, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Bade, D. Targeted Proteomic Analysis Revealed Kinome Reprogramming during Acquisition of Radioresistance in Breast Cancer Cells. J. Proteome Res. 2021, 20, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Khozooei, S.; Lettau, K. Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J. Exp. Clin. Cancer Res. 2022, 41, 256. [Google Scholar] [CrossRef]
- Lamb, R.; Fiorillo, M. Doxycycline down-regulates DNA-PK and radiosensitizes tumor initiating cells: Implications for more effective radiation therapy. Oncotarget 2015, 6, 14005–14025. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; Liao, Z. Exosomal Proteome Profiling: A Potential Multi-Marker Cellular Phenotyping Tool to Characterize Hypoxia-Induced Radiation Resistance in Breast Cancer. Proteomes 2013, 1, 87–108. [Google Scholar] [CrossRef]
- Yadav, P.; Pandey, V.K. Proteomic analysis of radio-resistant breast cancer xenografts: Increased TGF-β signaling and metabolism. Cell Biol. Int. 2021, 45, 804–819. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Pan, L.; Wu, Q. Characterization and mechanisms of radioresistant lung squamous cell carcinoma cell lines. Thorac. Cancer 2023, 14, 1239–1250. [Google Scholar] [CrossRef]
- Lei, Z.; He, J. Global profiling of transcriptome, proteome and 2-hydroxyisobutyrylome in radioresistant lung adenocarcinoma cell. BMC Genom. 2024, 25, 923. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, Q. HDGF and PRKCA upregulation is associated with a poor prognosis in patients with lung adenocarcinoma. Oncol. Lett. 2019, 18, 4936–4946. [Google Scholar] [CrossRef]
- Li, K.; Wang, S. Prognostic Biomarkers after Radiotherapy for Nonsmall Cell Lung Cancer Based on Bioinformatics Analysis. BioMed Res. Int. 2022, 2022, 6405228. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Su, W. Effect and Mechanism of Tanshinone I on the Radiosensitivity of Lung Cancer Cells. Mol. Pharm. 2018, 15, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, L.; Hellevik, T. Cancer-associated fibroblasts from lung tumors maintain their immunosuppressive abilities after high-dose irradiation. Front. Oncol. 2015, 5, 87. [Google Scholar] [CrossRef]
- Huang, W.; Ding, X. Serum biomarkers analyzed by LC-MS/MS as predictors for short outcome of non-small cell lung cancer patients treated with chemoradiotherapy. Neoplasma 2013, 60, 11–18. [Google Scholar] [CrossRef]
- Kuai, X.; Lv, J. Serpin Family A Member 1 Is Prognostic and Involved in Immunological Regulation in Human Cancers. Int. J. Mol. Sci. 2023, 24, 11566. [Google Scholar] [CrossRef]
- Walker, M.J.; Zhou, C. Discovery and Validation of Predictive Biomarkers of Survival for Non-small Cell Lung Cancer Patients Undergoing Radical Radiotherapy: Two Proteins With Predictive Value. EBioMedicine 2015, 2, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Chang, L.; Ni, J. Identification of protein biomarkers and signaling pathways associated with prostate cancer radioresistance using label-free LC-MS/MS proteomic approach. Sci. Rep. 2017, 7, 41834. [Google Scholar] [CrossRef]
- Odiase, P.; Ma, J. The Role of Fetuin-A in Tumor Cell Growth, Prognosis, and Dissemination. Int. J. Mol. Sci. 2024, 25, 12918. [Google Scholar] [CrossRef]
- Kurganovs, N.; Wang, H. A proteomic investigation of isogenic radiation resistant prostate cancer cell lines. Proteom. Clin. Appl. 2021, 15, e2100037. [Google Scholar] [CrossRef]
- Challis, D.; Lippis, T. Multiomics analysis of adaptation to repeated DNA damage in prostate cancer cells. Epigenetics 2023, 18, 2214047. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Graham, P. Proteomic identification of the lactate dehydrogenase A in a radioresistant prostate cancer xenograft mouse model for improving radiotherapy. Oncotarget 2016, 7, 74269–74285. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.P.; Gulati, T. Exploring the oncoproteomic response of human prostate cancer to therapeutic radiation using data-independent acquisition (DIA) mass spectrometry. Prostate 2018, 78, 563–575. [Google Scholar] [CrossRef]
- Tirinato, L.; Marafioti, M.G. Lipid droplets and ferritin heavy chain: A devilish liaison in human cancer cell radioresistance. Elife 2021, 10, e72943. [Google Scholar] [CrossRef]
- Wu, X.; Yu, X. Fibrinogen and tumors. Front. Oncol. 2024, 14, 1393599. [Google Scholar] [CrossRef]
- Muniyan, S.; Chaturvedi, N.K. Human prostatic acid phosphatase: Structure, function and regulation. Int. J. Mol. Sci. 2013, 14, 10438–10464. [Google Scholar] [CrossRef]
- Williams, S.G.; Aw Yeang, H.X. Immune molecular profiling of a multiresistant primary prostate cancer with a neuroendocrine-like phenotype: A case report. BMC Urol. 2020, 20, 171. [Google Scholar] [CrossRef]
- Gao, A.; He, C. TMT-Based Quantitative Proteomic Profiling of Human Esophageal Cancer Cells Reveals the Potential Mechanism and Potential Therapeutic Targets Associated with Radioresistance. Proteom. Clin. Appl. 2025, 19, e202400010. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zheng, W. SDC1-TGM2-FLOT1-BHMT complex determines radiosensitivity of glioblastoma by influencing the fusion of autophagosomes with lysosomes. Theranostics 2023, 13, 3725–3743. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, K. PYGB Promoted Tumor Progression by Regulating Wnt/β-Catenin Pathway in Gastric Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820926592. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Q. Vitamin D Enhances Radiosensitivity of Colorectal Cancer by Reversing Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2021, 9, 684855. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, A.R.; Landemberger, M.C. Rab5C enhances resistance to ionizing radiation in rectal cancer. J. Mol. Med. 2019, 97, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Rajendra, J.; Datta, K.K. Enhanced proteasomal activity is essential for long term survival and recurrence of innately radiation resistant residual glioblastoma cells. Oncotarget 2018, 9, 27667–27681. [Google Scholar] [CrossRef] [PubMed]
- Clavreul, A.; Guette, C. Proteomics of tumor and serum samples from isocitrate dehydrogenase-wildtype glioblastoma patients: Is the detoxification of reactive oxygen species associated with shorter survival? Mol. Oncol. 2024, 18, 2783–2800. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perico, D.; Mauri, P. Deciphering Radiotherapy Resistance: A Proteomic Perspective. Proteomes 2025, 13, 25. https://doi.org/10.3390/proteomes13020025
Perico D, Mauri P. Deciphering Radiotherapy Resistance: A Proteomic Perspective. Proteomes. 2025; 13(2):25. https://doi.org/10.3390/proteomes13020025
Chicago/Turabian StylePerico, Davide, and Pierluigi Mauri. 2025. "Deciphering Radiotherapy Resistance: A Proteomic Perspective" Proteomes 13, no. 2: 25. https://doi.org/10.3390/proteomes13020025
APA StylePerico, D., & Mauri, P. (2025). Deciphering Radiotherapy Resistance: A Proteomic Perspective. Proteomes, 13(2), 25. https://doi.org/10.3390/proteomes13020025








