Chromosome X Open Reading Frame 38 (CXorf38) Is a Tumor Suppressor and Potential Prognostic Biomarker in Lung Adenocarcinoma: The First Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmids and Transfection
2.3. Immunoblotting Analysis
2.4. Cell Proliferation Assay
2.5. Focus Formation Assay
2.6. Kaplan–Meier (KM) Survival Analysis and Detection of CXorf38 Level in Smokers
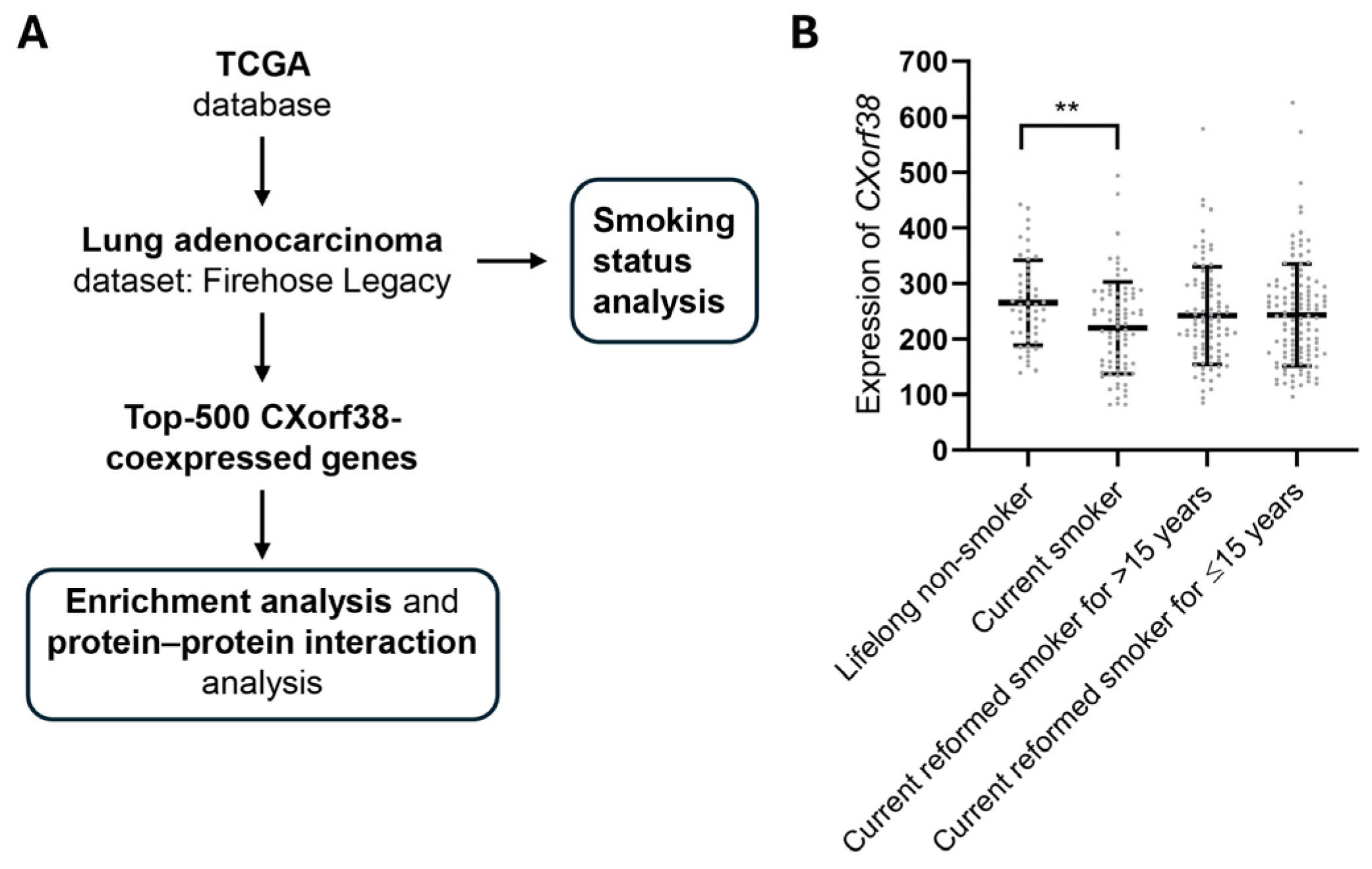
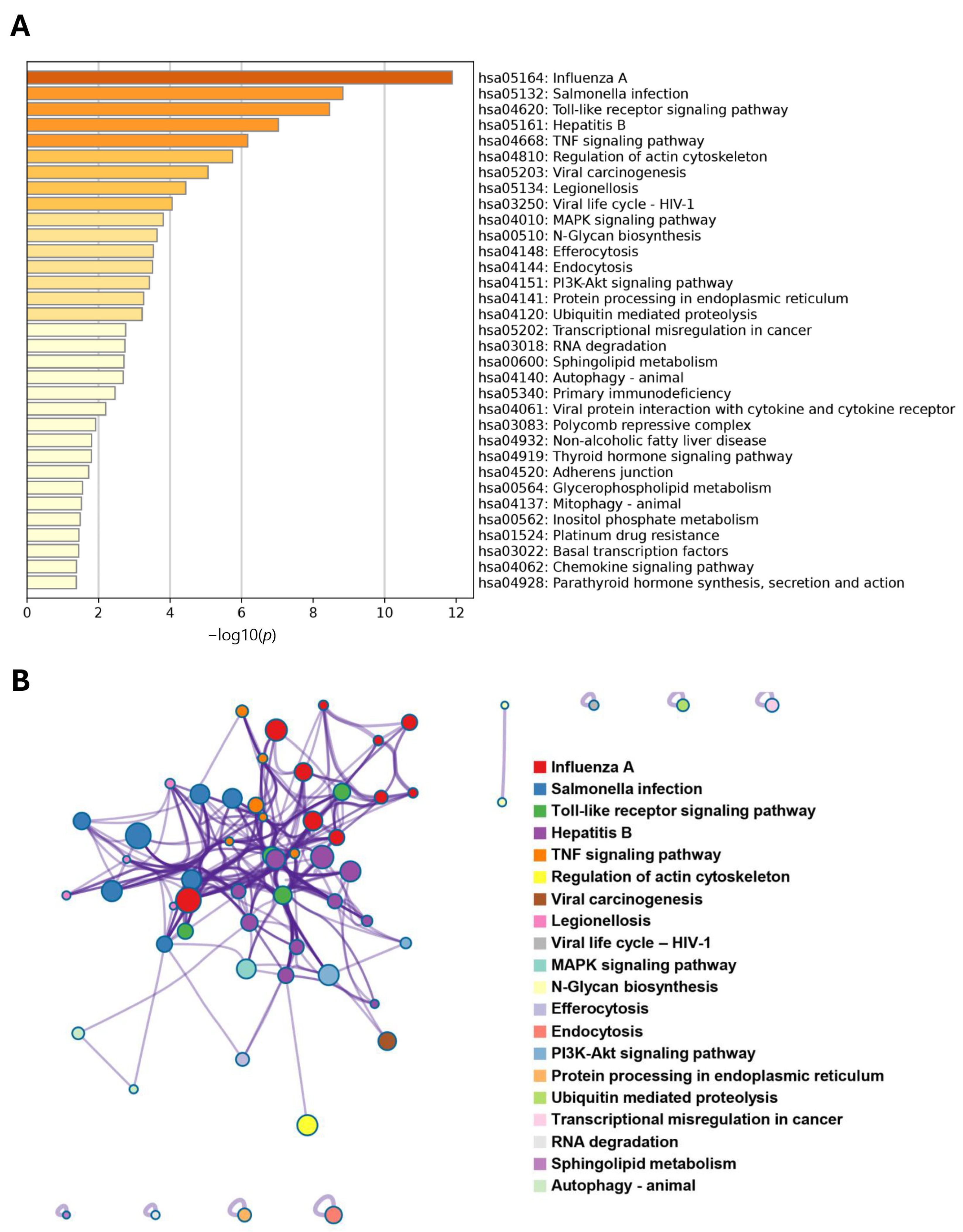
2.7. Identification of CXorf38-Coexpressed Genes, Enrichment Analysis, and PPI
2.8. Immune Cell Infiltration (ICI) Analysis
2.9. Statistical Analysis
3. Results and Discussion
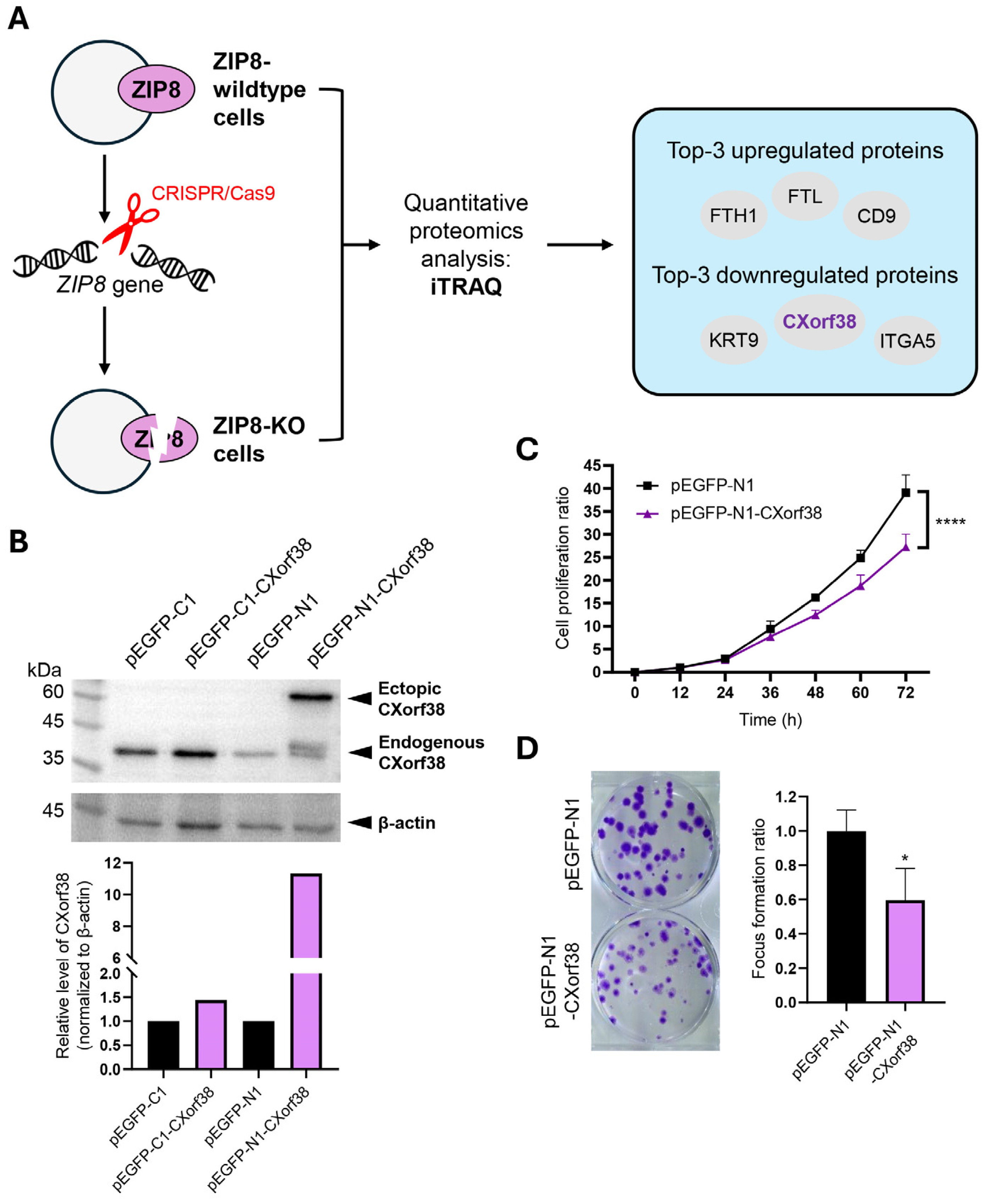
3.1. Proteomics Reveals Significant Downregulation of CXorf38 in ZIP8-KO Cells
3.2. CXorf38 Suppresses Growth and Colony Formation in Lung Cancer Cells
3.3. CXorf38 as a Prognostic Biomarker for Lung Adenocarcinoma but Not for Lung Squamous Cell Carcinoma
3.4. Clinical Database Analysis: CXorf38 Is Downregulated in Lung Cancer Tissue of Smokers
3.5. Bioinformatics Analysis Unveils the Potential Roles of CXorf38 in the Tumor Immune Microenvironment
3.6. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTMs | Post-Translational Modifications |
| PPI | Protein–Protein Interaction |
| ORF | Open Reading Frame |
| KO | Knockout |
| iTRAQ | Isobaric Tags for Relative and Absolute Quantitation |
| CXorf38 | Chromosome X Open Reading Frame 38 |
| ATCC | American Type Culture Collection |
| NBB | Naphthol Blue Black |
| RTCA | Real-Time Cell Analysis |
| TCGA | The Cancer Genome Atlas |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ICI | Immune Cell Infiltration |
References
- Tan, H.W.; Xu, Y.M.; Wu, D.D.; Lau, A.T.Y. Recent insights into human bronchial proteomics–how are we progressing and what is next? Expert Rev. Proteom. 2018, 15, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications–cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Cheng, C.; Zhang, L. High-throughput proteomics: A methodological mini-review. Lab. Investig. 2022, 102, 1170–1181. [Google Scholar] [CrossRef]
- Wu, C.; Lu, X.; Lu, S.; Wang, H.; Li, D.; Zhao, J.; Jin, J.; Sun, Z.; He, Q.Y.; Chen, Y.; et al. Efficient detection of the alternative spliced human proteome using translatome sequencing. Front. Mol. Biosci. 2022, 9, 895746. [Google Scholar] [CrossRef]
- Duek, P.; Lane, L. Worming into the uncharacterized human proteome. J. Proteome Res. 2019, 18, 4143–4153. [Google Scholar] [CrossRef]
- Xu, Y.M.; Lee, M.H.; Wong, C.M.; Lau, A.T.Y. Editorial: Characterizing the uncharacterized human proteins. Front. Genet. 2023, 14, 1203691. [Google Scholar] [CrossRef]
- Delcourt, V.; Staskevicius, A.; Salzet, M.; Fournier, I.; Roucou, X. Small proteins encoded by unannotated ORFs are rising stars of the proteome, confirming shortcomings in genome annotations and current vision of an mRNA. Proteomics 2018, 18, e1700058. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.I.; Pueyo, J.I.; Fouix, S.; Bishop, S.A.; Couso, J.P. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007, 5, e106. [Google Scholar] [CrossRef]
- Slavoff, S.A.; Heo, J.; Budnik, B.A.; Hanakahi, L.A.; Saghatelian, A. A human short open reading frame (sORF)-encoded polypeptide that stimulates DNA end joining. J. Biol. Chem. 2014, 289, 10950–10957. [Google Scholar] [CrossRef]
- D’Lima, N.G.; Ma, J.; Winkler, L.; Chu, Q.; Loh, K.H.; Corpuz, E.O.; Budnik, B.A.; Lykke-Andersen, J.; Saghatelian, A.; Slavoff, S.A. A human microprotein that interacts with the mRNA decapping complex. Nat. Chem. Biol. 2017, 13, 174–180. [Google Scholar] [CrossRef]
- Liang, Z.L.; Tan, H.W.; Wu, J.Y.; Chen, X.L.; Wang, X.Y.; Xu, Y.M.; Lau, A.T.Y. The Impact of ZIP8 disease-associated variants G38R, C113S, G204C, and S335T on selenium and cadmium accumulations: The first characterization. Int. J. Mol. Sci. 2021, 22, 11399. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.S.; Xu, Y.M.; Lau, A.T.Y. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol. Res. 2016, 5, 987–1002. [Google Scholar] [CrossRef]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.K.; Nguyen, T.T.; Gupta, N.; Iwase, S.; Seo, Y.A. Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Sci. Rep. 2018, 8, 3163. [Google Scholar] [CrossRef]
- Tan, H.W.; Xu, Y.M.; Liang, Z.L.; Cai, N.L.; Wu, Y.Y.; Lau, A.T.Y. Single-gene knockout-coupled omics analysis identifies C9orf85 and CXorf38 as two uncharacterized human proteins associated with ZIP8 malfunction. Front. Mol. Biosci. 2022, 9, 991308. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Tan, H.W.; Zhao, X.Y.; Wu, J.Y.; Zhong, Q.H.; Wang, X.Y.; Cai, N.L.; Xu, Y.M.; Lau, A.T.Y. A methionine/aspartate-rich synthetic peptide delineated from N-terminal region of nucleophosmin protein effectively protects against cadmium-induced toxicity. Environ. Int. 2025, 199, 109443. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhong, Q.H.; Tan, H.W.; Yan, R.; Wang, X.Y.; Cai, N.L.; Ji, Y.C.; Lau, A.T.Y.; Xu, Y.M. Non-cytotoxic levels of resveratrol enhance the anticancer effects of cisplatin by increasing the methyltransferase activity of CARM1 in human cancer cells. Phytomedicine 2024, 135, 156127. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Winslow, J.W.W.; Limesand, K.H.; Zhao, N. The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis. Int. J. Mol. Sci. 2020, 21, 3304. [Google Scholar] [CrossRef]
- Lin, W.; Vann, D.R.; Doulias, P.T.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J.; et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investig. 2017, 127, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkol, Y.; Lam, E.W. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Jamali, M.; Chetty, R. Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: An overview and the value of Ki-67 immunostaining. Endocr. Pathol. 2008, 19, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Che, Y.; Zhang, C.; Huang, J.; Lei, Y.; Lu, Z.; Sun, N.; He, J. PLAU directs conversion of fibroblasts to inflammatory cancer-associated fibroblasts, promoting esophageal squamous cell carcinoma progression via uPAR/Akt/NF-κB/IL8 pathway. Cell Death Discov. 2021, 7, 32. [Google Scholar] [CrossRef]
- Győrffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation 2024, 5, 100625. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, T.; Xie, F.; Wang, L.; Liang, Z.; Li, D.; Liang, Y.; Zhao, K.; Qi, X.; Yang, X.; et al. Evaluating the biological functions of the prognostic genes identified by the Pathology Atlas in bladder cancer. Oncol. Rep. 2021, 45, 191–201. [Google Scholar] [CrossRef]
- Tan, H.W.; Liang, Z.L.; Yao, Y.; Wu, D.D.; Mo, H.Y.; Gu, J.; Chiu, J.F.; Xu, Y.M.; Lau, A.T.Y. Lasting DNA damage and aberrant DNA repair gene expression profile are associated with post-chronic cadmium exposure in human bronchial epithelial cells. Cells 2019, 8, 842. [Google Scholar] [CrossRef]
- Tan, H.W.; Seen, D.L.T.; Xu, Y.M.; Lau, A.T.Y. Cadmium, cellular senescence, and cancer. Rev. Environ. Contam. Toxicol. 2023, 261, 21. [Google Scholar] [CrossRef]
- Sasco, A.J.; Secretan, M.B.; Straif, K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung Cancer 2004, 45, S3–S9. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Baralle, F.E.; Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017, 18, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, A.; Komatsu, S. Impact of post-translational modifications of crop proteins under abiotic stress. Proteomes 2016, 4, 42. [Google Scholar] [CrossRef]
- Ito, Y.; Hart, J.R.; Vogt, P.K. Isoform-specific activities of the regulatory subunits of phosphatidylinositol 3-kinases-potentially novel therapeutic targets. Expert Opin. Ther. Targets 2018, 22, 869–877. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, R.; Tan, H.-W.; Cai, N.-L.; Yu, L.; Gao, Y.; Xu, Y.-M.; Lau, A.T.Y. Chromosome X Open Reading Frame 38 (CXorf38) Is a Tumor Suppressor and Potential Prognostic Biomarker in Lung Adenocarcinoma: The First Characterization. Proteomes 2025, 13, 22. https://doi.org/10.3390/proteomes13020022
Yan R, Tan H-W, Cai N-L, Yu L, Gao Y, Xu Y-M, Lau ATY. Chromosome X Open Reading Frame 38 (CXorf38) Is a Tumor Suppressor and Potential Prognostic Biomarker in Lung Adenocarcinoma: The First Characterization. Proteomes. 2025; 13(2):22. https://doi.org/10.3390/proteomes13020022
Chicago/Turabian StyleYan, Rui, Heng-Wee Tan, Na-Li Cai, Le Yu, Yan Gao, Yan-Ming Xu, and Andy T. Y. Lau. 2025. "Chromosome X Open Reading Frame 38 (CXorf38) Is a Tumor Suppressor and Potential Prognostic Biomarker in Lung Adenocarcinoma: The First Characterization" Proteomes 13, no. 2: 22. https://doi.org/10.3390/proteomes13020022
APA StyleYan, R., Tan, H.-W., Cai, N.-L., Yu, L., Gao, Y., Xu, Y.-M., & Lau, A. T. Y. (2025). Chromosome X Open Reading Frame 38 (CXorf38) Is a Tumor Suppressor and Potential Prognostic Biomarker in Lung Adenocarcinoma: The First Characterization. Proteomes, 13(2), 22. https://doi.org/10.3390/proteomes13020022





