HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethanolic Extract Preparation of Ocimum sanctum
2.2. Parasite Collection and Culture
2.3. Preparation of S. cervi Homogenate
2.4. Estimation of Viability
2.5. Estimation of Glutathione (GSH) Level
2.6. Estimation of Glutathione-S-Transferase (GST) Activity
2.7. Estimation of Glutathione Reductase (GR) Activity
2.8. Estimation of Thioredoxin Reductase (TrxR) Activity
2.9. Estimation of Protein Carbonyl Content
2.10. Estimation of Lipid Peroxidation
2.11. Estimation of NADPH Oxidase Activity
2.12. Two-Dimensional Gel Electrophoresis of S. cervi Protein Samples After EOS Treatment
2.12.1. Sample Preparation
2.12.2. Two-Dimensional Electrophoresis
2.13. Image Analysis and Quantitation
2.14. Reduction and Trypsin Digestion of Differentially Expressed Spots from 2D Gels
2.15. High-Resolution Accurate Mass Spectrometry (HRAMS) Analysis
2.16. Statistical Analysis
2.17. String Analysis of Differentially Expressed Proteins
2.18. Retrieval of Targeted Protein Structures
2.19. Protein Model Validation
2.20. Retrieval of Ligand
2.21. Molecular Docking
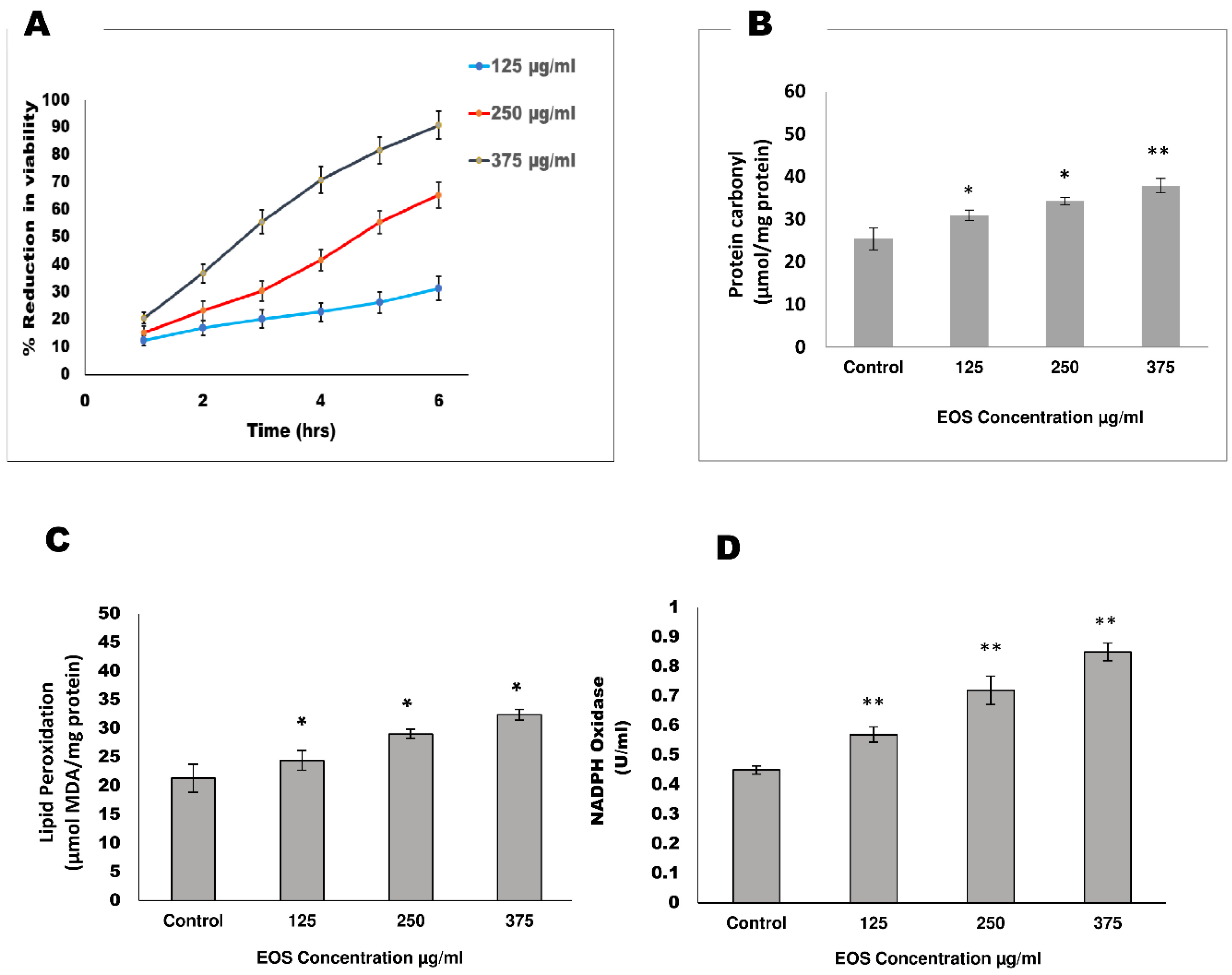
3. Results
3.1. Viability
3.2. Effect of EOS on Oxidative Stress Markers
3.3. Proteforms Profile of S. cervi After EOS Treatment
3.4. Protein Networks and Functional Analysis
3.5. Target Protein Retrieval and Validation
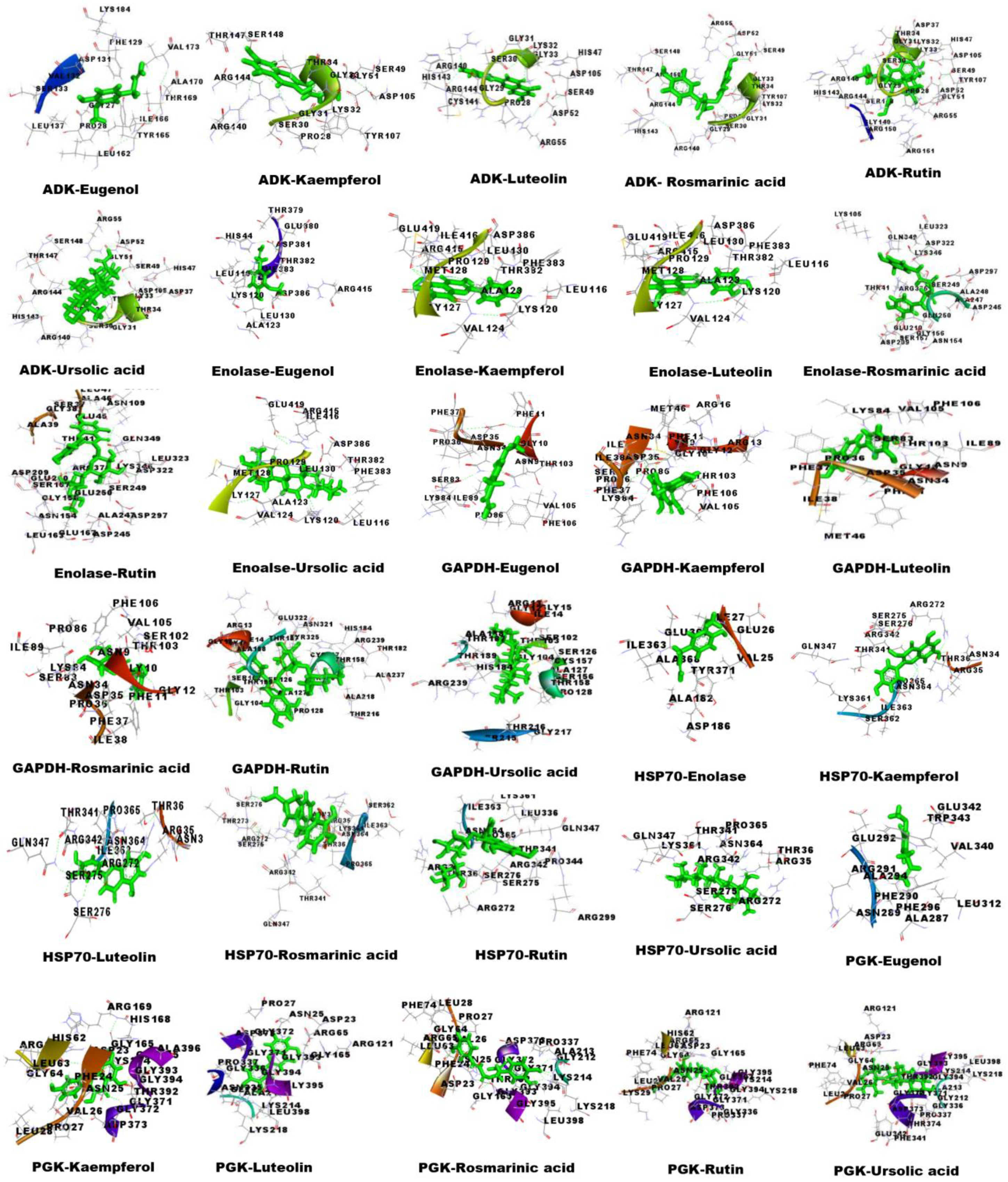
3.6. Molecular Docking Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 2 December 2024).
- Ton, T.G.; Mackenzie, C.; Molyneux, D.H. The burden of mental health in lymphatic filariasis. Infect. Dis. Poverty 2015, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, M.; Singh, N.; Rathaur, S. Inhibition of cathepsin B by E-64 induces oxidative stress and apoptosis in filarial parasite. PLoS ONE 2014, 9, e93161. [Google Scholar] [CrossRef] [PubMed]
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature. PLoS Negl. Trop. Dis. 2018, 12, e0006454. [Google Scholar] [CrossRef] [PubMed]
- Behera, D.R.; Bhatnagar, S. Filariasis: Role of medicinal plant in lymphatic filariasis. Int. J. Herb. Med. 2018, 6, 40–46. [Google Scholar]
- Cobo, F. Determinants of parasite drug resistance in human lymphatic filariasis. Rev. Española Quimioter. 2016, 29, 288–295. [Google Scholar]
- Singh, A.; Mishra, A.; Chaudhary, R.; Kumar, V. Role of herbal plants in prevention and treatment of parasitic diseases. J. Sci. Res. 2020, 64, 50–58. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef]
- Baliga, M.S.; Jimmy, R.; Thilakchand, K.R.; Sunitha, V.; Bhat, N.R.; Saldanha, E.; Rao, S.; Rao, P.; Arora, R.; Palatty, P.L. Ocimum sanctum L. (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr. Cancer 2013, 65 (Suppl. S1), 26–35. [Google Scholar] [CrossRef]
- Pandey, R.; Chandra, P.; Srivastava, M.; Mishra, D.K.; Kumar, B. Simultaneous quantitative determination of multiple bioactive markers in Ocimum sanctum obtained from different locations and its marketed herbal formulations using UPLC-ESI-MS/MS combined with principal component analysis. Phytochem. Anal. 2015, 26, 383–394. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sharma, S.; Mittal, A.; Gupta, S.; Dua, A. Phytochemical and antioxidant profiling of Ocimum sanctum. J. Food Sci. Technol. 2020, 57, 3852–3863. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, V.; Singh, A. Deciphering the anti-filarial potential of bioactive compounds from Ocimum sanctum: A combined experimental and computational study. Pharm. Biol. 2022, 60, 2237–2252. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Sinha Babu, S.P. Antifilarial effects of polyphenol rich ethanolic extract from the leaves of Azadirachta indica through molecular and biochemical approaches describing reactive oxygen species (ROS) mediated apoptosis of Setaria cervi. Exp. Parasitol. 2014, 136, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rathaur, S. Identification and characterization of a selenium dependent glutathione peroxidase in Setaria cervi. Biochem. Biophys. Res. Commun. 2005, 331, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ahmad, F.; Singh, A.; Rathaur, S. Identification of glucose regulated protein94 (GRP94) in filarial parasite S. cervi and its expression under ER stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2022, 258, 110683. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of picogram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1970, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization glutathione reductase from calf liver: An improved procedure for affinity chromatography on 29 59-ADP sepharose 4B. Anal. Biochem. 1981, 116, 531–536. [Google Scholar] [CrossRef]
- Holmgren, A.; Björnstedt, M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, R.A.; Vercauteren, R.E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J. Leukoc. Biol. 1984, 36, 751–759. [Google Scholar] [CrossRef]
- Kumar, V.; Mishra, A.; Yadav, A.K.; Rathaur, S.; Singh, A. Lymphatic filarial serum proteome profiling for identification and characterization of diagnostic biomarkers. PLoS ONE 2022, 17, e0270635. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Costanzo, L.D.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Willard, L.; Ranjan, A.; Zhang, H.; Monzavi, H.; Boyko, R.F.; Sykes, B.D.; Wishart, D.S. VADAR: A web server for quantitative evaluation of protein structure quality. Nucleic Acids Res. 2003, 31, 3316–3319. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Jayaram, B.; Singh, T.; Mukherjee, G.; Mathur, A.; Shekhar, S.; Shekhar, V. Sanjeevini: A freely accessible web-server for target directed lead molecule discovery. BMC Bioinform. 2012, 13, S7. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. Correction to “admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties”. J. Chem. Inf. Model. 2019, 59, 4959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mishra, A.; Singh, V.K.; Singh, A. Targeting HIV-1 Tat and Human Tat Protein Complex through Natural Products: An In silico Docking and Molecular Dynamic Simulation Approach. Lett. Drug Des. Discov. 2022, 19, 982–995. [Google Scholar] [CrossRef]
- Musthaba, M.; Baboota, S.; Athar, T.M.; Thajudeen, K.Y.; Ahmed, S.; Ali, J. Patented herbal formulations and their therapeutic applications. Recent Pat. Drug Deliv. Formul. 2010, 4, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Al-Abd, N.M.; Nor, Z.M.; Al-Adhroey, A.H.; Suhaimi, A.; Sivanandam, S. Recent advances on the use of biochemical extracts as filaricidal agents. Evid. Based Complement. Altern. Med. 2013, 2013, 986573. [Google Scholar] [CrossRef]
- Kustiati, U.; Ratih, T.S.D.; Agung, N.D.A.; Kusindarta, D.L.; Wihadmadyatami, H. In silico molecular docking and in vitro analysis of ethanolic extract Ocimum sanctum Linn.: Inhibitory and apoptotic effects against non-small cell lung cancer. Vet. World 2021, 14, 3175–3187. [Google Scholar] [CrossRef]
- Inbaneson, S.J.; Sundaram, R.; Suganthi, P. In vitro antiplasmodial effect of ethanolic extracts of traditional medicinal plant Ocimum species against Plasmodium falciparum. Asian Pac. J. Trop. Med. 2012, 5, 103–106. [Google Scholar] [CrossRef]
- Kaur, S.; Bhardwaj, K.; Sachdeva, H. Antileishmanial efficacy of Boerhaavia diffusa L. and Ocimum sanctum L. against experimental visceral leishmaniasis. Indian J. Exp. Biol. 2015, 53, 522–529. [Google Scholar]
- Sonar, V.P.; Corona, A.; Distinto, S.; Maccioni, E.; Meleddu, R.; Fois, B.; Floris, C.; Malpure, N.V.; Alcaro, S.; Tramontano, E.; et al. Natural product-inspired esters and amides of ferulic and caffeic acid as dual inhibitors of HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2017, 130, 248–260. [Google Scholar] [CrossRef]
- Nandini, H.S.; Krishna, K.L.; Apattira, C. Combination of Ocimum sanctum extract and Levetiracetam ameliorates cognitive dysfunction and hippocampal architecture in rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2022, 120, 102069. [Google Scholar] [CrossRef]
- Venuprasad, M.P.; Kandikattu, H.K.; Razack, S.; Amruta, N.; Khanum, F. Chemical composition of Ocimum sanctum by LC-ESI–MS/MS analysis and its protective effects against smoke induced lung and neuronal tissue damage in rats. Biomed. Pharmacother. 2017, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magesh, V.; Lee, J.C.; Ahn, K.S.; Lee, H.J.; Lee, H.J.; Lee, E.O.; Shim, B.S.; Jung, H.J.; Kim, J.S.; Kim, D.K.; et al. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother. Res. 2009, 23, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Agrawal, S.K.; Sharma, P.R.; Chadha, B.S.; Khosla, M.K.; Saxena, A.K. Cytotoxic and apoptotic activity of essential oil from Ocimumviride towards COLO 205 cells. Food Chem. Toxicol. 2010, 48, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rathaur, S. Combination of DEC plus aspirin induced mitochondrial mediated apoptosis in filarial parasite Setaria cervi. Biochimie 2010, 92, 894–900. [Google Scholar] [CrossRef]
- Sharma, S.; Ahmad, F.; Singh, A.; Rathaur, S. Role of anti-filarial drugs in inducing ER stress mediated signaling in bovine filarial parasitosis Setaria cervi. Vet. Parasitol. 2021, 290, 109357. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S.; Ahmad, F.; Rathaur, S. Antifilarial efficacy of green silver nanoparticles synthesized using Andrographis paniculate. J. Drug Deliv. Sci. Technol. 2020, 56, 101557. [Google Scholar] [CrossRef]
- Kanojiya, D.; Shanker, D.; Sudan, V.; Jaiswal, A.K.; Parashar, R. Anthelmintic activity of Ocimum sanctum leaf extract against ovine gastrointestinal nematodes in India. Res. Vet. Sci. 2015, 99, 165–170. [Google Scholar] [CrossRef]
- Chiumiento, L.; Bruschi, F. Enzymatic antioxidant systems in helminth parasites. Parasitol. Res. 2009, 105, 593. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, A.K. Glutathione metabolism of filarial worms: A vulnerable target for the design and synthesis of new antifilarial agents. Med. Sci. Monit. 2006, 12, HY1–HY9. [Google Scholar]
- Tiwari, S.; Wadhawan, M.; Singh, N.; Rathaur, S. Effect of CDNB on filarial thioredoxin reductase: A proteomic and biochemical approach. J. Proteom. 2015, 113, 435–446. [Google Scholar] [CrossRef]
- Taylor, J.P.; Tse, H.M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021, 48, 102159. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Wadhawan, M.; Tiwari, S.; Kumar, R.; Rathaur, S. Inhibition of Setaria cervi protein tyrosine phosphatases by Phenylarsine oxide: A proteomic and biochemical study. Acta Trop. 2016, 159, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srikanth, E.; Liebau, E.; Rathaur, S. Identification of Setaria cervi heat shock protein 70 by mass spectrometry and its evaluation as diagnostic marker for lymphatic filariasis. Vaccine 2010, 28, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Huang, M.Y.; Park, J.; Yenari, M.A.; Lee, J.E. Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 2020, 9, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zinsser, V.L.; Hoey, E.M.; Trudgett, A.; Timson, D.J. Biochemical characterisation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from the liver fluke, Fasciola hepatica. Biochim. Biophys. Acta 2014, 1844, 744–749. [Google Scholar] [CrossRef]
- Dzeja, P.; Terzic, A. Adenylate kinase and AMP signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 2009, 10, 1729–1772. [Google Scholar] [CrossRef]
- Rathaur, S.; Yadav, M.; Singh, N.; Singh, A. Effect of diethylcarbamazine, butylated hydroxy anisole and methyl substituted chalcone on filarial parasite Setaria cervi: Proteomic and biochemical approaches. J. Proteom. 2011, 74, 1595–1606. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Pattanaik, S.; Pattnaik, G.; Mallick, S.; Mohapatra, R. Review on the use of Molecular Docking as the First Line Tool in Drug Discovery and Development. Indian J. Pharm. Sci. 2022, 84, 1334–1337. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Kalani, K.; Kushwaha, V.; Sharma, P.; Verma, R.; Srivastava, M.; Khan, F.; Murthy, P.K.; Srivastava, S.K. In Vitro, In Silico and In Vivo Studies of Ursolic Acid as an Anti-Filarial Agent. PLoS ONE 2014, 9, e11124. [Google Scholar] [CrossRef]
- Saini, P.; Gayen, P.; Kumar, D.; Nayak, A.; Mukherjee, N.; Mukherjee, S.; Pal, B.C.; Babu, S.P. Antifilarial effect of ursolic acid from Nyctanthes arbortristis: Molecular and biochemical evidences. Parasitol. Int. 2014, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Xia, A.; Cheng, W.; Zhou, M.; Wang, J.; Shi, T.; Cai, C.; Jin, W.; Zhou, M.; Liao, Y.; et al. Rutin Promotes Pancreatic Cancer Cell Apoptosis by Upregulating miRNA-877-3p Expression. Molecules 2022, 27, 2293. [Google Scholar] [CrossRef]
- Lakshmi, V.; Joseph, S.K.; Srivastava, S.; Verma, S.K.; Sahoo, M.K.; Dube, V.; Mishra, S.K.; Murthy, P.K. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop. 2010, 116, 127–133. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef]
- Jain, M.; Dhariwal, R.; Patil, N.; Ojha, S.; Tendulkar, R.; Tendulkar, M.; Dhanda, P.S.; Yadav, A.; Kaushik, P. Unveiling the Molecular Footprint: Proteome-Based Biomarkers for Alzheimer’s Disease. Proteomes 2023, 11, 33. [Google Scholar] [CrossRef]



| Samples | GSH (µM/mg Protein) | GST (U/mL) | GR (U/mL) | TRxR |
|---|---|---|---|---|
| Control | 6.50 ± 0.120 | 22.86 ± 0.568 | 16.02 ± 1.110 | 18.12 ± 0.832 |
| 125 µg/mL | 5.82 ± 0.513 | 20.34 ± 0.862 * | 11.89 ± 0.636 ** | 11.35 ± 1.232 ** |
| (−10.46%) | (−11.02%) | (−25.78%) | (−37.36%) | |
| 250 µg/mL | 4.24 ± 0.982 | 15.56 ± 1.101 * | 9.54 ± 0.350 ** | 5.82 ± 0.345 *** |
| (−34.76%) | (−31.93%) | (−40.44%) | (−67.88%) | |
| 375 µg/mL | 3.95 ± 0.832 * | 11.32 ± 1.926 * | 6.38 ± 0.832 ** | 3.99 ± 0.127 *** |
| (−39.23%) | (−50.48%) | (−60.17%) | (−77.98%) |
| S.N. | Protein a (Accession Number) | Function | MW/pI | Identified Peptide | Fold Change b,c | |
|---|---|---|---|---|---|---|
| Expected | Experimental | |||||
| Cytosol stress response protein/chaperons | ||||||
| 1 | Heat-shock protein 70 [P27541] | Act as molecular chaperone; help in protein folding, transport, and assembly; and protect cell against stress | 72/5.6 | 70.2/5.47 | [K].MKETAEAFLGHAVK.[D] [RH].NVLIFDLGGGTFDVSILTIEDGIFEVK.[S] [R].IINEPTAAAIAYGLDK.[K] | +1.9 * |
| 2 | p27 [A0A4E9ERK7] | Cell cycle regulation | 25.86/7.9 | 24.1/6.32 | [K].IDVTPSNYSVLDTEFGSMR.[E] [R].AVFREYNQEFMLPR.[G] | −2.4 ** |
| Cytosol energy metabolism proteins | ||||||
| 3 | Adenylate kinase isoenzyme 1 [J9AQV1] | Catalyzes phosphoryl transferase, having a role in metabolic monitoring and AMP signaling | 22.95/8.8 | 22.8/8.34 | [R].LHTYITATAPVVDYYQK.[Q] [K].YGLTHLSSGDLLRAEVK.[S] [K].ANVPIFFIVGGPGSGKGTQCDKIVAK.[Y] | −3.6 *** |
| 4 | Enolase [Q5GTG4] | Role in glycolysis and gluconeogenesis | 47/5.9 | 46.4/5.67 | [R].LAKYNELIR.[I] | −3.3 *** |
| 5 | Glyceraldehyde−3-phosphate dehydrogenase [A0A4E9FA01] | Role in energy metabolism | 36/7.1 | 36.1/7.84 | [K].LTGMAFRVPTPDVSVVDLTCR.[L] [R].VPTPDVSVVDLTCR.[L] [K].AVGKVIPDLNGKLTGMAFR.[V] | −3.3 *** |
| 6 | Phosphoglycerate kinase [A0A4E9EYJ5] | Glycolytic enzyme | 44/7.68 | 44.6/7.88 | [K].MEFTLEPVAAELK.[A] [R].AKTIVWNGPAGVFEWENFSK.[G] [R].KMEFTLEPVAAELK.[A] | −3.2 *** |
| Antioxidant protein/enzymes | ||||||
| 7 | Glutathione S-transferase [E3UV59] | Antioxidant enzyme; detoxification of endogenous and xenobiotics compounds | 25/5.88 | 24.1/6.68 | [K].DILPVELAKFEK.[L] [K].FEKLLATR.[D] | −2.6 ** |
| 8 | Thioredoxin domain-containing protein [A0A4E9FJK0] | Antioxidant enzyme | 22.1/7.09 | 22.1/7.06 | [R].LIQAFQFVDKHGEVCPANWHPGSETIKPGVK.[E] [K].GKYVVLFFYPLDFTFVCPTEIIAFSDR.[I] | +1.5 * |
| Signaling protein | ||||||
| 9 | Coiled-coil domain-containing protein 6 [A0A1I8E9M6] | Structural motifs involved in a variety of important interactions | 60.92/5.19 | 60.9/5.26 | [R].AFAASETTRENDEDNCMAALLNR.[M] | +2.4 ** |
| Protein Digestion and folding protein | ||||||
| 10 | Calreticulin precursor [A0A0J9XSV8] | Calcium-binding chaperone role in transcription regulation | 47.42/4.78 | 49.4/4.87 | [K].KVHVIFHYKGR.[N] [K].HKDDFGKWEISHGK.[F] | −3.5 *** |
| Receptor | Name of Ligand | Binding Energy (Kcal/mol) | Dissociation Constant (µm) | GSC Score | AI Area |
|---|---|---|---|---|---|
| ADK | Eugenol | 5.7090 | 65.3502 | 3286 | 381.30 |
| Kaempferol | 7.4400 | 3.5189 | 4244 | 473.50 | |
| Luteolin | 7.5920 | 2.7226 | 4280 | 497.40 | |
| Rosmarinic acid | 7.9160 | 1.5757 | 5100 | 636.50 | |
| Rutin | 9.0130 | 0.2473 | 5614 | 630.70 | |
| Ursolic acid | 7.6660 | 2.4029 | 6176 | 791.80 | |
| Albendazole | 5.9560 | 43.0719 | 4426 | 518.70 | |
| DEC | 5.0650 | 193.7785 | 4038 | 475.00 | |
| Enolase | Eugenol | 5.4220 | 106.0770 | 3484 | 388.50 |
| Kaempferol | 8.0820 | 1.1907 | 4062 | 388.50 | |
| Luteolin | 7.630 | 2.4252 | 3760 | 438.70 | |
| Rosmarinic acid | 7.6640 | 2.4110 | 4634 | 540.60 | |
| Rutin | 8.7070 | 0.4146 | 5488 | 711.60 | |
| Ursolic acid | 8.0270 | 1.3065 | 5800 | 699.80 | |
| Albendazole | 5.6240 | 75.4316 | 3994 | 480.80 | |
| DEC | 4.9930 | 218.8177 | 3764 | 421.30 | |
| GAPDH | Eugenol | 6.1610 | 30.4738 | 3124 | 331.50 |
| Kaempferol | 7.5610 | 2.8688 | 3664 | 406.20 | |
| Luteolin | 7.9720 | 1.4336 | 3710 | 426.50 | |
| Rosmarinic acid | 7.6930 | 2.2959 | 4252 | 494.10 | |
| Rutin | 8.5540 | 0.5368 | 5538 | 651.60 | |
| Ursolic acid | 9.2360 | 0.1697 | 5352 | 578.60 | |
| Albendazole | 5.8850 | 48.5554 | 3730 | 451.10 | |
| DEC | 5.0210 | 208.7171 | 3500 | 372.30 | |
| HSP70 | Eugenol | 4.8890 | 260.8047 | 3232 | 3555.30 |
| Kaempferol | 6.6860 | 12.5631 | 3914 | 441.00 | |
| Luteolin | 7.0660 | 6.6153 | 3888 | 417.10 | |
| Rosmarinic acid | 7.2870 | 4.5557 | 4590 | 548.30 | |
| Rutin | 7.9120 | 1.5864 | 5268 | 719.70 | |
| Ursolic acid | 7.8070 | 1.8940 | 5276 | 658.40 | |
| Albendazole | 6.0360 | 37.6316 | 3830 | 438.10 | |
| DEC | 4.8560 | 275.7432 | 3676 | 418.90 | |
| PGK | Eugenol | 5.5360 | 7.5101 | 3162 | 335.40 |
| Kaempferol | 8.0840 | 1.1872 | 3546 | 373.70 | |
| Luteolin | 8.4230 | 0.6696 | 3528 | 390.70 | |
| Rosmarinic acid | 8.6300 | 0.4722 | 4396 | 479.90 | |
| Rutin | 8.9930 | 0.2558 | 4968 | 551.60 | |
| Ursolic acid | 10.2220 | 0.0321 | 4860 | 548.70 | |
| Albendazole | 6.1310 | 32.0565 | 3904 | 443.90 | |
| DEC | 5.7720 | 58.7581 | 3398 | 353.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Kumar, V.; Kumar, S.; Singh, H.; Singh, A. HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing. Proteomes 2025, 13, 2. https://doi.org/10.3390/proteomes13010002
Mishra A, Kumar V, Kumar S, Singh H, Singh A. HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing. Proteomes. 2025; 13(1):2. https://doi.org/10.3390/proteomes13010002
Chicago/Turabian StyleMishra, Ayushi, Vipin Kumar, Sunil Kumar, HariOm Singh, and Anchal Singh. 2025. "HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing" Proteomes 13, no. 1: 2. https://doi.org/10.3390/proteomes13010002
APA StyleMishra, A., Kumar, V., Kumar, S., Singh, H., & Singh, A. (2025). HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing. Proteomes, 13(1), 2. https://doi.org/10.3390/proteomes13010002







